Abstract
Origanum vulgare L. is a widely used aromatic plant, especially due to its content in essential oil, mainly rich in carvacrol and thymol. The ethnopharmacological uses of Origanum vulgare L. essential oil (OEO) comprise digestive, respiratory, or dermatological disorders. The review focuses on the increasing number of recent studies investigating several biological activities of OEO. The bioactivities are in tight relation to the phytochemical profile of the essential oil, and also depend on taxonomic, climatic, and geographical characteristics of the plant material. The antibacterial, antifungal, antiparasitic, antioxidant, anti-inflammatory, antitumor, skin disorders beneficial effects, next to antihyperglycemic and anti-Alzheimer activities were reported and confirmed in multiple studies. Moreover, recent studies indicate a positive impact on skin disorders of OEO formulated as nanocarrier systems in order to improve its bioavailability and, thus, enhancing its therapeutic benefits. The review brings an up to date regarding the phytochemistry and bioactivity of Origanum vulgare L. essential oil, underlining also the most successful pharmaceutical formulation used for skin disorders.
1. Introduction
Origanum vulgare L., commonly oregano, is one of the most renowned aromatic species, with a strong traditional background as a spice and medicinal plant, but also as a well-established source of valuable plant-based drugs in modern phytotherapy. This Lamiaceae plant is native to Europe, the North of the African continent, and most of temperate Asia, but the hotspot of its diversity is situated in the Mediterranean region and predominantly in Turkey [1]. It has also been introduced in North America, where it grows in several states along the East and West coast [2]. Commercial O. vulgare is either wild-collected or cultivated, with Turkey being the most important supplier of Mediterranean oregano. However, the common name of “oregano” is employed for several other species of the genus (Origanum onites or Turkish oregano, O. majorana or sweet marjoram, O. minutiflorum or Spartan oregano, O. syriacum var. bevanii or Israeli oregano) or even belonging to other genus (Thymus, Satureja, Lippia, Coridothymus) [3].
Botanically, O. vulgare is a perennial plant with a woody base and herbaceous stems, growing to a height of 20–80 cm. Its leaves are opposite, egg-shaped, 1–4 cm long, and 0.5–2.5 cm wide; the leaf margin is smooth and the tip varies from rounded to pointed. The flowers are relatively small, grouped in terminal and lateral paniculate inflorescences. Their corolla consists of 5 united petals, 0.4–0.8 cm long, having a white to purple color. The sepals are as well reunited; the inner part of the flower contains fours stamens and the pistil is constituted by two fused carpels. The fruits are small nutlets [4]. Origanum vulgare is a highly variable species, encompassing several subspecies, varieties, chemotypes, and hybrids. Six subspecies are recognized: Origanum vulgare subsp. glandulosum (Desf.) Ietsw., subsp. gracile (K.Koch) Ietsw., subsp. hirtum (Link) A.Terracc., subsp. virens (Hoffmanns. & Link) Ietsw., subsp. viridulum (Martrin-Donos) Nyman, and subsp. vulgare [2,5]. The first three subspecies are typical for the southern range of the species and considered to be of high quality and rich in volatile oils, as opposed to the last three subspecies vegetating in more Northern regions, and poorer in essential oils [6].
The essential oils, the most relevant constituents for the medicinal value of O. vulgare, are synthesized in peltate glandular trichomes, which occur on the surface of stems, leaves, and flowers (sepals, petals). These trichomes have an enlarged secretory head, made up of 12–16 glandulous cells covered by a common cuticle (Figure 1); volatile oils are released upon rupture of the cuticle. Except for these peltate hairs, much smaller capitate hairs also occur on both sides of the leaves and the epidermis of the kalyx. They have a 1–2 celled head and seem to be involved in the synthesis of other more hydrophilic metabolites like phenolic compounds and polysaccharides [7]. The factors that influence the density and size of glandular trichomes, and, thus, directly impact the yield in essential oil, are defined both genetically and environmentally. Greek oregano displays an increased density and size of peltate trichomes, in contrast to common oregano [8]. With increasing altitude, the frequency of peltate glands decreases, leading to the decline of the essential oil quantity. Interestingly, the chemical profile of the essential oil also varies with the altitude: in the Venetian region, lowland plants were shown to synthesize monoterpene and sesquiterpene hydrocarbon-rich essential oils, while high-altitude plants contained high amounts of aromatic and hydrocarbon monoterpenes and low amounts of sesquiterpenes [7]. Reduced water and nitrogen supply augment the density of peltate trichomes [9], while plant density does not affect the number of glandular hairs per leaf surface unit [10].

Figure 1.
Origanum vulgare. Left image: flowering plant. The highlighted portion of the leaf surface is detailed in the middle image (short red arrow). Middle: Tectory and glandular trichomes on the lower leaf surface. The highlighted portion represents a secretory trichome, enlarged in the right image (long red arrow). Right: Peltate secretory trichome containing essential oil.
2. Traditional Uses
Uses of oregano in ethnomedicine have been linked to stomachic, carminative, expectorant, and emmenagogue properties [11]. It has been indicated as tinctures or teas in respiratory and digestive disorders, but also as ointments to treat wounds [12]. Its traditional uses include indigestions, diarrhea, cough, and bronchitis [13]. It has also been used as a remedy against pruritus, headaches, and depression [14]. Particular uses have been mentioned for different parts of the plant: while the aerial parts have been used in pain, cough, or sexual dysfunction, the seeds were used in urinary tract infections or menstrual disorders and the flowering branches were used externally by rubbing in place of fractures or to treat toothaches [15]. An ethnobotanical study showed that tea made from Origanum vulgare herb was traditionally used in Transylvania to treat sore throat [16]. In Turkey, the flowering branches and the leaves were prepared as infusions and used for cold, flu, headache, or toothache [17]. Arial parts of oregano represent a habitual spice, especial in Mediterranean countries [18]. In addition to culinary and medicinal uses, oregano was also used in perfumery or as flavoring for alcoholic beverages [19].
3. Chemical Composition
The aerial parts of O. vulgare contain essential oils with variable composition, as well as a diversity of flavonoids, tannins, phenolic glycosides, and terpenoids [20]. Luteolin-O-glucuronide and luteolin-7-O-glucoside are the main flavonoid derivatives found in the hydroalcoholic extracts, decoctions, and infusions of O. vulgare¸ while rosmarinic acid is the main phenolic acid [21]. The presence of caffeic acid, protocatechuic acid, vanillic acid, and o-coumaric acid has also been reported [22].
The essential oil is the central type of oregano’s chemical constituents and has extensively been studied. As O. vulgare is a variable species, there are also variations in the chemical composition [23]. The volatile oil contains monoterpenes and sesquiterpene hydrocarbons, as well as phenolic compounds [14]. Terpenes such as thymol, carvacrol, p-cymene, γ-terpinene, and linalool are the main constituents (Figure 2). Depending on the major compounds, several chemotypes have been defined [24]. Investigation of 502 plants from 17 European countries led to the identification of three main O. vulgare chemotypes, depending on the proportions of acyclic linalool/linalyl acetate, cymyl and sabinyl compounds [23].
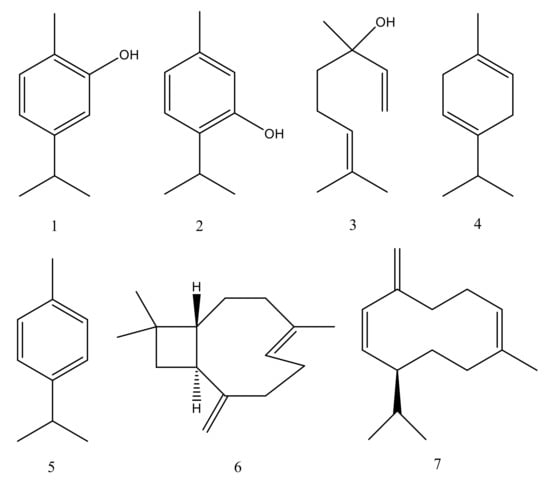
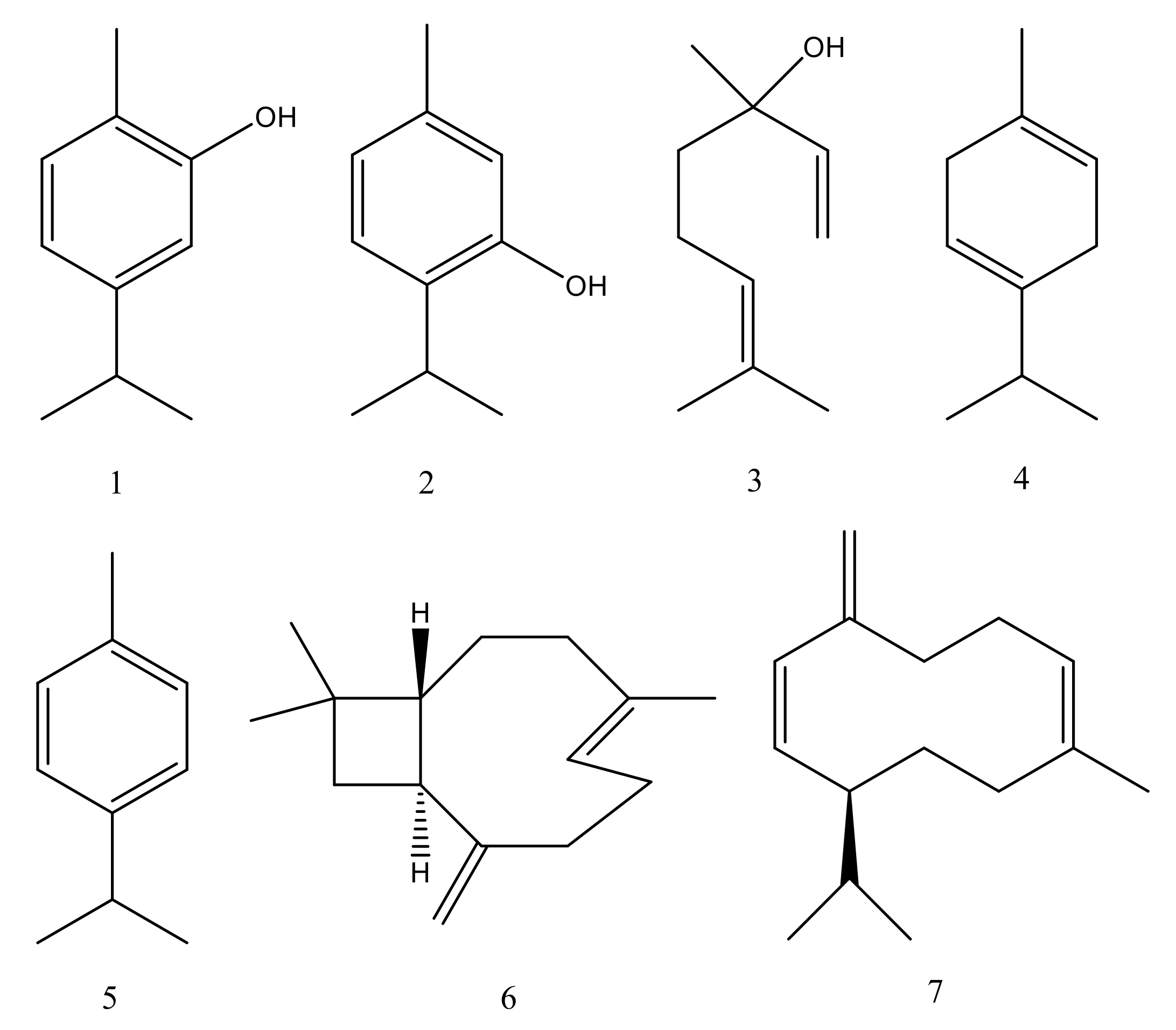
Figure 2.
Chemical structures of main constituents of Origanum vulgare essential oil: (1) Carvacrol, (2) Thymol, (3) Linalool, (4) γ-Terpinene, (5) p-Cymene, (6) β-Caryophyllene, (7) Germacrene D.
The essential oil composition varies according to environmental conditions, geographical area, harvesting time, and stage of plant maturity. The essential oil from plants in different countries has been evaluated in terms of chemical composition and major components have been identified depending on the area of origin [14]. Essential oils obtained from plants collected in Italy allowed the detection of 37 compounds, among which in greater quantities were thymol, carvacrol, linalyl acetate, and γ-terpinene [25]. Plants from Portugal revealed the presence of carvacrol, thymol, γ-terpinene, and β-fenchyl alcohol as main compounds in the essential oil [26], while the one from Montenegro had germacrene D, β-caryophyllene, linalyl acetate, and α-terpineol as major constituents [27]. The differences in the composition of essential oil in different organs of the plant have also been investigated, revealing variations in the content of certain compounds. For example, the stem oil was poorer in monoterpene hydrocarbons compared to leaf oil [28].
Plants with a higher essential oil content (Origanum vulgare subsp. hirtum, Origanum vulgare subsp. glandulosum and Origanum vulgare subsp. gracile) are characterized by the presence of carvacrol, thymol, p-cymene, and γ-terpinene, while the ones with lower content by the presence of sabinene, linalool, borneol, and sesquiterpenes [22]. The extensive investigation of O. vulgare essential oil led to the conclusion that the remarkable chemical variability of oregano can be explained by the up-/down regulation of the metabolic biosynthetic pathways. This research substantiated the fact that chemotype composition is in direct correlation with the climatic factors, with plants from the Mediterranean climate presenting an active cymyl- and/or linalool pathway, while plants growing in areas characterized by Continental climate are poorer in monoterpenes and display a more active sabinyl-pathway [23].
4. Pharmacological Activities
4.1. Antimicrobial Activity
The effectiveness of Origanum vulgare L. essential oil (OEO) against a wide range of pathogenic bacteria has been extensively studied. As it has been shown by an increased number of studies in the field, OEO represents an efficient alternative as an antimicrobial agent against both Gram-positive and Gram-negative bacterial strain [28,29,30]. Because essential oils are hydrophobic molecules, they have a greater permeability through the cell membrane and cause expansion of the cellular content. The death of the bacteria occurs by the drainage of crucial molecules and ions from the bacterial cell [29]. The disinfectant and antibacterial properties of the OEO were attested for the first time in ancient Greece, where it was frequently utilized for treating bacterial skin and wound contaminations. It was also used as a food preservative [30]. Carvacrol and thymol represent the two primary phenols, constituting almost 78–85% of the OEO and they are responsible for the plant’s antimicrobial properties [31]. The antibacterial activity of the OEO results from the high level of thymol which, according to Lambert et al. ties to membrane proteins and increases the permeability of the bacterial cell membrane [32]. In the same way, carvacrol acts on bacterial cells causing structural and functional damage, which increases bacterial cell membrane permeability [33]. The antibacterial activities explored for Origanum vulgare L. essential oil are represented in Table 1 for Gram-positive and Table 2 for Gram-negative bacteria.

Table 1.
Antibacterial activity against Gram-positive bacteria.

Table 2.
Antibacterial activity against Gram-negative bacteria.
Lu M. et al. investigated the efficacy of a commercial Origanum vulgare L. essential oil against multidrug-resistant (MDR) microbes such as methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa in vitro and in vivo, in a mouse model of burn injury. OEO also exhibited comparable antibacterial activities against built-up biofilms formed by the 13 bacterial strains, with total inactivation of the biofilms of Pseudomonas aeruginosa, and MRSA at the concentrations of 1.0, and 0.4 mg/mL, respectively. Moreover, transmission electron microscopy (TEM) showed ultrastructural damages in Pseudomonas aeruginosa cells. They displayed a severe spillage of intracellular substances resulted from the contraction of the cell membrane after exposure to OEO for 1 h at 0.56 mg/mL. Noteworthy, the treatment of burn infection in BALB/c mice with OEO using a dose of 5 or 10 mg/mL, was a success. [37].
In a comprehensive study, Scandorieiro et al. described the synergistic and additive interactions of a two-drug combination of Origanum vulgare EO and green silver nanoparticles (bio-AgNP) produced by Fusarium oxysporum against multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus, b-lactamase- and carbapenemase-producing, Escherichia coli, Escherichia coli ESBL and Escherichia coli KPC. In combination, OEO and bio-AgNP showed significantly lower minimum inhibitory concentration (MIC) values when compared with individual treatment (p < 0.05), where the two compounds together resulted in synergistic or additive antibacterial potential. Besides, the combination of OEO and bio-AgNP led to a faster reduction of CFU/mL than in individual treatment with bio-AgNP. Moreover, scanning electron microscope (SEM) revealed similar morphological alterations in non-methicillin-resistant Staphylococcus aureus cells exposed to three different treatments (OEO, bio-AgNP, and combination of the two), which appeared on cell surface blebbing after 6 h of treatment. Individual and combined treatments showed a reduction in cell density and a decrease in the exopolysaccharide matrix compared to untreated bacterial cells [35].
OEO with higher content in carvacrol and thymol, alone or in combination with silver nanoparticles, acts efficiently against Gram-positive and Gram-negative bacterial strains including multiresistant gram-positive and gram-negative bacteria S. aureus, E. coli, P. aeruginosa, K. pneumoniae.
4.2. Antifungal Activity
The antifungal activity of OEO is based on the presence of a high amount of thymol and carvacrol. Their antifungal action is related to the disturbance of the fungal cell wall integrity and with the interference of ergosterol synthesis [48]. Studies about the antifungal properties of Origanum vulgare L. essential oil are shown in Table 3.

Table 3.
Antifungal activity of Origanum vulgare L. essential oil (OEO).
4.3. Antiparasitic Activity
Relevant studies on the antiparasitic activity of OEO are shown in Table 4. This effect of OEO may be due to the presence of phenolic compounds (thymol and carvacrol) that interact with the permeability of the cytoplasmic cell membrane [53].

Table 4.
Antiparasitic activity of Origanum vulgare L. essential oil (OEO).
Cryptosporidium parvum is the second leading cause of persistent diarrhea among children living in poor households. Gaur et al. showed the potential anti-cryptosporidial effect of Origanum vulgare L. essential oil (origin: Turkey, steam extracted) in HCT-8 cells (Human colon adenocarcinoma). The immunodetection of infectivity using phase-contrast/fluorescent microscopy showed that OEO inhibited (p < 0.05) Cryptosporidium parvum growth in a dose-dependent manner: 55.6 ± 10.4% at 60 μg/mL OEO after 24 h incubation, without any apparent toxicity to the HCT-8 cells [54].
Santoro et al. explored the impact of the essential oil obtained from Origanum vulgare L. (harvested from Lavras, Brazil) on development and ultrastructure of Trypanosoma cruzi. Within 24 h incubation with OEO (from 25 to 250 μg/mL), the epimastigotes and trypomastigotes presented lysis of the cells and the IC50 values were 115μg/mL for trypomastigotes and 175 μg/mL for epimastigotes. Moreover, the examination of oregano-treated parasites (115 μg/mL) assessed by TEM showed slight morphological alterations in the plasma and flagellar membrane [55].
Pensel et al. demonstrated in vitro the effect of OEO (Buenos Aires Province, Argentina) against Echinococcus granulosus protoscoleces and cysts. The essential oil was added to the medium contained 10 μg/mL thymol. OEO diminished (p < 0.01) the viability of protoscoleces to 22.3 ± 1.2% after 60 days of incubation. TUNEL assay noticed DNA fragmentation and apoptosis of parasites treated with OEO for 16 h [56].
4.4. Antioxidant Activity
The antioxidant activity of Origanum vulgare L. essential oil is attributed due to the presence of carvacrol, thymol, and p-cymene, each one having the property to form chemical complexes with metal ions and free radicals (Table 5) [57]. The application of this bioactivity can be valued in food and pharmaceutical industries, as a safer alternative to the synthetic antioxidants [58].

Table 5.
Antioxidant activity of Origanum vulgare L. essential oil (OEO).
4.5. Anti-Inflammatory Activity
OEO can inhibit the secretion of pro-inflammatory cytokines and also, down-regulates the expression of inflammatory genes due to the presence of carvacrol [65]. The anti-inflammatory activities studied for Origanum vulgare L. essential oil are shown in Table 6.

Table 6.
Anti-inflammatory activity of Origanum vulgare L. essential oil (OEO).
Cheng et al. determined the anti-inflammatory effect on lipopolysaccharide (LPS) murine macrophage cells (RAW264.7) after treatment with OEO (acquired from Meritech Bioengineering Co. Ltd. Guangzhou, China). ELISA commercial kits revealed the decreased levels of IL-1β, IL-6, and TNF-α after 12 h treatment with OEO (2.5, 5, 10 μg/mL). Chemiluminescence assay measured the ROS (reactive oxygen species) level using luminol. Moreover, pretreatment with OEO (2.5, 5, 10 μg/mL) restrained the ROS generation within the RAW264.7 cells in the presence of lipopolysaccharide. Noteworthy, LPS may initiate an inflammatory response through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation. Furthermore, ROS production can be counteracted by inhibitors against NADPH oxidase. Therefore, the research team evaluated the NADPH oxidase activity of RAW264.7 cells treated with OEO, using ELISA kits. The results have shown that the OEO inhibited the lipopolysaccharide inflammatory response driven by NADPH oxidase and oxidative stress [67].
Laothaweerungsawat et al. tested a microemulsion of O. Vulgare essential oil (ME, 5% w/w OEO) on Raw 264.7 cells (murine macrophage cells). Origanum vulgare L. was harvested from Chaing Mai, Thailand and HPLC assay attested carvacrol as a major component of OEO. The microemulsion was investigated for the irritation properties in comparison with nonencapsulated OEO using HET-CAM (hen egg test-chorioallantoic membrane) assay. ME initiated a lower irritation score (IS = 3.1 ± 0.10) than nonemulsified OEO (IS = 4.8 ± 0.02) (p < 0.01). Furthermore, the ME showed higher inhibitory potential against IL-6 than the OEO (IC50 = 6.8 ± 2.0 and 15.5 ± 3.3 μg/mL, respectively (p < 0.05). Regarding the inhibition of TNF-α, ME had comparable inhibition to dexamethasone with the IC50 values of 5.4 ± 2.3 and 1.1 ± 0.9 μg/mL, respectively (p > 0.05) [68].
Carrasco et al. studied the anti-inflammatory activity of essential oils from 3 types of oregano harvested from Supra-Mediterranean and upper Meso-Mediterranean bioclimatic areas (Murcia, Spain). OEO samples were analyzed by GC-MS to determine their composition. (E)-β-Caryophyllene (0.5–4.9%), thymol (0.2–5.8%), p-cymene (3.8–8.2%), γ-terpinene (2.1–10.7%), and carvacrol (58.7–77.4%) were determined as main molecules. Sample from the Upper Meso-Mediterranean bioclimatic zone had the greatest inhibitory activity against lipoxygenase (LOX) (IC50 = 251.5 ± 1.4 µL EO/L) due to the presence of carvacrol and γ-terpinene as major compounds [69].
4.6. Antitumoral Activity
The potential therapeutic effects of plant essential oils in anticancer treatment has been inquired by many researchers in the field, due to the increased multidrug resistance and also the negative side effects of traditional chemotherapeutic agents [70]. The antiproliferative and cytotoxic effects of OEO have been demonstrated in the studies presented in Table 7.

Table 7.
In vitro anticancer activity of Origanum vulgare L. essential oil (OEO).
Bioactive principles of OEO (purchased from Berjé USA) were evaluated for their apoptotic effects against human stomach cancer cell lines (AGS) by Balusamy et al. Antiproliferative property of the OEO in adenocarcinoma gastric cell line (AGS) was determined by MTT assay. After 48 h of incubation with OEO (5, 10, 25, 50 and 100 μg/mL), IC50 was 13.4 μg/mL. The best antiproliferative activity of OEO was found to be 100 μg/mL, where almost 100% of cells were induced by cell damage by OEO treatment. Apoptosis detection of stomach cancer cell lines treated with OEO was performed by Hoechst and PI staining. Hoechst staining showed typical apoptosis characteristic of cancer cells treated with 10, 25, 50, and 100 μg/mL, including darkly stained nuclei, nuclei shrinkage, and DNA fragmentation, segregated bodies, the formation of apoptotic bodies in the inner surface of the nuclei. Moreover, detection of OEO induced apoptosis using propidium iodide staining indicated that cells treated with 25 μg/mL and 50 μg/mL of OEO reduced cell viability and cell. Moreover, they studied the molecular mechanism associated with the mitochondrial-mediated apoptosis controlled by the B-cell lymphoma protein-2 (BCL-2) family members that stimulate pro-apoptotic proteins including Bcl-2-associated X protein (BAX). BAX expression gradually increased at the concentration of 10 μg/mL OEO (2.37-fold) and reached its maximum transcript accumulation at 50 μg/mL of 4.32-fold, respectively [72].
Elansary et al. published data regarding the anticancer activity of Origanum vulgare L. (northern Egypt) essential oil (50, 100, 200, 300, and 400 μg/mL) against several cancer cell lines using MTT assay: breast adenocarcinoma (MCF-7), cervical adenocarcinoma (HeLa), T-cell lymphoblast (Jurkat), colon adenocarcinoma (HT-29), and urinary bladder carcinoma (T24) and human cell line (HEK-293). Compared to positive controls (vinblastine sulfate and taxol) OEO presented significant antitumoral activities against MCF-7 (IC50 = 8.11 µg/mL, taxol-IC50 = 0.08 µg/mL), HeLa(IC50 = 13.41 µg/mL, vinblastine-IC50 = 2.5 µg/mL), Jurkat(IC50 = 27,05 µg/mL, vinblastine-IC50 = 0.1 µg/mL), HT-29(IC50 = 12.18 µg/mL, vinblastine-IC50 = 12.18 µg/mL), T24(IC50 = 105.5 µg/mL, vinblastine-IC50 = 63.31 µg/mL) cancer cell lines [38].
Carvacrol as a major phytocompound in OEO may be responsible, in part, for the antiproliferative potential of OEO, as indicated by others, acting as suppressor of kinase ERK1/2 (extracellular signal-regulated protein kinase1/2) and AKT (Protein kinase B) proteins while upregulating BAX and phosphor c-Jun N-terminal kinase (p-JNK) protein expression [18].
4.7. Beneficial Activity on Skin Disorders
The therapeutical proprieties of Origanum vulgare L. essential oil gained attention for skincare items and scientific research regarding its impacts on human skin cells. Recent approaches in this sense are presented in Table 8.

Table 8.
Beneficial effects of Origanum vulgare L. essential oil (OEO) on skin disorders.
In a comprehensive study, Avola et al. investigated the biological effects of OEO (provided by Esperis S.p.A., Milan, Italy) in the restoring of the physiological cell homeostasis during wound and inflammation phenomena. To accomplish this, human keratinocyte cell line NCTC 2544 was used as an inflammatory in vitro model obtained by interferon-gamma (IFN-γ) and histamine stimulation or as a wound model attained by scratching the confluent monolayer of keratinocytes. The cultures treated with OEO showed a significantly lower level of ROS compared to the control. Furthermore, analysis regarding the amount of mRNA and proteins of ICAM-1, iNOS, and COX-2 were assessed by RT-PCR and Western blot. The results marked that the addition of OEO (25 µg/mL) for 72 h significantly reduced mRNA of ICAM-1, iNOS, and COX-2, suggesting that OEO enables cell homeostasis restoration during inflammatory events. Besides, keratinocytes stimulated with IFN-γ and histamine showed high levels of MMP-12 (a skin elastase) involved in the metabolism of elastic fibers and associated with the decrease in skin elasticity and consequent formation of wrinkles in various types of tissues during acute or chronic inflammatory disease. Compared to indomethacin, OEO (25 μg/mL) inhibited the MMP-12 expression to a higher degree. Evaluation by immunoblotting, under inflammatory condition in human keratinocyte cell line, showed the increased expression levels of proliferating cell nuclear antigen (PCNA), a marker of proliferation, whereas the amount of PCNA signal was significantly decreased by treatment with OEO (25 μg/mL). These results indicated that OEO acts as both an inducer of cell proliferation and a supporter of wound healing by PCNA modulation [73].
Laothaweerungsawat et al. revealed the cosmeceutical potential of two samples of Origanum vulgare L. essential oil as a skin-aging retarding agent. They compared OEO from Chiang Mai, Thailand obtained by hydrodistillation and a commercial oil purchased from Botanic essence (Product of Spain). Using ascorbic acid as positive control in anti-collagenase and anti-elastase activity determination and oleanolic acid as positive control in the determination of anti-hyaluronidase, the research group showed that OEO from Chiang Mai, possessed a significant higher anti-skin-aging activity compared to ascorbic acid (p < 0.01), with inhibition against collagenase, elastase, and hyaluronidase of 92.0 ± 9.7%, 53.1 ± 13.3%, and 16.7 ± 0.3%, at the concentration of 67, 25, and 4 µg/mL, respectively. However, the anti-hyaluronidase activity of both essential oils was mitigated when compared to oleanolic acid. The retardation of collagen and elastin loss may occur due to the phytocompound carvacrol identified in the highest amount in both essential oils OEO from a highland area of a tropical country (79.5%) and commercial OEO (64.6%) [63].
4.8. Effects on Melanin Production
Melanin biosynthesis is controlled by tyrosinase (polyphenol oxidase). An imbalance in the activity of this enzyme can lead to hyperpigmentation of the skin. Hydroxyl groups of phenolic phytocompounds in Origanum vulgare L. EO can bind the active site of the enzyme, which can inhibit enzymatic activity [75]. Moghrovyan et al. tested Origanum vulgare EO as a whitening agent for hyperpigmentation using colorimetric tyrosinase inhibition assay. The medicinal plant was collected from Gegharkunik province, Armenia. The values for tyrosinase inhibitory activity of Origanum vulgare EO and arbutin (positive control) were 26.5 ± 0.3% and 50.0 ± 0.1%, respectively [62].
Essential oils from Origanum vulgare subsp. vulgare (OVV) (Kesan-Edirne, Turkey) and Origanum vulgare subsp. hirtum (OVH) (Ermenek-Karaman, Turkey) were analysed by Sarikurkcu et al. for their inhibitory properties against the tyrosinase enzyme, which is responsible for melanin biosynthesis. The chemical composition of OVH was carried out by gas GC-FID and GC-MS techniques. The most abundant components were linalool (96.31%) and β-caryophyllene (1.27%). The anti-tyrosinase activity of the EOs was spectrophotometrically measured and the results were expressed as kojic acid equivalents (mgKAEs/g oil). Origanum vulgare subsp. hirtum was the most potent inhibitor with 45.60 mg KAEs/g oil when compared to OVV (8.30 mg KAEs/g oil). It was reported that linalool-rich essential oils could inhibit [75,76].
4.9. Hypoglycemic Activity
The inhibition of α-Amylase and α-glucosidase enzymes is an important strategy for maintaining blood glucose values. Essential oils extracted from 2 species of Origanum vulgare L.: Origanum vulgare subsp. hirtum from Ermenek-Karaman, Turkey, and Origanum vulgare subsp. vulgare from Kesan-Edirne, Turkey were investigated by Sarikuku et al. for their antidiabetic activity. The highest α-glucosidase inhibitory activity was recorded for Origanum vulgare subsp. vulgare with 6.04 mmol ACEs/g oil. As to α-amylase inhibitory activity, the EOs exhibited similar activity (0.14 for Origanum vulgare subsp. hirtum and 0.13 mmol ACEs/g oil for Origanum vulgare subsp. vulgare) [75].
4.10. Effects on Human Sperm Mobility
New data regarding the biological activities of Origanum vulgare L. essential oil was reported by Mbaye et al. The research team studied the impact of oregano (harvested from Fes, Marocco) essential oil on sperm’s motility and vitality. The mobility was assessed by a Computer Assisted Sperm Analysis and the evaluation of sperm vitality was performed by eosin 2% staining and analyzed via optical microscopy. Following 5 min of incubation OEO (range of concentrations: 10−1, 10−2, 10−3, and 10−9) gave promising values of mobility and vitality (73 ± 0.07% respectively 74 ± 0.07%) suggesting that OEO can be a therapeutic agent in qualitative abnormalities of human sperm [77].
Mbaye et al. continued to study Origanum vulgare L. essential oil (Fes, Marocco) on the advanced parameters of mobility and the integrity of the sperm DNA of 25 male infertile volunteers. The data obtained during experiments was the subject of a statistical study. The results of OEO (0.2% (w/v) sterile agar solution) supplementation on the characteristic parameters of mobility and nuclear quality were obtained by the Student t-test. The experiment has shown a significant stimulating effect on advanced mobility parameters: curvilinear velocity (p < 0.01), linear velocity (p < 0.01), mean path velocity (p < 0.01), and amplitude of displacement (p < 0.01) [78].
4.11. Anti-Alzheimer Activity
The ability of phytocompounds present in essential oils to inhibit acetylcholinesterase is interesting, as it can be used as possible treatment of some nervous illnesses such as Alzheimer’s disease [79]. Carrasco et al. tested in vitro cholinesterase inhibitory activity of essential oils obtained from 2 samples of Origanum vulgare L. grown in Supra Mediterranean and Upper Meso-Mediterranean bioclimatic regions in Murcia, Spain. Quantitative determination of the relative and chiral distribution of the main components of each EO was performed by fast gas chromatography/mass spectrometry (FGS/MS). Results showed that myrcene, γ-terpinene, and thymol were found in high proportion in OEO from Supra Mediterranean bioclimatic region, whereas high concentrations of 1,8-cineole, linalool, borneol, carvacrol, and β-bisabolene were found in OEO Meso-Mediterranean field. Concerning inhibitory the activity on acetylcholinesterase (AChE) Origanum vulgare L. grown in Supra Mediterranean region had the greatest inhibitory activity (IC50 = 73.7 ± 0.5 µL EO/L), followed by OEO from Meso-Mediterranean region (IC50 = 61.5 ± 0.5 µL EO/L) [69].
The acetylcholinesterase inhibitory effect of the EO obtained from 2 species of Origanum vulgare L.: Origanum vulgare subsp. vulgare (OVV) and Origanum vulgare subsp. hirtum (OVH) harvested from Turkey was reported by Sarikurcku et al. The EOs showed a similar action both on AChE (1.57 for OVH and 1.64 mg GALAEs/g oil for OVV) and BChE inhibitory activities (1.74 for OVH and 1.75 mg GALAEs/g oil for OVV). The research team analyzed the oils by GC-FID and gas chromatography/mass spectrophotometry GC-MS techniques. Thus, the inhibitory properties of OVV may be explained by the great amount of thymol and carvacrol, while the inhibitory activity of OVH on AChE and BChE may be due to the high amount of linalool [75].
5. Drug Delivery Systems for OEO Topical Application
Due to its multiple pharmacological activities, as shown in Figure 3, as reviewed above, but especially due to its antimicrobial, anti-inflammatory, and antitumor effects, oregano essential oil can be used as natural alternative to synthetic drugs, being a promising candidate for therapy of various conditions with microbial, inflammatory and tumor etiology [80]. Although, nowadays oregano essential oil is frequently used in traditional medicine and several “Do It Yourself” recipes are available, there is a lack of pharmaceutical preparations containing this essential oil mainly because, like other volatile oils, its incorporation into pharmaceutical topical dosage forms, especially hydrophilic ones, being confronted to technological limitations. These constraints are determined by the hydrophobic, volatile, and reactive nature of oregano essential oil bioactive components [81]. However, in the recent years some attempts were made to incorporate this volatile oil as active ingredient in different nanosystems (nanoparticles, nanoemulsions, and microemulsions) as colloidal drug carriers.
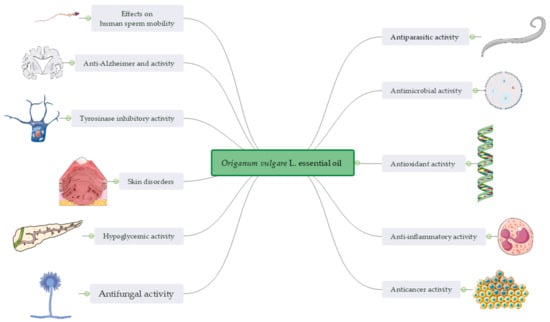
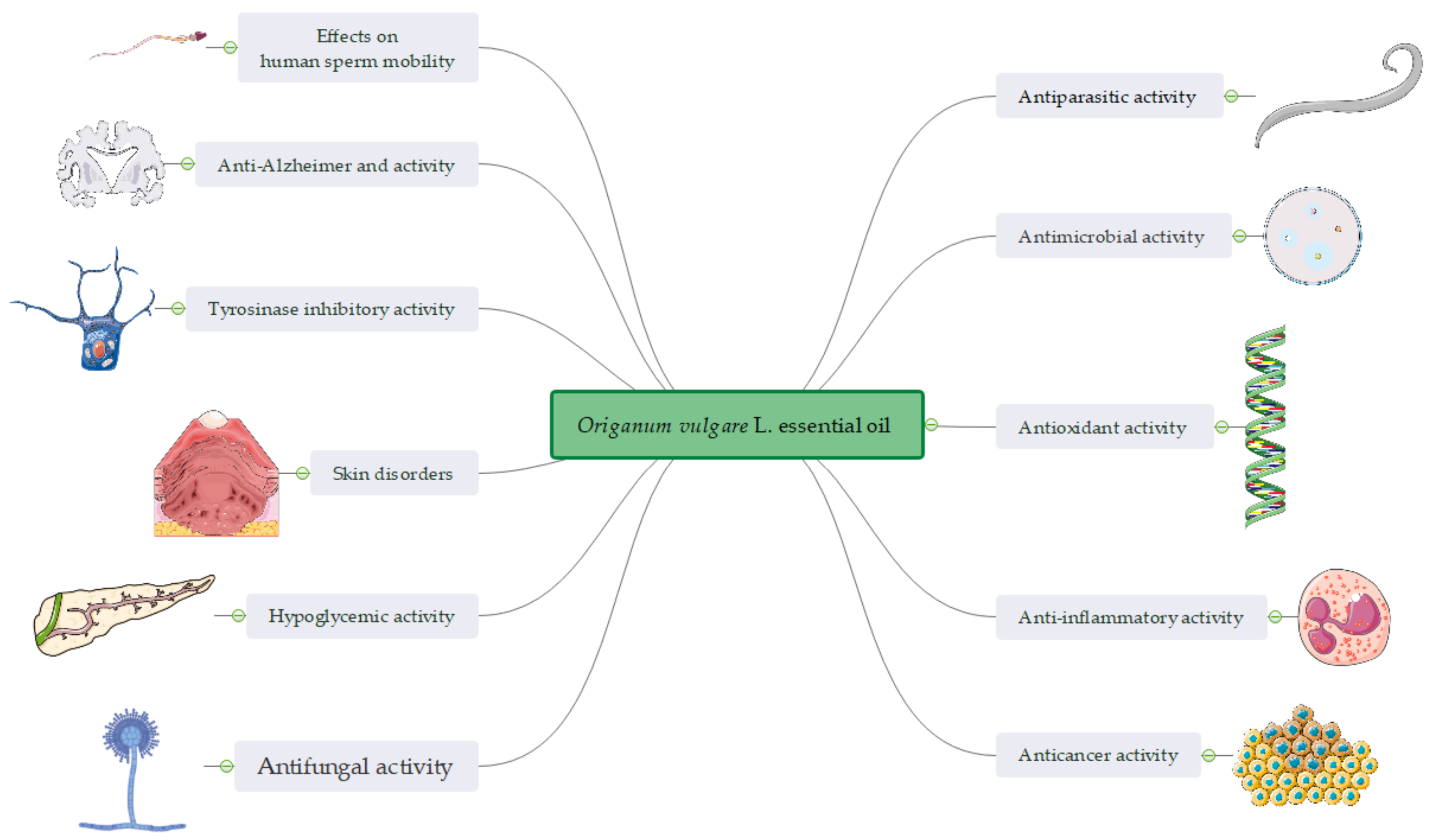
Figure 3.
A snapshot of the bioactivity of Origanum vulgare L. essential oil.
OEO was encapsulated into chitosan-Tween 80 nanoparticles prepared via a two-step method, consisting in the formation of an oil-in-water emulsion, followed by ionic gelation. The OEO-loaded chitosan nanoparticles showed regular distribution, spherical shape, an average diameter ranging from 309.8 nm to 402.2 nm and the loading capacity and encapsulation efficiency in the range of 1.32–2.12% and 5.45–24.72%, respectively. By increasing the OEO content of chitosan-nanoparticles from 0.1 to 0.8 g/g chitosan, their loading capacity increased, whereas their encapsulation efficiency decreased. The in vitro release profile of OEO from chitosan-nanoparticles indicated an initial burst effect (at low OEO concentration) followed by a subsequent slower release (at higher OEO concentrations) and suggested their potential as controlled release nanosystems for OEO [82].
Taleb et al. reported the development of a pharmaceutical oil-in water (O/W) nanoemulsion for dermal delivery of OEO to improve its poor water solubility and, thus, extending the application of OEO in aqueous formulations with antimicrobial activity useful in acne treatment. In this study, OEO was selected as active ingredient of the nanoemulsion, because among all tested EO, it exhibited the highest antimicrobial effect against acne-causing bacteria in vitro [39]. The topical O/W nanoemulsion consisting of 95% (w/w) water and 5% (w/w) mixture of OEO and Pluronic F127 (0.5% essential oil and 4.5% of Pluronic F127) was prepared by a low energy method at room temperature. The obtained O/W nanoformulation presented a low size distribution indicated by a particle size of 39.54 nm and a polydispersity index of 0.285, and remained clear and stable for four weeks at ambient temperature, without any signs of cloudiness, creaming, or phase separation. Following in vivo epicutaneous application of the proposed OEO nanoemulsion on a standardized acne mouse model, both inflammation and bacterial load decreased significantly and the tissue healing was superior in comparison with the 2% erythromycin solution used as a positive control. Authors suggested that antibacterial effects of OEO, like those of other essential oils [83], were improved by its formulation as O/W nanoemulsion and proposed the developed OEO nanoemulsion as new natural and effective alternative anti-acne treatment to avoid the drawbacks associated with the often-prescribed antibiotics.
Laothaweerungsawat et al. investigated the effect of OEO encapsulation in microemulsion-based systems on in vitro transdermal delivery of carvacrol, the major component of this essential oil, possessing analgesic and anti-inflammatory activity [68]. Microemulsion systems containing OEO were successfully obtained using Tween 60 and butylene glycol as surfactant and co-surfactant, respectively. From the pseudoternary phase diagram constructed using water dilution method, three microemulsion formulations were selected and evaluated for different physicochemical properties, including appearance, particle size and size distribution, zeta potential, viscosity, pH, and stability. Furthermore, irritation potential of OEO microemulsions (expressed as Irritation Score) versus that of a 5% (w/w) OEO solution in butylene glycol were investigated using the hen’s egg test. Transdermal absorption and skin maintenance of carvacrol from the formulation was studied in vitro. Finally, the IL-6 and TNF-α secretion was determined to evaluate the anti-inflammatory activity of OEO containing microemulsions. According to the results of physicochemical characterization, the optimal formulation was considered the microemulsion containing 5% OEO as oil phase and active component, 25% Tween 60 as surfactant, 25% butylene glycol as cosurfactant, and 45% deionized water. With the narrowest polydispersity index (0.30 ± 0.07) and the lowest surfactant matter, this formulation of OEO displayed the smallest droplet size (179.5 ± 27.9 nm). The skin irritation effect of OEO was reduced to a large extent by this formulation (IS = 3.1 ± 0.10 versus IS = 4.8 ± 0.02 of blank formulation and IS = 5.0 ±0.01 of 5% OEO solution). Furthermore, this OEO microemulsion released carvacrol in a sustained manner, delivered 6.5 times more carvacrol through the skin than the OEO solution and produced the retention of a significant amount of carvacrol in the skin layer (2.6 ± 1.3%). The OEO anti-inflammatory activity was greatly improved by its incorporation in this microemulsion, which exhibited more potency in inhibiting IL-6 secretion and a comparable inhibitory effect on TNF-α secretion to that of dexamethasone [68].
6. Conclusions
Oregano vulgare L. is an important Mediterranean aromatic plant of great value in traditional phytotherapy, next to its widespread dietary uses. Ethnopharmacological recommendations associated to the use of oregano include digestive, respiratory, and dermatological complaints. Owing to the content of carvacrol and thymol rich essential oils, the major associated pharmacological effects are the antimicrobial properties. This review was tailored to cover important details regarding the source of Origanum vulgare L. plant material, focusing on the phytochemistry of the essential oil content, linking it to the lately investigated bioactivities, but also to current approaches in dermal drug carrier systems using OEO.
Origanum vulgare is a highly variable species. The important variability of the essential oil composition depends on climate, environmental conditions, and geographical area. The phytochemical profile of OEO was extensively studied, and three main O. vulgare chemotypes were established, depending on the proportions of acyclic linalool/linalyl acetate, cymyl, and sabinyl compounds. The most investigated OEO are characterized by the presence of higher concentrations of carvacrol, thymol. Still, standardization of OEO according to main compounds is of great importance for relevant correlation with its biological activity.
OEO particularly high in carvacrol and thymol represents an efficient alternative as antimicrobial agent against both Gram-positive and Gram-negative bacterial strains including multiresistant Gram-positive and Gram-negative bacteria S. aureus, E. coli, P. aeruginosa, K. pneumoniae. The antioxidant activity of OEO is attributed to the presence of the same compounds. OEO decreases the expression of pro-inflammatory cytokines and of inflammatory genes, due to the presence of carvacrol, emphasizing multiple mechanisms of action. The antitumor effects of OEO were reported for several types of cancer, including breast, colon, hepatic, or cervical cancer, with IC50 values ranging approximatively from 8 to 300 µg/mL.
Furthermore, some of the common skin disorders such as acne, wound repair, or aging, were also shown to benefit from the antibacterial, anti-inflammatory, and antioxidative properties of OEO. The inhibition of hyaluronidase, collagenase, and elastase are correlated especially to the carvacrol content, while the antimelanin effect by tyrosinase inhibition was observed for linalool-rich essential oil type.
Only in recent years few promising approaches in the OEO use as pharmaceutical preparations have been reported. Mainly referring to dermal formulations, as topical application of OEO they were proven to be effective, generally safe and useful in various skin conditions. From our knowledge, nanocarriers of two categories, namely polymeric nanoparticles and lipid carriers including nano- and microemulsions, were proposed as drug delivery systems for OEO. OEO nanoencapsulation in such colloidal drug carriers is attractive because it allows to overcome the major disadvantage of OEO use, by decreasing its volatility, improving its stability and hydro solubility, and enhancing its bioavailability and therapeutic efficacy.
OEO is a highly valuable source of active phytocompounds with important biological effects. Beside the well depicted biological effects, reviewing the literature leads to the conclusion that there is a need of future preclinical and clinical studies, oriented towards the development of topical OEO-loaded nano delivery systems with improved bioavailability and important antimicrobial, anti-inflammatory, antitumor, and wound-healing effects.
Author Contributions
Conceptualization, C.D., A.L., S.A., C.S. and C.A.D.; writing—original draft preparation: chapter 1, D.A. and F.A.; chapter 2, D.A. and F.A.; chapter 3, A.L., D.A. and F.A.; chapter 4, A.L., I.Z.P., S.A., L.V., A.-M.M. and Z.D.; chapter 5, L.V. and A.-M.M. chapter 6, S.A., C.D.; writing—review and editing, S.A., C.D., C.A.D. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project PN-III-P1-1.1-TE-2019-0130, Contract number TE47, Project Director Associate Professor Dr. Pharm. Danciu Corina.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 2D-SDS-PAGE | Two-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| ABTS | 2,2′-Azino-Bis (3-Ethylbenzothiazoline)-6- Sulfonic acid |
| AChE | Acetylcholinesterase |
| AGS | Adenocarcinoma gastric cell line |
| BAX | Apoptosis regulator |
| BChE | Butyrylcholinesterase |
| BCL-2 | B-cell lymphoma protein 2 |
| bio-AgNP | Green silver nanoparticles |
| CCK-8 | Cell counting kit-8 |
| CFU | Colony forming units |
| CO | Commercial oil from the Mediterranean region |
| CZD | Clear zone diameter |
| DNA | Deoxyribonucleic acid |
| EGRF | Epidermal growth factor receptor |
| ELISA | Enzyme-linked immunosorbent assay |
| EO | Essential oil |
| ESBL | Extended-spectrum beta-lactamase |
| FGC/MS | Fast gas chromatography/mass spectrometry |
| GC-MS/FID | Gas chromatography-mass spectrometry/flame ionization detector |
| GC-MS | Gas chromatography-mass spectrometry |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| HaCaT | Healthy human keratinocytes |
| HCT-8 cells | Human colon adenocarcinoma |
| HeLa | Cervical adenocarcinoma |
| HepG2 | Hepatocarcinoma cell line |
| HO | Essential oil from a highland area of a tropical country |
| HT-29 | Human colon adenocarcinoma |
| IC50 | The half maximal inhibitory concentration |
| ICAM-1 | Intracellular cell adhesion molecule 1 |
| IFN-γ | Interferon-gamma |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxidesynthase |
| INU-CHI | Inulin-chitosan |
| IP-10 | Interferon gamma-induced protein 10 |
| IS | Irritation score |
| I-TAC | Interferon-inducible T-cell alpha chemoattractant |
| Jurkat | T-cell lymphoblast |
| KAEs | Kojic acid equivalents |
| KPC | K. Pneumoniae carbapenemase |
| LOX | Lipoxygenase |
| MBC | Minimum bactericidal concentration |
| MBEC | Minimal biofilm eradication concentration |
| MBIC | Minimum biofilm inhibitory concentration |
| MCF-7 | Human breast adenocarcinoma |
| MCP-1 | Monocyte chemoattractant protein 1 |
| M-CSF | Macrophage colony-stimulating factor |
| MDR | Multidrug-resistant |
| ME | Microemulsion of O. Vulgare essential oil |
| MFC | Minimum fungicidal concentration |
| MIC | Minimum inhibitory concentration |
| MIG | Monokine induced by gamma interferon |
| MLC | Minimal lethal concentration |
| MMP-1 | Matrix metalloproteinase 1 |
| MMP-2 | Matrix metalloproteinase 2 |
| mRNA | Messenger Ribonucleic acid |
| MRSA | Methicillin-resistant S. Aureus |
| MtP | Microtiter-plate test |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NCTC 2544 | Human keratinocyte cell line |
| NF | Nanofibrous membrane |
| NLC | Nanostructured lipid carriers |
| NO | Nitric oxide |
| OEO | Origanum vulgare L. essential oil |
| OT | Oregano and thyme essential oils |
| OVH | Origanum vulgare subsp.hirtum |
| OVV | Origanum vulgare subsp. Vulgare |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PCNA | Proliferating cell nuclear antigen |
| PI | Propidium iodide |
| PLCL/SF | Poly l-lactic acid- co-e-caprolactone/Silk Fibroin |
| PZ | precipitation zone |
| RAW 264.7 cells | Murine macrophage cell line |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscope |
| T24 | Urinary bladder carcinoma |
| TAS | The total antioxidant status |
| TEM | Transmission electron microscopy |
| TIMP-1 | Tissue inhibitor of metalloproteinase |
| TNF-α | Tumor necrosis factor |
| TUNEL assay | Terminal deoxynucleotidyl transferase dutp nick end labeling |
| VCAM-1 | Vascular cell adhesion molecule 1 |
References
- Arabaci, T.; Çelenk, S.; Özcan, T.; Martin, E.; Yazici, T.; Açar, M.; Üzel, D.; Dirmenci, T. Homoploid Hybrids of Origanum (Lamiaceae) in Turkey: Morphological and Molecular Evidence for a New Hybrid. Plant Biol. 2020, 1–13. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:453395-1 (accessed on 5 October 2020).
- Bejar, E. Adulteration of Oregano Herb and Essential Oil. Bot. Adulterants Prev. Bull. 2019, 1–10. Available online: http://cms.herbalgram.org/BAP/BAB/OreganoHerbandEOBulletin.html?ts=1608160808&signature=a474e37d3291079d017ae38fc0825ff7 (accessed on 17 December 2020).
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- Lotti, C.; Ricciardi, L.; Rainaldi, G.; Ruta, C.; Tarraf, W.; De Mastro, G. Morphological, Biochemical, and Molecular Analysis of Origanum vulgare L. Open Agric. J. 2019, 13, 116–124. [Google Scholar] [CrossRef]
- Kokkini, S. Taxonomy, diversity and distribution of Origanum. In Proceedings of the IPGRI International Workshop on Oregano Institute of Plant Genetics and Crop Plant Research, Bari, Italy, 8–12 May 1996; Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1996; pp. 8–12. [Google Scholar]
- Giuliani, C.; Maggi, F.; Papa, F.; Malecibini, L. Congruence of Phytochemical and Morphological Profiles along an Altitudinal Gradient in Origanum vulgare Ssp. Vulgare from Venetian Region (NE Italy). Chem. Biodivers. 2013, 10, 569–583. [Google Scholar] [CrossRef]
- Shafiee-Hajiabad, M.; Hardt, M.; Honermeier, B. Comparative Investigation about the Trichome Morphology of Common Oregano (Origanum vulgare L. Subsp. Vulgare) and Greek Oregano (Origanum vulgare L. Subsp. Hirtum). J. Appl. Res. Med. Aromat. Plants 2014, 1, 50–58. [Google Scholar] [CrossRef]
- Shafiee-Hajiabad, M.; Honermeier, B. Morphology and density of trichomes of Origanum vulgare L. in response to soil moisture regimes and nitrogen supply. Mitt. Ges. Pflanzenbauwiss. 2012, 24, 318–319. [Google Scholar]
- Tuttolomondo, T.; La Bella, S.; Leto, C.; Bonsangue, G.; Leone, R.; Gennaro, M.C.; Virga, G.; Inguanta, R.; Licata, M. Effects of Plant Density on the Number of Glandular Trichomes and on Yield and Quality of Essential Oils from Oregano. Nat. Prod. Commun. 2016, 11, 1934578X1601100638. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare Ssp. Vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Ličina, B.; Stefanovic, O.; Vasić, S.; Radojevic, I.; Dekic, M.; Čomić, L. Biological Activities of the Extracts from Wild Growing Origanum vulgare L. Food Control 2013, 33, 498–504. [Google Scholar] [CrossRef]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanum vulgare) Extract for Food Preservation and Improvement in Gastrointestinal Health. Int. J. Nutr. 2019, 3, 43–52. [Google Scholar] [CrossRef]
- Fikry, S.; Khalil, N.; Salama, O. Chemical Profiling, Biostatic and Biocidal Dynamics of Origanum vulgare L. Essential Oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Khaksarian, M.; Rafieian-kopaei, M.; Abbasi, N. Overview of the Therapeutic Effects of Origanum vulgare and Hypericum Perforatum Based on Iran’s Ethnopharmacological Documents. J. Clin. Diagn. Res. 2018, 12. [Google Scholar] [CrossRef]
- Papp, N.; Bartha, S.; Boris, G.; Balogh, L. Traditional Uses of Medicinal Plants for Respiratory Diseases in Transylvania. Nat. Prod. Commun. 2011, 6, 1459–1460. [Google Scholar] [CrossRef] [PubMed]
- Polat, R.; Satıl, F. An Ethnobotanical Survey of Medicinal Plants in Edremit Gulf (Balıkesir-Turkey). J. Ethnopharmacol. 2012, 139, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Berkay Yılmaz, Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy Venil, C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phyther. Res. 2020, 1–27. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Trivic, S. Antioxidant Capacity of Ocimum basilicum L. and Origanum vulgare L. Extracts. Molecules 2011, 16, 7401–7414. [Google Scholar] [CrossRef]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An Update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Decoction, Infusion and Hydroalcoholic Extract of Origanum vulgare L.: Different Performances Regarding Bioactivity and Phenolic Compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- Kosakowska, O.; Czupa, W. Morphological and Chemical Variability of Common Oregano (Origanum vulgare L. Subsp. Vulgare) Occurring in Eastern Poland. Herba Pol. 2018, 64, 11–21. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential Oil Diversity of Origanum vulgare L. Populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Stešević, D.; Jaćimović, Ž.; Šatović, Z.; Šapčanin, A.; Jančan, G.; Kosović, M.; Damjanović-Vratnica, B. Chemical Characterization of Wild Growing Origanum vulgare Populations in Montenegro. Nat. Prod. Commun. 2018, 13, 1934578X1801301031. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical Diversity in Leaf and Stem Essential Oils of Origanum vulgare L. and Their Effects on Microbicidal Activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicine 2017, 4, 58. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial Activity of Essential Oils of Cultivated Oregano (Origanum vulgare), Sage (Salvia Officinalis), and Thyme (Thymus Vulgaris) against Clinical Isolates of Escherichia Coli, Klebsiella Oxytoca, and Klebsiella Pneumoniae. Microb. Ecol. Heal. Dis. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn Essential Oils of Greek Oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.M.A.; Elbanna, K.; Al-Thubiani, A.; Ramadan, M.F. Cold-Pressed Oregano (Origanum vulgare) Oil: A Rich Source of Bioactive Lipids with Novel Antioxidant and Antimicrobial Properties. Eur. Food Res. Technol. 2016, 242, 1013–1023. [Google Scholar] [CrossRef]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Yaldiz, G.; Arici, Y.; Yilmaz, G. Phytochemical Analysis, Antioxidant and Antibacterial Activities of Four Lamiaceae Species Cultivated in Barnyard Manure. Tarim Bilim. Dergisi J. Agric. Sci. 2017, 23, 95–108. [Google Scholar]
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective Antioxidant, Antimicrobial and Anticancer Activities of Essential Oils of Horticultural Aromatic Crops in Northern Egypt. BMC Complement. Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and in Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial Activity of Essential Oils and Carvacrol, and Synergy of Carvacrol and Erythromycin, against Clinical, Erythromycin-Resistant Group A Streptococci. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential Oils from Origanum vulgare and Salvia Officinalis Exhibit Antibacterial and Anti-Biofilm Activities against Streptococcus Pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef]
- Sharifi, A.; Ahmadi, A.; Mohammadzadeh, A. Streptococcus Pneumoniae Quorum Sensing and Biofilm Formation Are Affected by Thymus Daenensis, Satureja Hortensis, and Origanum vulgare Essential Oils. Acta Microbiol. Immunol. Hung. 2018, 65, 345–359. [Google Scholar] [CrossRef]
- Jnaid, Y.; Yacoub, R.; Al-Biski, F. Antioxidant and Antimicrobial Activities of Origanum vulgare Essential Oil. Int. Food Res. J. 2016, 23, 1706–1710. [Google Scholar]
- Moshayedi, S.; Shahraz, F.; Schaffner, D.W.; Khanlarkhani, A.; Shojaee-Aliabadi, S.; Shahnia, M.; Khaksar, R. In Vitro Control of Enterococcus Faecalis by Zataria Multilfolira Boiss, Origanum vulgare l and Mentha Pulegium Essential Oils. J. Food Saf. 2013, 33, 327–332. [Google Scholar] [CrossRef]
- Mazzarrino, G.; Paparella, A.; Chaves-López, C.; Faberi, A.; Sergi, M.; Sigismondi, C.; Compagnone, D.; Serio, A. Salmonella Enterica and Listeria Monocytogenes Inactivation Dynamics after Treatment with Selected Essential Oils. Food Control 2015, 50, 794–803. [Google Scholar] [CrossRef]
- Lesjak, M.; Simin, N.; Orcic, D.; Franciskovic, M.; Knezevic, P.; Beara, I.; Aleksic, V.; Svircev, E.; Buzas, K.; Mimica-Dukic, N. Binary and Tertiary Mixtures of Satureja Hortensis and Origanum vulgare Essential Oils as Potent Antimicrobial Agents Against Helicobacter Pylori. Phyther. Res. 2016, 30, 476–484. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; de Leite, A.L.; de Pontes, L.G.; dos Santos, L.D.; et al. Proteomic Analysis and Antibacterial Resistance Mechanisms of Salmonella Enteritidis Submitted to the Inhibitory Effect of Origanum vulgare Essential Oil, Thymol and Carvacrol. J. Proteom. 2020, 214, 103625. [Google Scholar] [CrossRef]
- Vahedi, G.; Khosravi, A.R.; Shokri, H.; Moosavi, Z.; Delirezh, N.; Sharifzadeh, A.; Barin, A.; Shahrokh, S.; Balal, A. Fungicidal Effect of Origanum vulgare Essential Oil against Candida Glabrata and Its Cytotoxicity against Macrophages. J. HerbMed Pharmacol. 2016, 5, 78–84. [Google Scholar]
- Pradebon Brondani, L.; Alves da Silva Neto, T.; Antonio Freitag, R.; Guerra Lund, R. Evaluation of Anti-Enzyme Properties of Origanum vulgare Essential Oil against Oral Candida Albicans. J. Mycol. Med. 2018, 28, 94–100. [Google Scholar] [CrossRef]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical Composition and in Vitro Activity of Origanum vulgare L., Satureja Hortensis L., Thymus Serpyllum L. And Thymus Vulgaris L. Essential Oils towards Oral Isolates of Candida Albicans and Candida Glabrata. Open Chem. 2020, 18, 108–118. [Google Scholar] [CrossRef]
- Hashemi, M.; Ehsani, A.; Aminzare, M.; Hassanzadazar, H. Antioxidant and Antifungal Activities of Essential Oils of Origanum vulgare Ssp. Gracile Flowers and Leaves from Iran. J. Food Qual. Hazards Control 2016, 3, 134–140. [Google Scholar]
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical Characterization and Antifungal Activity of Origanum vulgare, Thymus Vulgaris Essential Oils and Carvacrol against Malassezia Furfur. Nat. Prod. Res. 2019, 33, 3273–3277. [Google Scholar] [CrossRef]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of Dietary Supplementation with Oregano Essential Oil on Performance of Broilers after Experimental Infection with Eimeria Tenella. Arch. Tierernahr. 2003, 57, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Andrade, J.E. Effect of Oregano Essential Oil and Carvacrol on Cryptosporidium Parvum Infectivity in HCT-8 Cells. Parasitol. Int. 2018, 67, 170–175. [Google Scholar] [CrossRef]
- Santoro, G.F.; Das Graças Cardoso, M.; Guimarães, L.G.L.; Salgado, A.P.S.P.; Menna-Barreto, R.F.S.; Soares, M.J. Effect of Oregano (Origanum vulgare L.) and Thyme (Thymus Vulgaris L.) Essential Oils on Trypanosoma Cruzi (Protozoa: Kinetoplastida) Growth and Ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Pensel, P.E.; Maggiore, M.A.; Gende, L.B.; Eguaras, M.J.; Denegri, M.G.; Elissondo, M.C. Efficacy of Essential Oils of Thymus Vulgaris and Origanum vulgare on Echinococcus Granulosus. Interdiscip. Perspect. Infect. Dis. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hać-Szymańczuk, E.; Cegiełka, A.; Karkos, M.; Gniewosz, M.; Piwowarek, K. Evaluation of Antioxidant and Antimicrobial Activity of Oregano (Origanum vulgare L.) Preparations during Storage of Low-Pressure Mechanically Separated Meat (BAADER Meat) from Chickens. Food Sci. Biotechnol. 2018, 28, 449–457. [Google Scholar] [CrossRef]
- Stanojević, L.P.; Stanojević, J.S.; Cvetković, D.J.; Ilić, D.P. Antioxidant activity of oregano essential oil (Origanum vulgare L.). Biologica Nyssana. 2016, 7, 131–139. [Google Scholar]
- Han, F.; Ma, G.-Q.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.-C.; Zhao, Z.-D.; Xu, H.-L. Chemical Composition and Antioxidant Activities of Essential Oils from Different Parts of the Oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef]
- Cao, T.L.; Yang, S.Y.; Song, K.B. Development of Burdock Root Inulin/Chitosan Blend Films Containing Oregano and Thyme Essential Oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar] [CrossRef]
- Khan, A.R.; Nadeem, M.; Aqeel Bhutto, M.; Yu, F.; Xie, X.; El-Hamshary, H.; El-Faham, A.; Ibrahim, U.A.; Mo, X. Physico-Chemical and Biological Evaluation of PLCL/SF Nanofibers Loaded with Oregano Essential Oil. Pharmaceutics 2019, 11, 386. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and Other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical Compositions and Anti-Skin-Ageing Activities of Origanum vulgare L. Essential Oil from Tropical and Mediterranean Region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kozics, K.; Bucková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. Molecules The E Ff Ect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; Machado, F.D.F.; Quintans-Júnior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean Essential Oils as Precious Matrix Components and Active Ingredients of Lipid Nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef] [PubMed]
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal Delivery Enhancement of Carvacrol from Origanum vulgare L. Essential Oil by Microemulsion. Int. J. Pharm. 2020, 579, 119052. [Google Scholar] [CrossRef]
- Carrasco, A.; Perez, E.; Cutillas, A.B.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Origanum vulgare and Thymbra Capitata Essential Oils from Spain: Determination of Aromatic Profile and Bioactivities. Nat. Prod. Commun. 2016, 11, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum vulgare L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Begnini, K.R.; Nedel, F.; Lund, R.G.; Carvalho, P.H.D.A.; Rodrigues, M.R.A.; Beira, F.T.A.; Del-Pino, F.A.B. Composition and Antiproliferative Effect of Essential Oil of Origanum vulgare against Tumor Cell Lines. J. Med. Food 2014, 17. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-Proliferative Activity of Origanum vulgare Inhibited Lipogenesis and Induced Mitochondrial Mediated Apoptosis in Human Stomach Cancer Cell Lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-Inflammatory, Tissue Remodeling, Immunomodulatory, and Anticancer Activities of Oregano (Origanum vulgare) Essential Oil in a Human Skin Disease Model. Biochim. Open 2017, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (Subsp. Vulgare and Subsp. Hirtum) Essential Oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Souza, P.M.; Elias, S.T.; Simeoni, L.A.; de Paula, J.E.; Gomes, S.M.; Guerra, E.N.S.; Fonseca, Y.M.; Silva, E.C.; Silveira, D.; Magalhães, P.O. Plants from Brazilian Cerrado with Potent Tyrosinase Inhibitory Activity. PLoS ONE 2012, 7, e48589. [Google Scholar] [CrossRef] [PubMed]
- Mbaye, M.M.; El Khalfi, B.; Addoum, B.; Mar, P.D.; Saadani, B.; Louanjli, N.; Soukri, A. The Effect of Supplementation with Some Essential Oils on the Mobility and the Vitality of Human Sperm. Sci. World J. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Mbaye, M.M.; El Khalfi, B.; Ouzamode, S.; Saadani, B.; Louanjli, N.; Soukri, A. Effect of Origanum vulgare Essential Oil Supplementation on the Advanced Parameters of Mobility and on the Integrity of Human Sperm DNA. Int. J. Reprod. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and Their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef]
- Singletary, K. Oregano: Overview of the Literature on Health Benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid. Based. Complement. Alternat. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and in Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Ghaderi, L.; Moghimi, R.; Aliahmadi, A.; McClements, D.J.; Rafati, H. Development of Antimicrobial Nanoemulsion-Based Delivery Systems against Selected Pathogenic Bacteria Using a Thymol-Rich Thymus Daenensis Essential Oil. J. Appl. Microbiol. 2017, 123, 832–840. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).