Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine
Abstract
1. Introduction
2. Results
2.1. The Number of VIP-Positive ENS Neurons
2.2. The Co-Localization of VIP with nNOS
2.3. The Co-Localization of VIP with SP
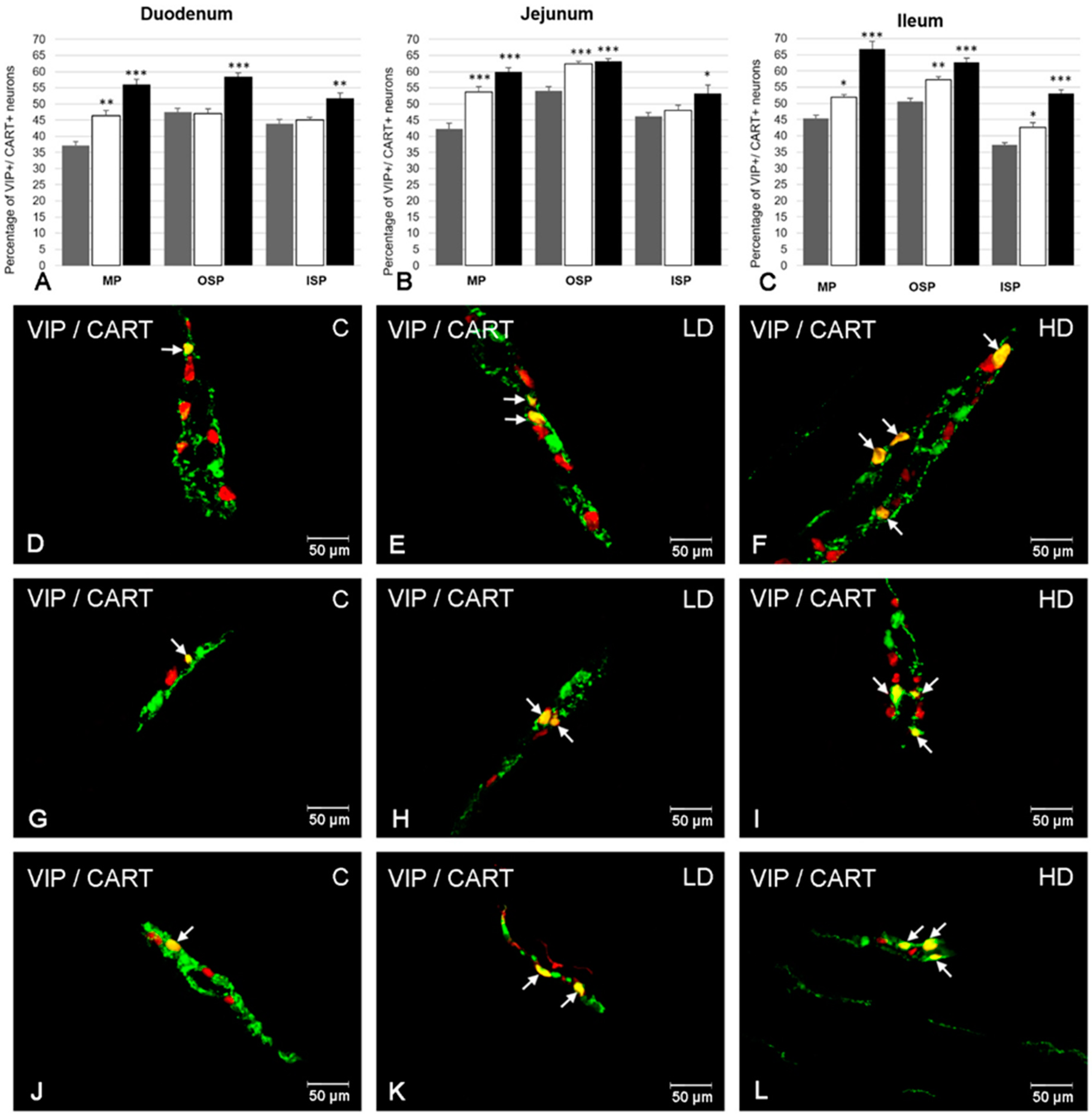
2.4. The Co-Localization of VIP with CART
3. Discussion
4. Material and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dybing, E.; Farmer, P.B.; Andersen, M.; Fennell, T.R.; Lalljie, S.P.; Müller, D.J.; Olin, S.; Petersen, B.J.; Schlatter, J.; Scholz, G.; et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Chemical modifications of peptides and their impact on food properties. Chem. Rev. 2011, 111, 7876–7903. [Google Scholar] [CrossRef]
- WHO. Health Implications of Acrylamide in Food; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2002; Available online: http://apps.who.int/iris/handle/10665/42563 (accessed on 15 April 2019).
- Shipp, A.; Lawrence, G.; Gentry, R.; McDonald, T.; Bartow, H.; Bounds, J.; Macdonald, N.; Clewell, H.; Allen, B.; Van Landingham, C. Acrylamide: Review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit. Rev. Toxicol. 2006, 36, 481–608. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.R.; Lee, J.Y.; Cho, J.H.; Song, H.M.; Kim, A.H.; Lee, W.; Lee, Y.; Chang, S.C.; Kim, H.S.; et al. Learning, memory deficits, and impaired neuronal maturation attributed to acrylamide. J. Toxicol. Environ. Health A 2018, 81, 254–265. [Google Scholar] [CrossRef]
- IARC. Acrylamide. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1994; Volume 60, pp. 389–433. [Google Scholar]
- Lo Pachin, R.M. The changing view of acrylamide neurotoxicity. Neurotoxicology 2004, 25, 617–630. [Google Scholar] [CrossRef]
- Zödl, B.; Schmid, D.; Wassler, G.; Gundacker, C.; Leibetseder, V.; Thalhammer, T.; Ekmekcioglu, C. Intestinal transport and metabolism of acrylamide. Toxicology 2007, 232, 99–108. [Google Scholar] [CrossRef]
- De Giorgio, R.; Barbara, G.; Pinto, D.; Cogliandro, R.; Elia, G.; Tomassetti, P.; Gizzi, G.; Stanghellini, V.; Corinaldesi, R. The innervation of the digestive tract: Its morphofunctional and neurochemical aspects. Minerva Gastroenterol. Dietol. 1996, 42, 83–91. [Google Scholar]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Gonkowski, S. Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 2013, 39, 542–551. [Google Scholar] [CrossRef]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Makowska, K. Chemically induced inflammation and nerve damage affect the distribution of vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nervous structures in the descending colon of the domestic pig. Neurogastroenterol Motil. 2018, 30, e13439. [Google Scholar] [CrossRef]
- Szymanska, K.; Gonkowski, S. Bisphenol A-Induced changes in the enteric nervous system of the porcine duodenum. Neurotoxicology 2018, 66, 78–86. [Google Scholar] [CrossRef]
- Said, S.I.; Mutt, V. Polypeptide with broad biological activity: Isolation from small intestine. Science 1970, 169, 1217–1218. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef]
- Waschek, J.A.; Bravo, D.T.; Sena, M.; Casillas, R.; Rodriguez, W.; Nguyen, T.; Colburn, S. Targeting of embryonic and postnatal autonomic and enteric neurons with a vasoactive intestinal peptide transgene. J. Neurochem. 1999, 73, 1739–1748. [Google Scholar] [CrossRef]
- Palle, C.; Ottesen, B.; Jørgensen, J.; Fahrenkrug, J. Peptide histidine methionine and vasoactive intestinal peptide: Occurrence and relaxant effect in the human female reproductive tract. Biol. Reprod. 1989, 41, 1103–1111. [Google Scholar] [CrossRef]
- Biancani, P.; Walsh, J.H.; Behar, J. Vasoactive intestinal polypeptide. A neurotransmitter for lower esophageal sphincter relaxation. J. Clin. Investig. 1984, 73, 963–967. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Xue, H.; Sun, H.J. Nervous mechanisms of restraint water-immersion stress-induced gastric mucosal lesion. World J. Gastroenterol. 2020, 26, 2533–2549. [Google Scholar] [CrossRef]
- Deng, G.; Jin, L. The effects of vasoactive intestinal peptide in neurodegenerative disorders. Neurol. Res. 2016, 39, 65–72. [Google Scholar] [CrossRef]
- Ekblad, E.; Bauer, A.J. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol. Motil. 2004, 16, 123–128. [Google Scholar] [CrossRef]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Palus, K.; Zielonka, Ł.; Gajęcka, M.; Całka, J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin-induced diabetes in the pig. World J. Gastroenterol. 2017, 23, 6088–6099. [Google Scholar] [CrossRef] [PubMed]
- Pimont, S.; Bruley Des Varannes, S.; Le Neel, J.C.; Aubert, P.; Galmiche, J.P.; Neunlist, M. Neurochemical coding of myenteric neurones in the human gastric fundus. Neurogastroenterol. Motil. 2003, 15, 655–662. [Google Scholar] [CrossRef]
- Toole, L.; Belai, A.; Burnstock, G. A neurochemical characterisation of the golden hamster myenteric plexus. Cell Tissue Res. 1998, 291, 385–394. [Google Scholar] [CrossRef]
- Matini, P.; Mayer, B.; Faussone-Pellegrini, M.S. Neurochemical differentiation of rat enteric neurons during pre- and postnatal life. Cell Tissue Res. 1997, 288, 11–23. [Google Scholar] [CrossRef]
- Coupar, I.M. Stimulation of sodium and water secretion without inhibition of glucose absorption in the rat jejunum by vasoactive intestinal peptide(VIP). Clin. Exp. Pharmacol. Physiol. 1976, 3, 615–618. [Google Scholar] [CrossRef]
- Toumi, F.; Neunlist, M.; Cassagnau, E.; Parois, S.; Laboisse, C.L.; Gal-miche, J.P.; Jarry, A. Human submucosal neurones regulate intestinal epithelial cell proliferation: Evidence from a novel co-culture model. Neurogastroenterol. Motil. 2003, 15, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef]
- Jayawardena, D.; Guzman, G.; Gill, R.K.; Alrefai, W.A.; Onyuksel, H.; Dudeja, P.K. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G16–G25. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Dąbrowski, M.; Całka, J. Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig. Int. J. Mol. Sci. 2019, 20, 1681. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Nezami, B.G.; Srinivasan, S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G949–G957. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.S.; Stevens, S.; LoPachin, R.M. Proteomic analysis of rat striatal synaptosomes during acrylamide intoxication at a low dose rate. Toxicol. Sci. 2007, 100, 157–167. [Google Scholar] [CrossRef]
- LoPachin, R.M., Jr.; Lehning, E.J. Acrylamide-induced distal axon degeneration: A proposed mechanism of action. Neurotoxicology 1994, 15, 247–259. [Google Scholar]
- Sickles, D.W.; Stone, J.D.; Friedman, M.A. Fast axonal transport: A site of acrylamide neurotoxicity? Neurotoxicology 2002, 23, 223–251. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular mechanism of acrylamide neurotoxicity: Lessons learned from organic chemistry. Environ. Health Perspect. 2012, 120, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, O.T.; Ay, H.; Aytan, N.; Carreras, I.; Kowall, N.W.; Dedeoglu, A.; Tuncel, N. Vasoactive Intestinal Peptide Decreases β-Amyloid Accumulation and Prevents Brain Atrophy in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2019, 68, 389–396. [Google Scholar] [CrossRef]
- Tunçel, N.; Korkmaz, O.T.; Tekin, N.; Şener, E.; Akyüz, F.; Inal, M. Antioxidant and anti-apoptotic activity of vasoactive intestinal peptide (VIP) against 6-hydroxy dopamine toxicity in the rat corpus striatum. J. Mol. Neurosci. 2012, 46, 51–57. [Google Scholar] [CrossRef]
- Sandgren, K.; Lin, Z.; Fex Svenningsen, A.; Ekblad, E. Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J. Neurosci. Res. 2003, 72, 595–602. [Google Scholar] [CrossRef]
- Rozza, A.L.; Moraes Tde, M.; Kushima, H.; Tanimoto, A.; Marques, M.O.; Bauab, T.M.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef]
- Yousef, M.I. El-Demerdash FM. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology 2006, 219, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, X.; Hu, X.; Li, S.; Wang, J. The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2020, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, N.; Oono, T.; Igarashi, H.; Ito, T.; Nakamura, T.; Uchida, M.; Coy, D.H.; Jensen, R.T.; Takayanagi, R. Vasoactive intestinal peptide reduces oxidative stress in pancreatic acinar cells through the inhibition of NADPH oxidase. Peptides 2011, 32, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Obremski, K.; Bulc, M.; Całka, J. The impact of low and high doses of acrylamide on the intramural neurons of the porcine ileum. Food Chem. Toxicol. 2019, 132, 110673. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Zapolska-Downar, D.; Ko’smider, A.; Nowicka, G.; Kozłowska-Wojciechowska, M.; Vikström, A.S.; Törnqvist, M. Chronic intake of potato chips in humans increases the production of reactive oxygen radicals by leukocytes and increases plasma C-reactive protein: A pilot study. Am. J. Clin. Nutr. 2009, 89, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Erendor, F.; Sahin, E.O.; Sanlioglu, A.D.; Balci, M.K.; Griffith, T.S.; Sanlioglu, S. Lentiviral gene therapy vectors encoding VIP suppressed diabetes-related inflammation and augmented pancreatic beta-cell proliferation. Gene Ther. 2020. [Google Scholar] [CrossRef]
- Banks, M.R.; Farthing, M.J.; Robberecht, P.; Burleigh, D.E. Antisecretory actions of a novel vasoactive intestinal polypeptide (VIP) antagonist in human and rat small intestine. Br. J. Pharmacol. 2005, 144, 994–1001. [Google Scholar] [CrossRef]
- Carniglia, L.; Ramírez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases. Mediators Inflamm. 2017, 2017, 5048616. [Google Scholar] [CrossRef]
- Li, X.B.; Chen, H.M.; Lu, H.; Zheng, Q.; Chen, X.Y.; Peng, Y.S.; Ge, Z.Z.; Liu, W.Z. Role of Helicobacter pylori infection on neuronal expression in the stomach and spinal cord of a murine model. J. Dig. Dis. 2009, 10, 286–292. [Google Scholar] [CrossRef]
- Arranz, A.; Abad, C.; Juarranz, Y.; Leceta, J.; Martinez, C.; Gomariz, R.P. Vasoactive intestinal peptide as a healing mediator in Crohn’s disease. Neuroimmunomodulation 2008, 15, 46–53. [Google Scholar] [CrossRef]
- Abad, C.; Cheung-Lau, G.; Coûté-Monvoisin, A.C.; Waschek, J.A. Vasoactive intestinal peptide-deficient mice exhibit reduced pathology in trinitrobenzene sulfonic acid-induced colitis. Neuroimmunomodulation 2015, 22, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can. J. Vet. Res. 2017, 81, 129–136. [Google Scholar] [PubMed]
- Yunker, A.M.; Galligan, J.J. Extrinsic denervation increases myenteric nitric oxide synthase-containing neurons and inhibitory neuromuscular transmission in guinea pig. J. Auton. Nerv. Syst. 1998, 71, 148–158. [Google Scholar] [CrossRef]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric. Oxide. 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sandgren, K.; Ekblad, E. Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton. Neurosci. 2004, 114, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.H.; Li, Q.; Sarna, S.K. Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn’s colitis and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G295–G302. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, J.V. Substance P and the regulation of inflammation in infections and inflammatory bowel disease. Acta Physiol. 2015, 213, 453–461. [Google Scholar] [CrossRef]
- Gonkowski, S.; Kamińska, B.; Bossowska, A.; Korzon, M.; Landowski, P.; Majewski, M. The influence of experimental Bacteroides fragilis infection on substance P and somatostatin-immunoreactive neural elements in the porcine ascending colon—A preliminary report. Folia Morphol. 2003, 62, 455–457. [Google Scholar]
- Nascimento, R.D.; Martins, P.R.; de Souza Lisboa, A.; Adad, S.J.; Morais da Silveira, A.B.; Reis, D. An imbalance between substance P and vasoactive intestinal polypeptide might contribute to the immunopathology of megaesophagus after Trypanosoma cruzi infection. Hum. Pathol. 2013, 44, 269–276. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Perera, D.S.; Burcher, E.; Liu, L. Hemokinin-1 and substance P stimulate production of inflammatory cytokines and chemokines in human colonic mucosa via both NK(1) and NK(2) tachykinin receptors. Neuropeptides 2020, 82, 102061. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Paige, C.J. Hemokinin-1 has Substance P-like function in U-251 MG astrocytoma cells: A pharmacological and functional study. J. Neuroimmunol. 2005, 164, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Fang, X.C.; Pan, G.Z. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004, 53, 1096–1101. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, X.; Bao, X.; Zhang, Y.; Xu, Y.; Sha, D. Cocaine- and amphetamine-regulated transcript protects synaptic structures in neurons after ischemic cerebral injury. Neuropeptides 2020, 81, 102023. [Google Scholar] [CrossRef]
- Gonkowski, S.; Burliński, P.; Szwajca, P.; Całka, J. Changes in cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) nerve struc-tures of the porcine descending colon during proliferative enteropathy. Bull. Vet. Inst. Pulawy 2012, 56, 199–203. [Google Scholar] [CrossRef]
- Burliński, P.J. Inflammation- and axotomy-induced changes in cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nervous structures in the porcine descending colon. Pol. J. Vet. Sci. 2012, 15, 517–524. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Z. Evaluation of density and distribution of CART-immunoreactive structures in gastrointestinal tract of hypertensive rats. Biofactors 2012, 38, 407–415. [Google Scholar] [CrossRef]
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like immunoreactivity in intramural neurons within the pig stomach following supplementation with low and high doses of acrylamide. Neurotoxicology 2018, 69, 47–59. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palus, K.; Bulc, M.; Całka, J. Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine. Int. J. Mol. Sci. 2020, 21, 9691. https://doi.org/10.3390/ijms21249691
Palus K, Bulc M, Całka J. Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine. International Journal of Molecular Sciences. 2020; 21(24):9691. https://doi.org/10.3390/ijms21249691
Chicago/Turabian StylePalus, Katarzyna, Michał Bulc, and Jarosław Całka. 2020. "Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine" International Journal of Molecular Sciences 21, no. 24: 9691. https://doi.org/10.3390/ijms21249691
APA StylePalus, K., Bulc, M., & Całka, J. (2020). Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine. International Journal of Molecular Sciences, 21(24), 9691. https://doi.org/10.3390/ijms21249691




