Suppression of Pax3–MITF-M Axis Protects from UVB-Induced Skin Pigmentation by Tetrahydroquinoline Carboxamide
Abstract
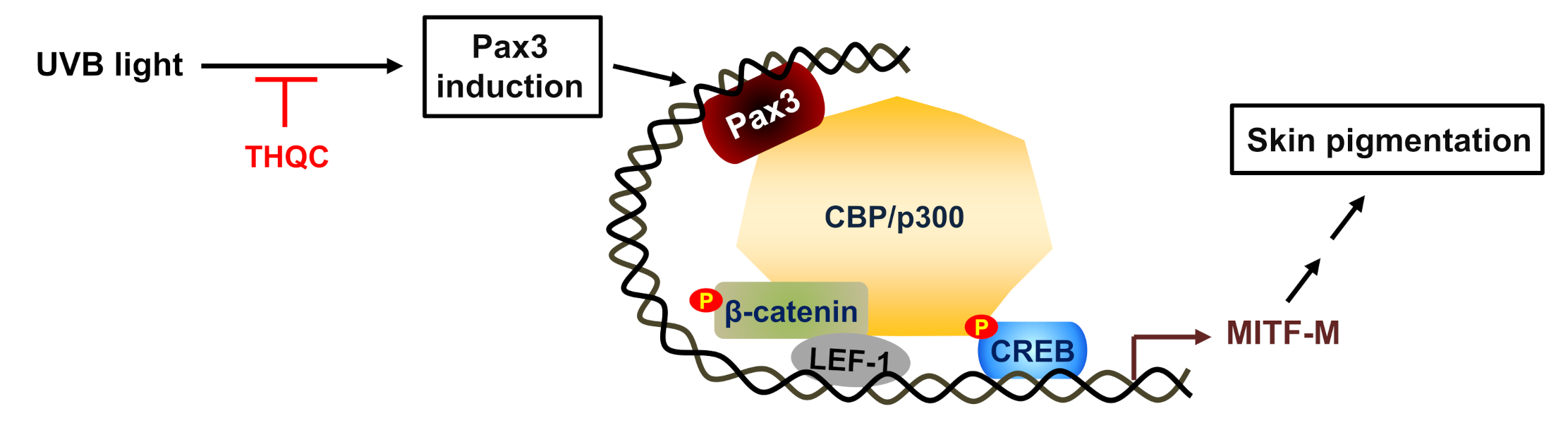
:1. Introduction
2. Results
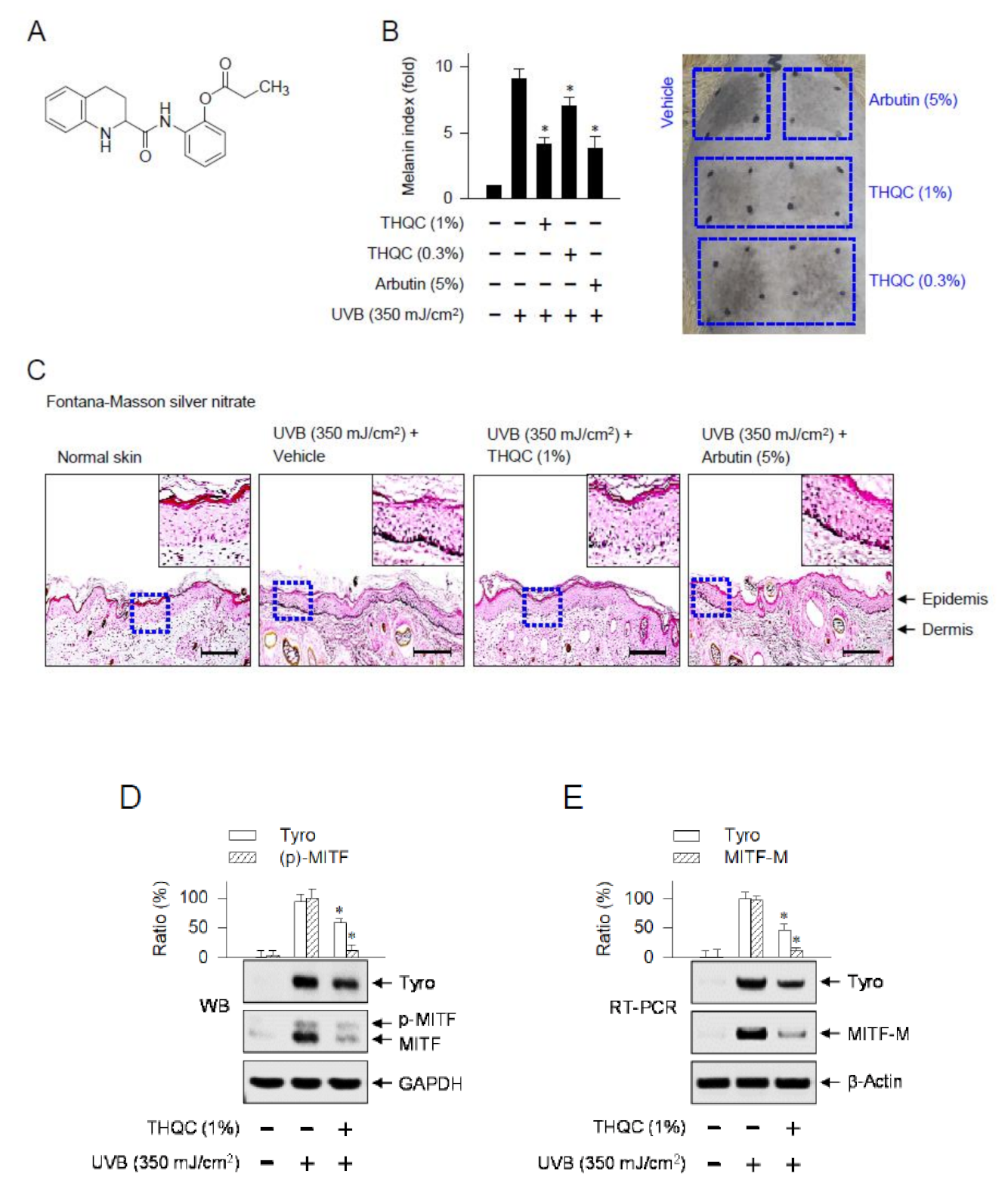
2.1. Topical Treatment with THQC Mitigated UVB-Induced Skin Pigmentation
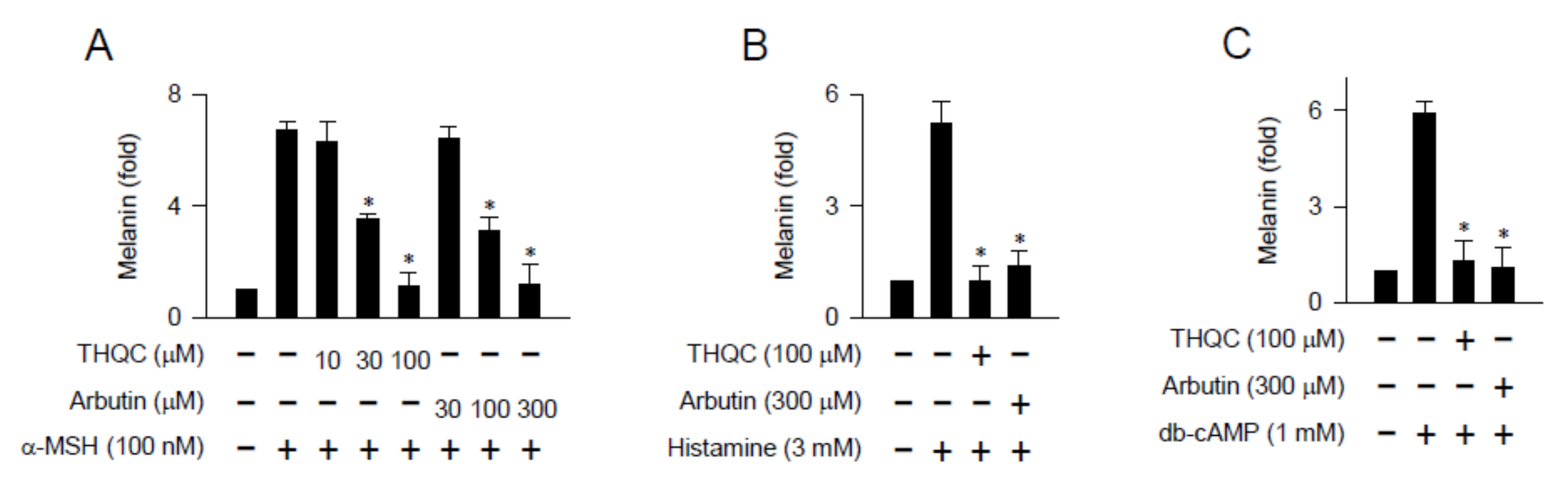
2.2. THQC Inhibited Melanin Production in cAMP-Elevated B16-F0 Culture
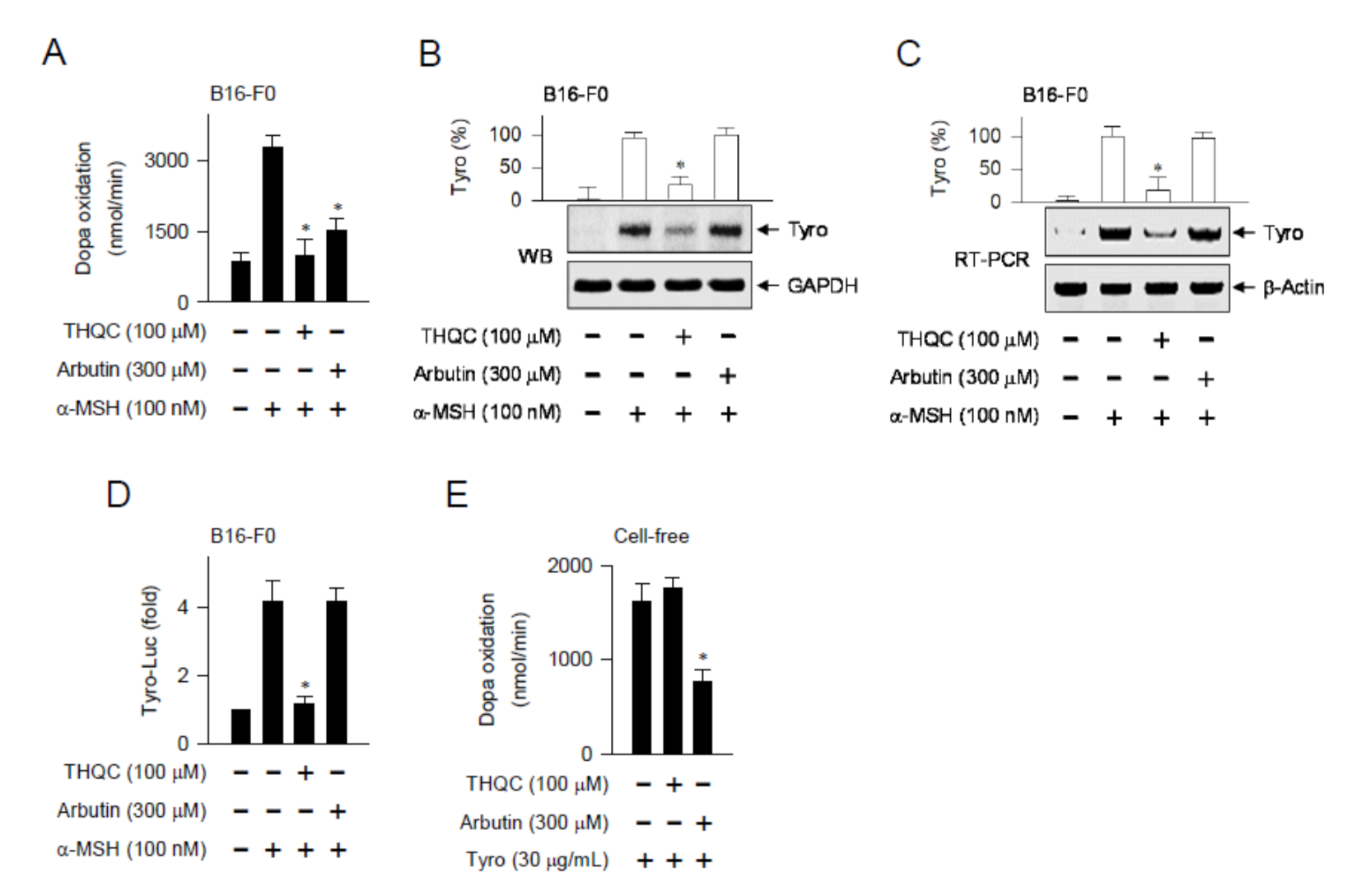
2.3. THQC Downregulated α-MSH-Induced Tyro Expression
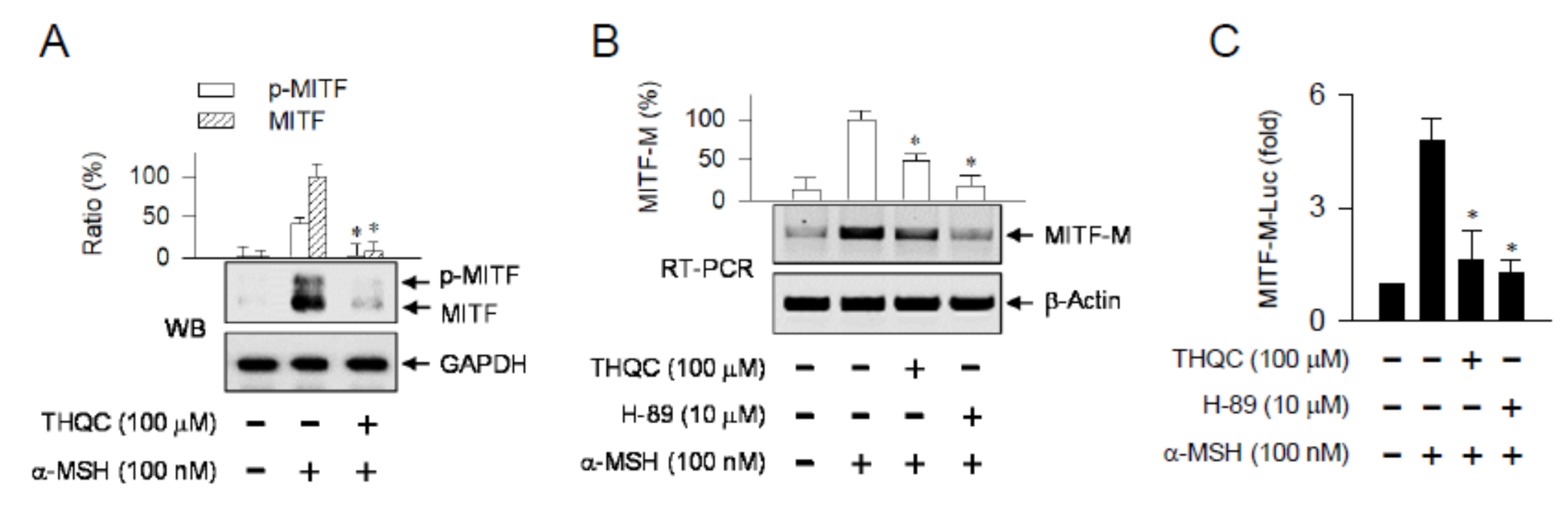
2.4. THQC Inhibited α-MSH-Induced Promoter Activity of the MITF-M Gene
2.5. THQC Decreased Pax3 Level for Inhibiting Facultative Melanin Pigmentation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Cell Culture
4.2. UVB-Induced Skin Pigmentation
4.3. Western Blot Analysis
4.4. RT-PCR Analysis
4.5. Melanin Quantification
4.6. Cell Viability Assay
4.7. Dopa Oxidation Assay
4.8. Luciferase Reporter Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CREB | cAMP responsive element-binding protein |
| db-cAMP | dibutyryl cyclic adenosine monophosphate |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| MITF-M | microphthalmia-associated transcription factor isoform M |
| MSH | melanocyte-stimulating hormone |
| Pax3 | paired box gene 3 |
| SOX10 | SRY-related HMG-box 10 |
| THQC | tetrahydroquinoline carboxamide |
| Tyro | tyrosinase |
| UVB | ultraviolet B |
References
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; Choi, W.; et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.H.; Jin, Z.H. Paracrine regulation of melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, L.E.; White, A. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 2007, 148, 4201–4207. [Google Scholar] [CrossRef] [PubMed]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postepy Hig. Med. Dosw. (Online) 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and function of human tyrosinase and tyrosinase-related proteins. Chemistry 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, R.; Takahashi, Y. Intercellular transfer of organelles during body pigmentation. Curr. Opin. Genet. Dev. 2017, 45, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Goding, C.R.; Arnheiter, H. MITF-the first 25 years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.J.; Fisher, D.E. Lighting a path to pigmentation: Mechanisms of MITF induction by UV. Pigment Cell Melanoma Res. 2010, 23, 741–745. [Google Scholar] [CrossRef]
- Buscà, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Kubic, J.D.; Young, K.P.; Plummer, R.S.; Ludvik, A.E.; Lang, D. Pigmentation PAX-ways: The role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008, 21, 627–645. [Google Scholar] [CrossRef] [Green Version]
- Shibahara, S.; Takeda, K.; Yasumoto, K.; Udono, T.; Watanabe, K.; Saito, H.; Takahashi, K. Microphthalmia-associated transcription factor (MITF): Multiplicity in structure, function, and regulation. J. Investig. Dermatol. Symp. Proc. 2001, 6, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Huber, W.E.; Price, E.R.; Widlund, H.R.; Du, J.; Davis, I.J.; Wegner, M.; Fisher, D.E. A tissue-restricted cAMP transcriptional response: SOX10 modulates a-melanocyte-stimulating hormone-triggered expression of microphthalmia-associated transcription factor in melanocytes. J. Biol. Chem. 2003, 278, 45224–45230. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Yasumoto, K.; Takeda, K.; Takahashi, K.; Yamamoto, H.; Shibahara, S. Microphthalmia-associated transcription factor in the Wnt signaling pathway. Pigment Cell Res. 2003, 16, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. In Vitro 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Bastien, N.; Millau, J.F.; Rouabhia, M.; Davies, R.J.; Drouin, R. The sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid photosensitizes the formation of oxidized guanines in cellulo after UV-A or UV-B exposure. J. Investig. Dermatol. 2010, 130, 2463–2471. [Google Scholar] [CrossRef] [Green Version]
- Guhl, S.; Stefaniak, R.; Strathmann, M.; Babina, M.; Piazena, H.; Henz, B.M.; Zuberbier, T. Bivalent effect of UV light on human skin mast cells-low-level mediator release at baseline but potent suppression upon mast cell triggering. J. Investig. Dermatol. 2005, 124, 453–456. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Takahashi, Y.; Inoue, S. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J. Investig. Dermatol. 2000, 114, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Lochner, A.; Moolman, J.A. The many faces of H89: A review. Cardiovasc. Drug Rev. 2006, 24, 261–274. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Nishimura, E.K.; Xin, H.; Zhou, A.; Guo, Y.; Dong, L.; Denning, M.F.; Nickoloff, B.J.; Cui, R. Inhibition of PAX3 by TGF-b modulates melanocyte viability. Mol. Cell 2008, 32, 554–563. [Google Scholar] [CrossRef]
- Lee, D.H.; Ahn, S.S.; Kim, J.B.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Downregulation of α-melanocyte-stimulating hormone-induced activation of the Pax3-MITF-tyrosinase axis by Sorghum ethanolic extract in B16F10 melanoma cells. Int. J. Mol. Sci. 2018, 19, 1640. [Google Scholar] [CrossRef] [Green Version]
- Solt, I.; Magyar, C.; Simon, I.; Tompa, P.; Fuxreiter, M. Phosphorylation-induced transient intrinsic structure in the kinase-inducible domain of CREB facilitates its recognition by the KIX domain of CBP. Proteins 2006, 64, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.W.; Gambee, J.E. Histone acetylation by p300 is involved in CREB-mediated transcription on chromatin. Biochim. Biophys. Acta 2001, 1541, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Tagashira, H.; Miyamoto, A.; Kitamura, S.; Tsubata, M.; Yamaguchi, K.; Takagaki, K.; Imokawa, G. UVB stimulates the expression of endothelin B receptor in human melanocytes via a sequential activation of the p38/MSK1/CREB/MITF pathway which can be interrupted by a French Maritime pine bark extract through a direct inactivation of MSK1. PLoS ONE 2015, 10, e0128678. [Google Scholar] [CrossRef] [Green Version]
- Bellei, B.; Pitisci, A.; Catricalà, C.; Larue, L.; Picardo, M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.; Hartman, M.G. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene 2001, 273, 1–11. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [Green Version]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin pigmentation and pigmentary disorders: Focus on epidermal/dermal cross-talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Rajanala, S.; Maymone, M.B.C.; Vashi, N.A. Melasma pathogenesis: A review of the latest research, pathological findings, and investigational therapies. Dermatol. Online J. 2019, 25, 1–6. [Google Scholar]
- Praetorius, C.; Sturm, R.A.; Steingrimsson, E. Sun-induced freckling: Ephelides and solar lentigines. Pigment Cell Melanoma Res. 2014, 27, 339–350. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents: Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A. TGF-b targets PAX3 to control melanocyte differentiation. Dev. Cell 2008, 15, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Choi, M.; Kumar, A.S.; Jung, Y.; Kim, S.; Yun, J.; Kang, J.S.; Kim, Y.; Han, S.B.; Jung, J.K.; et al. Development of novel 1,2,3,4-tetrahydroquinoline scaffolds as potent NF-κB inhibitors and cytotoxic agents. ACS Med. Chem. Lett. 2016, 7, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-P.; Kim, G.H.; Kim, S.-H.; Maeng, J.; Lee, H.; Han, S.-B.; Kim, K.H.; Kim, Y. Suppression of Pax3–MITF-M Axis Protects from UVB-Induced Skin Pigmentation by Tetrahydroquinoline Carboxamide. Int. J. Mol. Sci. 2020, 21, 9631. https://doi.org/10.3390/ijms21249631
Choi Y-P, Kim GH, Kim S-H, Maeng J, Lee H, Han S-B, Kim KH, Kim Y. Suppression of Pax3–MITF-M Axis Protects from UVB-Induced Skin Pigmentation by Tetrahydroquinoline Carboxamide. International Journal of Molecular Sciences. 2020; 21(24):9631. https://doi.org/10.3390/ijms21249631
Chicago/Turabian StyleChoi, Yong-Pyo, Ga Hyun Kim, Song-Hee Kim, Jongseo Maeng, Heesoon Lee, Sang-Bae Han, Ki Ho Kim, and Youngsoo Kim. 2020. "Suppression of Pax3–MITF-M Axis Protects from UVB-Induced Skin Pigmentation by Tetrahydroquinoline Carboxamide" International Journal of Molecular Sciences 21, no. 24: 9631. https://doi.org/10.3390/ijms21249631





