Transient Receptor Potential Vanilloid 6 (TRPV6) Proteins Control the Extracellular Matrix Structure of the Placental Labyrinth
Abstract
:1. Introduction
2. Results
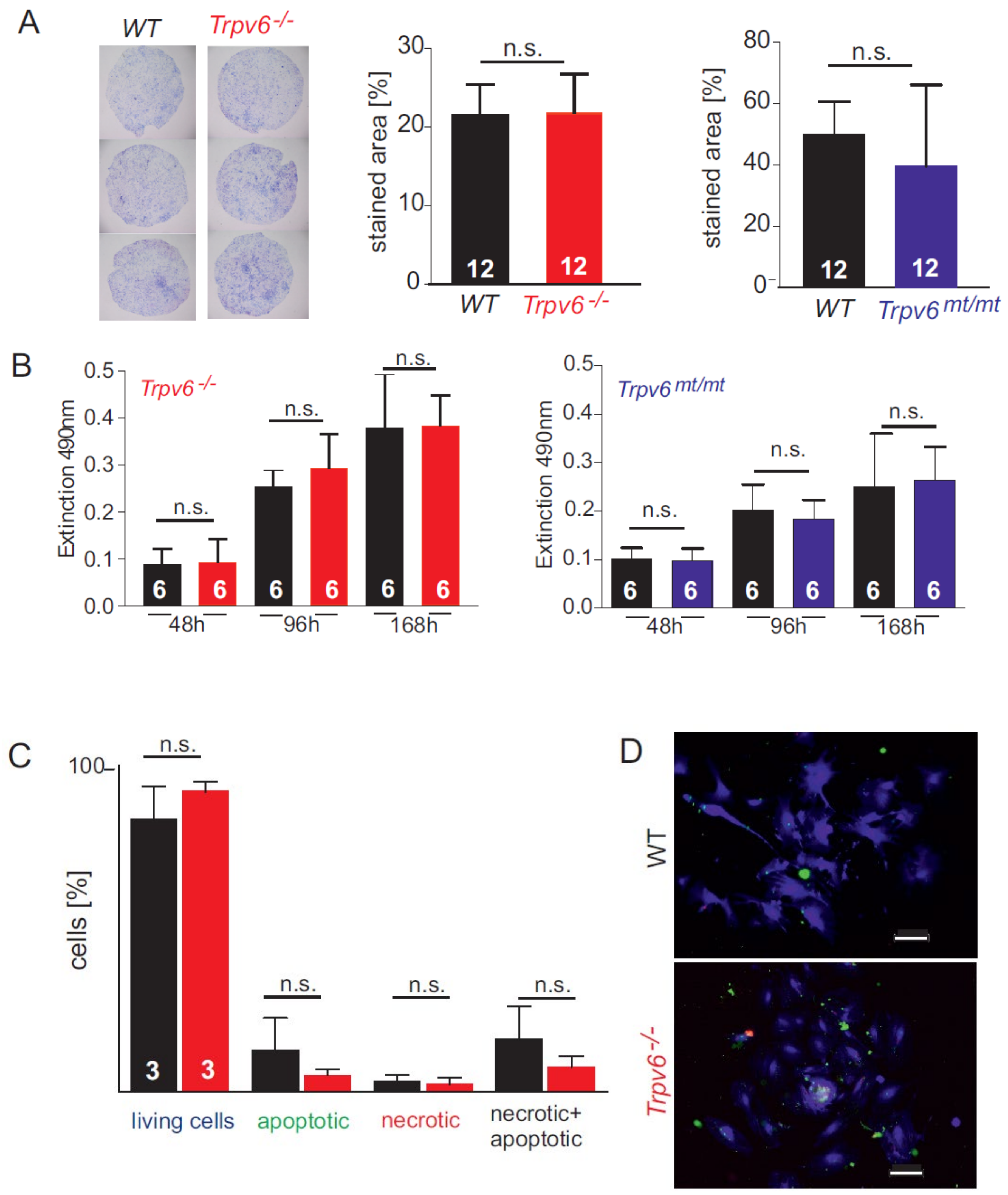
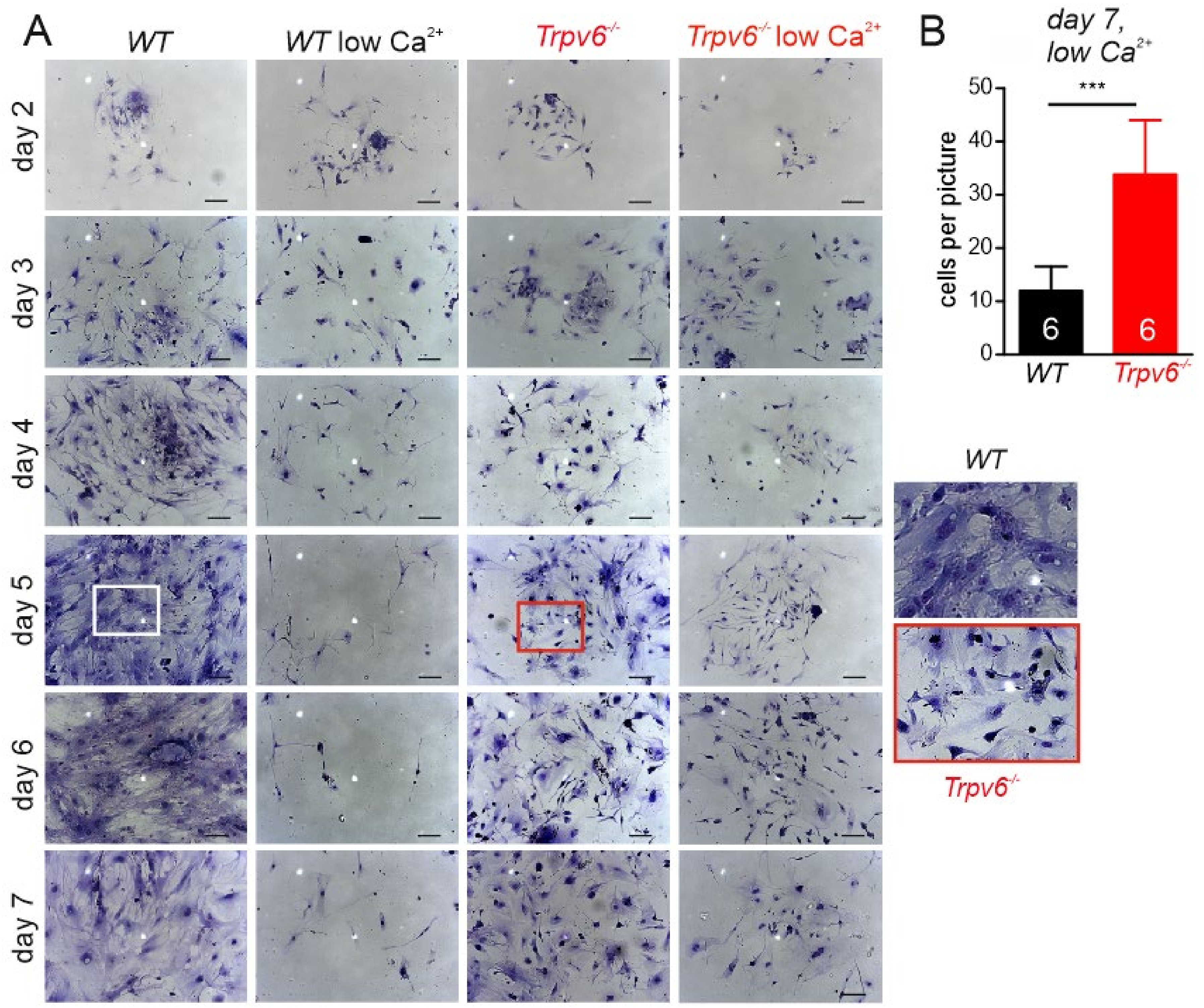
2.1. Calcium-Dependent Cell Growth, Viability, and Migration Behavior of wt and Trpv6-/- Trophoblast Cells
2.2. Proteome Analysis of Primary Trophoblasts from wt and Trpv6-/- Placentae at E14.5
2.3. Proteases Are More-Abundantly Expressed in TRPV6-Deficient Trophoblasts
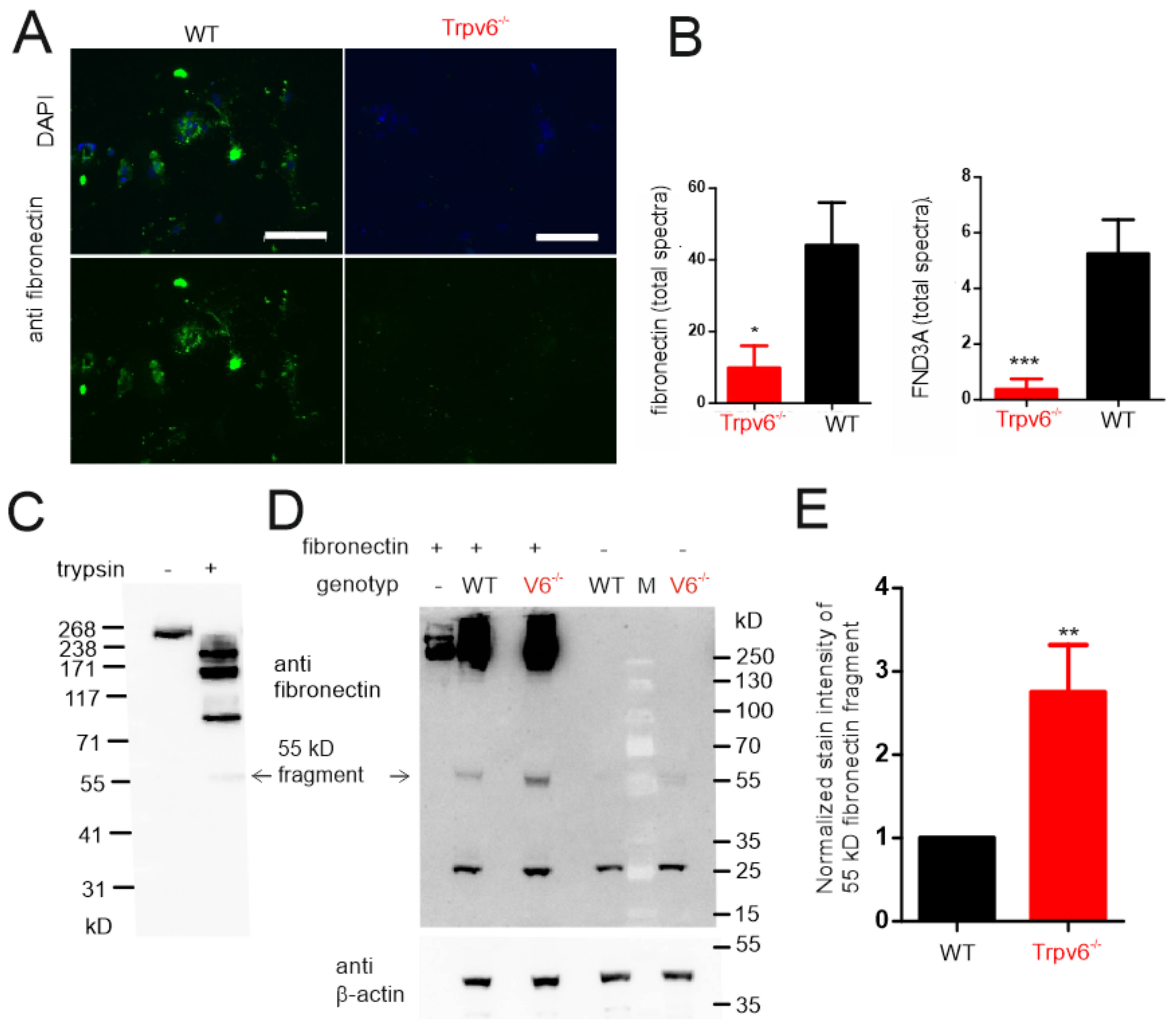
2.4. The amount of Extracellular Matrix Proteins Is Reduced in TRPV6-Deficient Trophoblasts
2.5. Increased Proteolytic Activity in Trpv6-/- Placentae Causes Increased Fibronectin Degradation
2.6. What Are the Potential Mechanisms Underlying Higher Protease Expression and Activity in Trpv6- Deficient Placentae?
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Isolation of Primary Trophoblast Cells from Pregnant Mice and Cell Culture
4.3. RIPA Lysate Preparation and Western Blot Analysis
4.4. Immunohistochemistry of MCT1 and MCT4 in the Mouse Placenta
4.5. Antibodies
4.6. Growth Analysis
4.7. Transwell Migration Assay
4.8. Viability Assay
4.9. Fibronectin Digestion Assay
4.10. Sample Preparation of Primary Trophoblast Lysates for Proteome Analysis
4.11. Mass Spectrometric Measurement (Nano-LC–MS/MS)
4.12. Raw Mass Spectrometrical Data Analysis
4.13. Flow Cytometry
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TRPV6 | Transient receptor potential channel |
| ECM | Extracellular matrix |
| LC–MS | Liquid chromatography–mass spectrometry |
| FND3A | Fibronectin-domain-containing protein 3A |
| HTRA1 | High-temperature requirement A serine protease |
References
- Fecher-Trost, C.; Lux, F.; Busch, K.M.; Raza, A.; Winter, M.; Hielscher, F.; Belkacemi, T.; van der Eerden, B.; Boehm, U.; Freichel, M.; et al. Maternal Transient Receptor Potential Vanilloid 6 (Trpv6) Is Involved in Offspring Bone Development. J. Bone Miner. Res. 2019, 34, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, P.; Kriebs, U.; Tsvilovskyy, V.; Olausson, J.; Kretz, O.; Stoerger, C.; Vennekens, R.; Wissenbach, U.; Middendorff, R.; Flockerzi, V.; et al. Male fertility depends on Ca(2)+ absorption by TRPV6 in epididymal epithelia. Sci. Signal. 2011, 4, ra27. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, P.; Kriebs, U.; Tsvilovskyy, V.; Olausson, J.; Kretz, O.; Stoerger, C.; Mannebach, S.; Wissenbach, U.; Vennekens, R.; Middendorff, R.; et al. Excision of the Trpv6 gene leads to severe defects in epididymal Ca2+ absorption and male fertility much alike the single D541A pore mutation. J. Biol. Chem. 2012, 287, 17930–17941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, R.; Hamel, A.; Daoud, G.; Simoneau, L.; Lafond, J. Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol. Reprod. 2002, 67, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Kovacs, C.S.; Takanaga, H.; Peng, J.B.; Landowski, C.P.; Hediger, M.A. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J. Bone Miner. Res. 2008, 23, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauberger, C.W.; Pitkin, R.M. Maternal-perinatal calcium relationships. Obstet. Gynecol. 1979, 53, 74–76. [Google Scholar] [PubMed]
- Adamson, S.L.; Lu, Y.; Whiteley, K.J.; Holmyard, D.; Hemberger, M.; Pfarrer, C.; Cross, J.C. Interactions between Trophoblast Cells and the Maternal and Fetal Circulation in the Mouse Placenta. Dev. Biol. 2002, 250, 358–373. [Google Scholar] [CrossRef]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef]
- Simmons, D.G.; Cross, J.C. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev. Biol. 2005, 284, 12–24. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, K.; Vriens, J. Establishing life is a calcium-dependent TRiP: Transient receptor potential channels in reproduction. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865 Pt 11 B, 1815–1829. [Google Scholar] [CrossRef]
- Wissenbach, U.; Niemeyer, B.A.; Fixemer, T.; Schneidewind, A.; Trost, C.; Cavalie, A.; Reus, K.; Meese, E.; Bonkhoff, H.; Flockerzi, V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J. Biol. Chem. 2001, 276, 19461–19468. [Google Scholar] [CrossRef] [Green Version]
- Fecher-Trost, C.; Wissenbach, U.; Beck, A.; Schalkowsky, P.; Stoerger, C.; Doerr, J.; Dembek, A.; Simon-Thomas, M.; Weber, A.; Wollenberg, P. The in vivo TRPV6 protein starts at a non-AUG triplet decoded as methionine upstream the canonical initiation at AUG. J. Biol. Chem. 2013, 288, 16629–16644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoerger, C.; Flockerzi, V. The transient receptor potential cation channel subfamily V member 6 (TRPV6): Genetics, biochemical properties, and functions of exceptional calcium channel proteins. Biochem. Cell Biol. 2014, 92, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Chitayat, D.; Sawada, H.; Deardorff, M.A.; McLaughlin, H.M.; Begtrup, A.; Millar, K.; Harrington, J.; Chong, K.; Roifman, M.; et al. TRPV6 Variants Interfere with Maternal-Fetal Calcium Transport through the Placenta and Cause Transient Neonatal Hyperparathyroidism. Am. J. Hum. Genet. 2018, 102, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, S.; Mizumoto, H.; Sawada, H.; Suzuki, Y.; Hata, D. TRPV6 Gene Mutation in a Dizygous Twin with Transient Neonatal Hyperparathyroidism. J. Endocr. Soc. 2019, 3, 602–606. [Google Scholar] [CrossRef]
- Burren, C.P.; Caswell, R.; Castle, B.; Welch, C.R.; Hilliard, T.N.; Smithson, S.F.; Ellard, S. TRPV6 compound heterozygous variants result in impaired placental calcium transport and severe undermineralization and dysplasia of the fetal skeleton. Am. J. Med. Genet. A 2018, 176, 1950–1955. [Google Scholar] [CrossRef] [Green Version]
- Almidani, E.; Elsidawi, W.; Almohamedi, A.; Bin Ahmed, I.; Alfadhel, A. Case Report of Transient Neonatal Hyperparathyroidism: Medically Free Mother. Cureus 2020, 12, e7000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, A.E.; Grier, D.; Smithson, S.F.; Burren, C.P.; Gradhand, E. Post-mortem histology in transient receptor potential cation channel subfamily V member 6 (TRPV6) under-mineralising skeletal dysplasia suggests postnatal skeletal recovery: A case report. BMC Med. Genet. 2020, 21, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, A.; Takebe, K.; Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta 2010, 31, 126–133. [Google Scholar] [PubMed] [Green Version]
- Moreau, J.L.; Artap, S.T.; Shi, H.; Chapman, G.; Leone, G.; Sparrow, D.B.; Dunwoodie, S.L. Cited2 is required in trophoblasts for correct placental capillary patterning. Dev. Biol. 2014, 392, 62–79. [Google Scholar] [CrossRef] [Green Version]
- Nie, G.; Li, Y.; Salamonsen, L.A. Serine protease HtrA1 is developmentally regulated in trophoblast and uterine decidual cells during placental formation in the mouse. Dev. Dyn. 2005, 233, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.Y.; Hampton, A.; Li, Y.; Findlay, J.K.; Salamonsen, L.A. Identification and cloning of two isoforms of human high-temperature requirement factor A3 (HtrA3), characterization of its genomic structure and comparison of its tissue distribution with HtrA1 and HtrA2. Biochem. J. 2003, 371 Pt 1, 39–48. [Google Scholar] [CrossRef]
- Lieberman, J. The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat. Rev. Immunol. 2003, 3, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, L.M.; Colucci, F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front. Immunol. 2017, 8, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Allen, M.P.; Nilsen-Hamilton, M. Granzymes D, E, F, and G are regulated through pregnancy and by IL-2 and IL-15 in granulated metrial gland cells. J. Immunol. 1998, 161, 2772–2779. [Google Scholar]
- Bots, M.; Medema, J.P. Granzymes at a glance. J. Cell Sci. 2006, 119 Pt 24, 5011–5014. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B.; Nair, S.; Pallas, J.; Williams, M.A. Fibronectin: A multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol. Rev. 2011, 35, 147–200. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qi, Y.; Li, S.; Hsu, S.C.; Saadat, S.; Hsu, J.; Rahimi, S.A.; Lee, L.Y.; Yan, C.; Tian, X. CREG1 Interacts with Sec8 to Promote Cardiomyogenic Differentiation and Cell-Cell Adhesion. Stem Cells 2016, 34, 2648–2660. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, A.; Yano, M.; Tocharus, J.; Kojima, H.; Fukumoto, M.; Kawaichi, M.; Oka, C. Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone 2005, 37, 323–336. [Google Scholar] [CrossRef]
- Grau, S.; Richards, P.J.; Kerr, B.; Hughes, C.; Caterson, B.; Williams, A.S.; Junker, U.; Jones, S.A.; Clausen, T.; Ehrmann, M. The role of human HtrA1 in arthritic disease. J. Biol. Chem. 2006, 281, 6124–6129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaleo, E.; Fantucci, P.; De Gioia, L. Effects of Calcium Binding on Structure and Autolysis Regulation in Trypsins. A Molecular Dynamics Investigation. J. Chem. Theory Comput. 2005, 1, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Savignac, M.; Mellstrom, B.; Naranjo, J.R. Calcium-dependent transcription of cytokine genes in T lymphocytes. Pflugers Arch. 2007, 454, 523–533. [Google Scholar] [CrossRef]
- Fan, D.X.; Zhou, W.J.; Jin, L.P.; Li, M.Q.; Xu, X.H.; Xu, C.J. Trophoblast-Derived CXCL16 Decreased Granzyme B Production of Decidual gammadelta T Cells and Promoted Bcl-xL Expression of Trophoblasts. Reprod. Sci. 2019, 26, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.J.; Revell, P.A.; Lu, Z.H.; Johnson, H.; Bredemeyer, A.J.; Ley, T.J. The orphan granzymes of humans and mice. Curr. Opin. Immunol. 2003, 15, 544–552. [Google Scholar] [CrossRef]
- Boivin, W.A.; Cooper, D.M.; Hiebert, P.R.; Granville, D.J. Intracellular versus extracellular granzyme B in immunity and disease: Challenging the dogma. Lab. Invest. 2009, 89, 1195–1220. [Google Scholar] [CrossRef] [Green Version]
- Buzza, M.S.; Bird, P.I. Extracellular granzymes: Current perspectives. Biol. Chem. 2006, 387, 827–837. [Google Scholar] [CrossRef]
- Hirst, C.E.; Buzza, M.S.; Sutton, V.R.; Trapani, J.A.; Loveland, K.L.; Bird, P.I. Perforin-independent expression of granzyme B and proteinase inhibitor 9 in human testis and placenta suggests a role for granzyme B-mediated proteolysis in reproduction. Mol. Hum. Reprod. 2001, 7, 1133–1142. [Google Scholar]
- Schulz, L.C.; Widmaier, E.P.; Qiu, J.; Roberts, R.M. Effect of leptin on mouse trophoblast giant cells. Biol. Reprod. 2009, 80, 415–424. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Ikawati, M.; Tocharus, J.; Kawaichi, M.; Oka, C. Abnormal development of placenta in HtrA1-deficient mice. Dev. Biol. 2015, 397, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, A.; Nishizawa, H.; Ota, S.; Suzuki, M.; Inuzuka, H.; Miyamura, H.; Sekiya, T.; Kurahashi, H.; Udagawa, Y. Upregulation of HtrA4 in the placentas of patients with severe pre-eclampsia. Placenta 2012, 33, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Marzioni, D.; Lorenzi, T.; Altobelli, E.; Giannubilo, S.R.; Paolinelli, F.; Tersigni, C.; Crescimanno, C.; Monsurro, V.; Tranquilli, A.L.; Di Simone, N. Alterations of maternal plasma HTRA1 level in preeclampsia complicated by IUGR. Placenta 2012, 33, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Wang, L.; Huang, P.; Shao, W.; Song, Y.; Gou, W. High temperature requirement A1 in placental tissues and serum from pre-eclamptic pregnancies with or without fetal growth restriction. Arch. Med. Sci. 2013, 9, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115 Pt 20, 3861–3863. [Google Scholar] [CrossRef] [Green Version]
- Grinnell, F.; Bennett, M.H. Fibroblast adhesion on collagen substrata in the presence and absence of plasma fibronectin. J. Cell Sci. 1981, 48, 19–34. [Google Scholar]
- Grinnell, F.; Billingham, R.E.; Burgess, L. Distribution of fibronectin during wound healing in vivo. J. Investig. Dermatol. 1981, 76, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Pommier, C.G.; Inada, S.; Fries, L.F.; Takahashi, T.; Frank, M.M.; Brown, E.J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J. Exp. Med. 1983, 157, 1844–1854. [Google Scholar]
- Liedtke, D.; Orth, M.; Meissler, M.; Geuer, S.; Knaup, S.; Koblitz, I.; Klopocki, E. ECM alterations in Fndc3a (Fibronectin Domain Containing Protein 3A) deficient zebrafish cause temporal fin development and regeneration defects. Sci. Rep. 2019, 9, 13383. [Google Scholar] [CrossRef] [Green Version]
- Obholz, K.L.; Akopyan, A.; Waymire, K.G.; MacGregor, G.R. FNDC3A is required for adhesion between spermatids and Sertoli cells. Dev. Biol. 2006, 298, 498–513. [Google Scholar] [CrossRef] [Green Version]
- Pennington, K.A.; Schlitt, J.M.; Schulz, L.C. Isolation of primary mouse trophoblast cells and trophoblast invasion assay. J. Vis. Exp. 2012, e3202. [Google Scholar] [CrossRef] [Green Version]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R.A. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, M.; Weissgerber, P.; Klein, K.; Lux, F.; Yildiz, D.; Wissenbach, U.; Philipp, S.E.; Meyer, M.R.; Flockerzi, V.; Fecher-Trost, C. Transient Receptor Potential Vanilloid 6 (TRPV6) Proteins Control the Extracellular Matrix Structure of the Placental Labyrinth. Int. J. Mol. Sci. 2020, 21, 9674. https://doi.org/10.3390/ijms21249674
Winter M, Weissgerber P, Klein K, Lux F, Yildiz D, Wissenbach U, Philipp SE, Meyer MR, Flockerzi V, Fecher-Trost C. Transient Receptor Potential Vanilloid 6 (TRPV6) Proteins Control the Extracellular Matrix Structure of the Placental Labyrinth. International Journal of Molecular Sciences. 2020; 21(24):9674. https://doi.org/10.3390/ijms21249674
Chicago/Turabian StyleWinter, Manuel, Petra Weissgerber, Karolin Klein, Femke Lux, Daniela Yildiz, Ulrich Wissenbach, Stephan E. Philipp, Markus R. Meyer, Veit Flockerzi, and Claudia Fecher-Trost. 2020. "Transient Receptor Potential Vanilloid 6 (TRPV6) Proteins Control the Extracellular Matrix Structure of the Placental Labyrinth" International Journal of Molecular Sciences 21, no. 24: 9674. https://doi.org/10.3390/ijms21249674
APA StyleWinter, M., Weissgerber, P., Klein, K., Lux, F., Yildiz, D., Wissenbach, U., Philipp, S. E., Meyer, M. R., Flockerzi, V., & Fecher-Trost, C. (2020). Transient Receptor Potential Vanilloid 6 (TRPV6) Proteins Control the Extracellular Matrix Structure of the Placental Labyrinth. International Journal of Molecular Sciences, 21(24), 9674. https://doi.org/10.3390/ijms21249674





