Nickel Sensitivity Is Associated with GH-IGF1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects
Abstract
:1. Introduction
2. Results
2.1. Demographic, Anthropometric and Metabolic Parameters
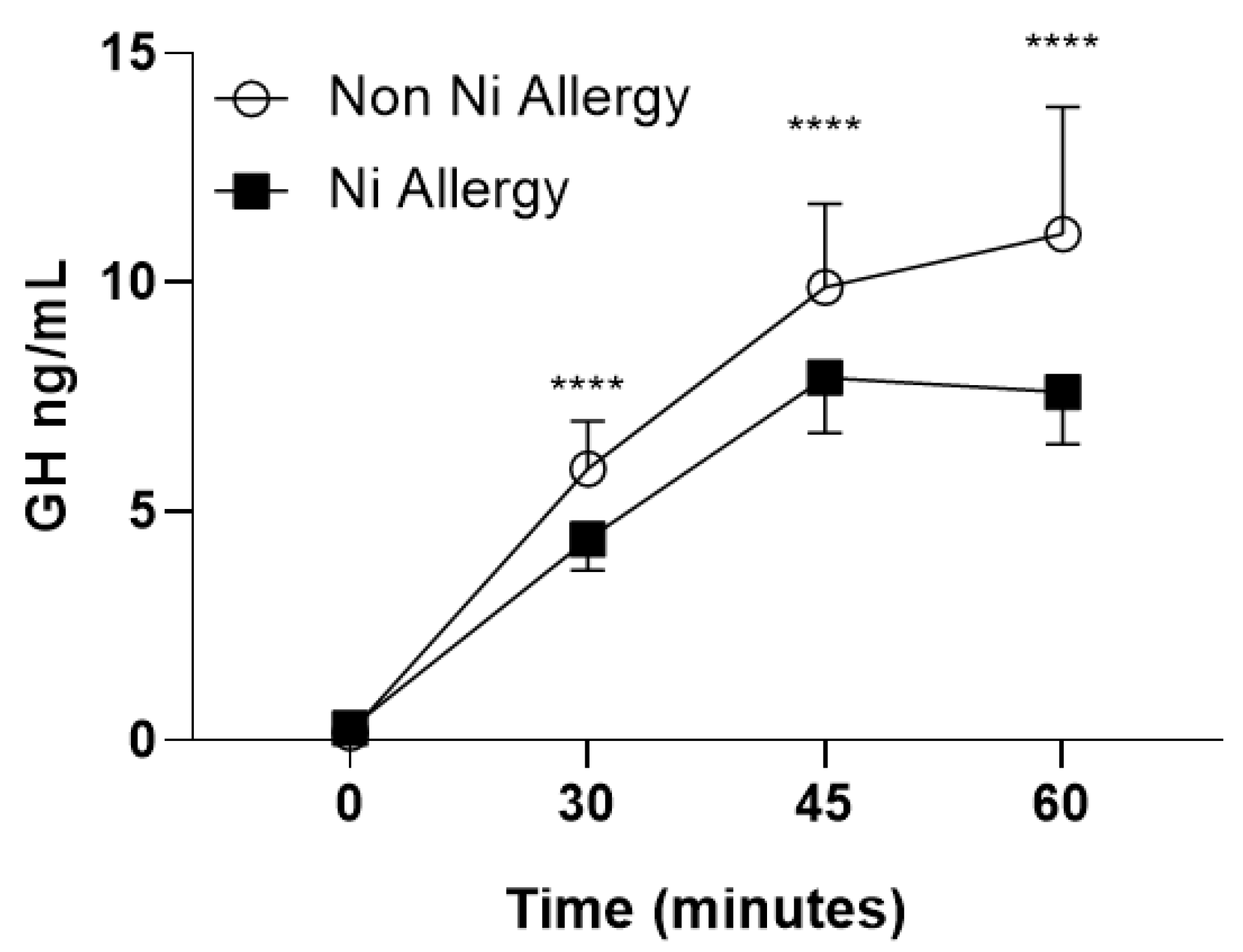
2.2. Pituitary Homonal Status
2.3. Pituitary Morphology on MRI
2.4. Bivariate and Partial Correlations between Ni Allergy, BMI, GH-IGF1 Axis and Inflammatory Markers in the Study Population
3. Discussion
4. Patients and Methods
4.1. Subjects
4.2. Anthropometric Measurements
4.3. Laboratory Assessments
4.4. Dynamic Test
4.5. Patch Test
4.6. Magnetic Resonance Imaging (MRI)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lubrano, C.; Saponara, M.; Barbaro, G.; Specchia, P.; Addessi, E.; Costantini, D.; Tenuta, M.; Di Lorenzo, G.; Genovesi, G.; Donini, L.M.; et al. Relationships between body fat distribution, epicardial fat and obstructive sleep apnea in obese patients with and without metabolic syndrome. PLoS ONE 2012, 7, e47059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty Liver Index Associates with Relative Sarcopenia and GH/ IGF- 1 Status in Obese Subjects. PLoS ONE 2016, 7, e0145811. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.K.; Dubey, P.; Cistola, D.P.; Reddy, S.Y. Association Between Obesity and Cardiovascular Outcomes: Updated Evidence from Meta-analysis Studies. Curr. Cardiol. Rep. 2020, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: A population-based longitudinal study. Int. J. Obes. (Lond.) 2019, 43, 139–148. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic Obesity and Metabolic Syndrome in Adult Caucasian Subjects. J. Nutr. Health Aging 2016, 20, 958–963. [Google Scholar] [CrossRef]
- Greco, E.A.; Fornari, R.; Rossi, F.; Santiemma, V.; Prossomariti, G.; Annoscia, C.; Aversa, A.; Brama, M.; Marini, M.; Donini, L.M.; et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int. J. Clin. Pract. 2010, 64, 817–820. [Google Scholar] [CrossRef]
- Romagnoli, E.; Lubrano, C.; Carnevale, V.; Costantini, D.; Nieddu, L.; Morano, S.; Migliaccio, S.; Gnessi, L. Lenzi A Assessment of trabecular bone score (TBS) in overweight/obese men: Effect of metabolic and anthropometric factors. Endocrine 2016, 54, 342–347. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Risi, R.; De Giorgi, F.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Lubrano, C.; Lenzi, A.; Gnessi, L. Obesity treatment within the tertiary Italian national healthcare system: What can we learn from it? Eat. Weight Disord. 2020. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Lubrano, C.; Cornoldi, A.; Pili, M.; Falcone, S.; Brandetti, F.; Fabbrini, E.; Ginanni-Corradini, S.; Eramo, A.; Marini, M.; Migliaccio, S.; et al. Reduction of risk factors for cardiovascular diseases in morbid-obese patients following biliary-intestinal bypass: 3 years’ follow-up. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1600–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Gangitano, E.; Francomano, D.; Addessi, E.; Toscano, R.; Costantini, D.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; et al. Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: A prospective randomized controlled pilot study. Nutrients 2018, 10, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets with Whey, Vegetable or Animal Protein in Patients with Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Risi, R.; Camajani, E.; Contini, S.; Persichetti, A.; Tuccinardi, D.; Ernesti, I.; Mariani, S.; Lubrano, C.; Genco, A.; et al. Baseline HOMA IR and Circulating FGF21 Levels Predict NAFLD Improvement in Patients Undergoing a Low Carbohydrate Dietary Intervention for Weight Loss: A Prospective Observational Pilot Study. Nutrients 2020, 12, 2141. [Google Scholar] [CrossRef]
- Basciani, S.; Costantini, D.; Contini, S.; Persichetti, A.; Watanabe, M.; Mariani, S.; Lubrano, C.; Spera, G.; Lenzi, A.; Gnessi, L. Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine 2015, 48, 863–870. [Google Scholar] [CrossRef]
- Watanabe, M.; Risi, R.; Masi, D.; Caputi, A.; Balena, A.; Rossini, G.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Manfrini, S.; et al. Current Evidence to Propose Different Food Supplements for Weight Loss: A Comprehensive Review. Nutrients 2020, 12, 2873. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267–296. [Google Scholar] [CrossRef] [Green Version]
- Lubrano, C.; Genovesi, G.; Specchia, P.; Costantini, D.; Mariani, S.; Petrangeli, E.; Lenzi, A.; Gnessi, L. Obesity and metabolic comorbidities: Environmental diseases? Oxid. Med. Cell. Longev. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular mechanisms of nickel allergy. Int. J. Mol. Sci. 2016, 17, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlström, M.G.; Thyssen, J.P.; Menné, T.; Johansen, J.D. Prevalence of nickel allergy in Europe following the EU Nickel Directive—A review. Contact Dermat. 2017, 77, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverberg, N.B.; Pelletier, J.L.; Jacob, S.E. Nickel Allergic Contact Dermatitis: Identification, Treatment, and Prevention. Pediatrics 2020, 145, e20200628. [Google Scholar] [CrossRef]
- Ricciardi, L.; Arena, A.; Arena, E.; Zambito, M.; Ingrassia, A.; Valenti, G.; Loschiavo, G.; D’Angelo, A.; Saitta, S. Systemic nickel allergy Syndrome: Epidemiological data from four Italian allergy units. Int. J. Immunopathol. Pharmacol. 2014, 27, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Kasper-Sonnenberg, M.; Sugiri, D.; Wurzler, S.; Ranft, U.; Dickel, H.; Wittsiepe, J.; Hölzer, J.; Lemm, F.; Eberwein, G.; Altmeyer, P.; et al. Prevalence of nickel sensitization and urinary nickel content of children are increased by nickel in ambient air. Environ. Res. 2011, 111, 266–273. [Google Scholar] [CrossRef]
- Panel, E.; Chain, F. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 1–202. [Google Scholar] [CrossRef] [Green Version]
- Antico, A.; Soana, R. Nickel sensitization and dietary nickel are a substantial cause of symptoms provocation in patients with chronic allergic-like dermatitis syndromes. Allergy Rhinol. 2015, 6, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Kasper-Sonnenberg, M.; Sugiri, D.; Wurzler, S.; Ranft, U.; Dickel, H.; Wittsiepe, J.; Hölzer, J.; Lemm, F.; Eberwein, G.; Altmeyer, P.; et al. Systemic nickel allergy syndrome: Nosologic framework and usefulness of diet regimen for diagnosis. Int. J. Immunopathol. Pharmacol. 2013, 26, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Patriarca, M.; Lyon, T.D.; Fell, G.S. Nickel metabolism in humans investigated with an oral stable isotope. Am. J. Clin. Nutr. 1997, 66, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, C.; Vrhovnik, P.; Fiket, Ž.; Salcedo-Bellido, I.; Echeverría, R.; Martín-Olmedo, P.; Kniewald, G.; Fernández, M.F.; Arrebola, J.P. Adipose tissue concentrations of arsenic, nickel, lead, tin, and titanium in adults from GraMo cohort in Southern Spain: An exploratory study. Sci. Total Environ. 2020, 719. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Nickel Air Quality Guidelines, 2nd ed.; WHO: Geneva, Switzerland, 2000; Chapter 6.10.
- Lusi, E.A.; Di Ciommo, V.M.; Patrissi, T.; Guarascio, P. High prevalence of nickel allergy in an overweight female population: A pilot observational analysis. PLoS ONE 2015, 10, e0123265. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Masieri, S.; Costantini, D.; Tozzi, R.; De Giorgi, F.; Gangitano, E.; Tuccinardi, D.; Poggiogalle, E.; Mariani, S.; Basciani, S.; et al. Overweight and obese patients with nickel allergy have a worse metabolic profile compared to weight matched non-allergic individuals. PLoS ONE 2018, 13, e0202683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijhawan, R.I.; Molenda, M.; Zirwas, M.J.; Jacob, S.E. Systemic Contact Dermatitis. Dermatol. Clin. 2009, 27, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J.; Peana, M. Metals, autoimmunity, and neuroendocrinology: Is there a connection? Environ. Res. 2020, 187, 109541. [Google Scholar] [CrossRef]
- Xu, X.; Rao, X.; Wang, T.Y.; Jiang, S.Y.; Ying, Z.; Liu, C.; Wang, A.; Zhong, M.; Deiuliis, J.A.; Maiseyeu, A.; et al. Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part. Fibre Toxicol. 2012, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Lian, I.B.; Kor, C.T.; Chang, C.C.; Su, P.Y.; Chang, W.T.; Liang, Y.F.; Su, W.W.; Soon, M.S. Association between soil heavy metals and fatty liver disease in men in Taiwan: A cross sectional study. BMJ Open 2017, 7, e014215. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Ahmad, N.; Husain, M.M.; Srivastava, R.C. Involvement of nitric oxide in nickel-induced hyperglycemia in rats. Nitric Oxide 2000, 4, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Dzugkoeva, F.S.; Mozhaeva, I.V.; Dzugkoev, S.G.; Margieva, O.I.; Tedtoeva, A.I.; Otiev, M.A. Oxidative stress and biochemical markers of endothelial dysfunction and organ damage under conditions of experimental nonferrous metal intoxication. Bull. Exp. Biol. Med. 2016, 162, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar] [PubMed]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy metals, islet function and diabetes development. Islets 2009, 1, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenson, M.Y.; Robson, D.L.; Jacobs, L.S. Divalent Cation Inhibition of Hormone Release from Isolated Adenohypophysial Secretory Granules. J. Biol. Chem. 1983, 258, 8618–8622. [Google Scholar]
- Carlson, H.E. Inhibition of prolactin and growth hormone secretion by nickel. Life Sci. 1984, 35, 1747–1754. [Google Scholar] [CrossRef]
- Dormer, R.L.; Kerbey, A.L.; McPherson, M.; Manley, S.; Ashcroft, S.J.; Schofield, J.G.; Randle, P.H. The effect of nickel on secretory systems. Studies on the release of amylase, insulin and growth hormone. Biochem. J. 1974, 140, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Labella., F.; Dular, R.; Lemon, P.; Vivian, S.; Queen, G. Prolactin Secretion is Specifically Inhibited by Nickel. Nature 1973, 245, 330–332. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Chiloiro, S.; Giampietro, A.; Bianchi, A.; Tartaglione, T.; Capobianco, A.; Anile, C.; De Marinis, L. Diagnosis of endocrine disease: Primary empty sella: A comprehensive review. Eur. J. Endocrinol. 2017, 177, R275–R285. [Google Scholar] [CrossRef]
- Rasmussen, M.H. Obesity, growth hormone and weight loss. Mol. Cell. Endocrinol. 2010, 316, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumrongpisutikul, N.; Chuajak, A.; Lerdlum, S. Pituitary height at magnetic resonance imaging in pediatric isolated growth hormone deficiency. Pediatr. Radiol. 2018, 48, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Kara, Ö.; Esen, İ.; Tepe, D.; Gülleroğlu, N.B.; Tayfun, M. Relevance of pituitary gland magnetic resonance imaging results with clinical and laboratory findings in growth hormone deficiency. Med. Sci. Monit. 2018, 24, 9473–9478. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Tenner, M.; Frey, M.; Noto, R. Pituitary volume in children with growth hormone deficiency, idiopathic short stature and controls. J. Pediatr. Endocrinol. Metab. 2016, 29, 1195–1200. [Google Scholar] [CrossRef]
- Lubrano, C.; Tenuta, M.; Costantini, D.; Specchia, P.; Barbaro, G.; Basciani, S.; Mariani, S.; Pontecorvi, A.; Lenzi, A.; Gnessi, L. Severe growth hormone deficiency and empty sella in obesity: A cross-sectional study. Endocrine 2015, 49, 503–511. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Aoki, S.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Contribution of metals to brain MR signal intensity: Review articles. Jpn. J. Radiol. 2016, 34, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Christoforidis, A.; Haritandi, A.; Perifanis, V.; Tsatra, I.; Athanassiou-Metaxa, M.; Dimitriadis, A.S. MRI for the determination of pituitary iron overload in children and young adults with β-thalassaemia major. Eur. J. Radiol. 2007, 62, 138–142. [Google Scholar] [CrossRef]
- Mousa, A.A.; Ghonem, M.; Elhadidy el, H.M.; Azmy, E.; Elbackry, M.; Elbaiomy, A.A.; Elzehery, R.R.; Shaker, G.A.; Saleh, O. Iron overload detection using pituitary and hepatic MRI in thalassemic patients having short stature and hypogonadism. Endocr. Res. 2016, 41, 81–88. [Google Scholar] [CrossRef]
- Lipsy, R.J. The National Cholesterol Education Program Adult Treatment Panel III guidelines. J. Manag. Care Pharm. 2003, 9 (Suppl. 1), 2–5. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.M.; Yuen, K.C.J.; Biller, B.M.K.; Kemp, S.F.; Vance, M.L. American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients—2009 update. Endocr. Pract. 2009, 15, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Tsou, T.C.; Liou, S.H.; Yeh, S.C.; Tsai, F.Y.; Chao, H.R. Crucial role of Toll-like receptors in the zinc/nickel-induced inflammatory response in vascular endothelial cells. Toxicol. Appl. Pharmacol. 2013, 273, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, M.E.; Sosa Ldel, V.; Petiti, J.P.; Mukdsi, J.H.; Mascanfroni, I.D.; Pellizas, C.G.; Gutiérrez, S.; Torres, A.I.; De Paul, A.L. Functional Toll-like receptor 4 expressed in lactotrophs mediates LPS-induced proliferation in experimental pituitary hyperplasia. Exp. Cell Res. 2013, 319, 3020–3034. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska-Zaremba, D.; Haziak, K.; Tomczyk, M.; Herman, A.P. Inflammation and LPS-binding protein enable the stimulatory effect of endotoxin on prolactin secretion in the ovine anterior pituitary: Ex vivo study. Mediat. Inflamm. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Lupi, I.; Zhang, J.; Gutenberg, A.; Landek-Salgado, M.; Tzou, S.C.; Mori, S.; Caturegli, P. From pituitary expansion to empty sella: Disease progression in a mouse model of autoimmune hypophysitis. Endocrinology 2011, 152, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Ihlebaek, C.; Eriksen, H.R.; Ursin, H. Prevalence of subjective health complaints (SHC) in Norway. Scand. J. Public Health 2002, 30, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Bunt, S.N.W.; Merelle, S.Y.M.; Steenhuis, I.H.M.; Kroeze, W. Predictors of need for help with weight loss among overweight and obese men and women in the Netherlands: A cross-sectional study. BMC Health Serv. Res. 2017, 17, 819. [Google Scholar] [CrossRef] [Green Version]
- Lachapelle, J.-M.; Maibach, H.I. Patch Testing and Prick Testing: A Practical Guide Official Publication of the ICDRG; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L. Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef]
- Ho, K.; On behalf of the 2007 GH Deficiency Consensus Workshop Participants. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: A statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur. J. Endocrinol. 2020, 157, 695–700. [Google Scholar] [CrossRef]
- Dinç, H.; Esen, F.; Demirci, A.; Sari, A.; Resit Gümele, H. Pituitary dimensions and volume measurements in pregnancy and post-partum: MR assessment. Acta Radiol. 1998, 39, 64–69. [Google Scholar] [CrossRef]
- Lurie, S.N.; Doraiswamy, P.M.; Husain, M.M.; Boyko, O.B.; Ellinwood, E.H., Jr.; Figiel, G.S.; Krishnan, K.R. In vivo assessment of pituitary gland volume with magnetic resonance imaging: The effect of age. J. Clin. Endocrinol. Metab. 1990, 71, 505–508. [Google Scholar] [CrossRef]

| Demographic, Anthropometric and Metabolic Parameters | Ni Allergy Confirmed (n = 42) | Ni Allergy Excluded (n = 25) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Age (years) | 48.1 ± 1.6 | 43.3 ± 1.7 |
| Gender (%F) | 95.9 | 84.6 |
| Weight (Kg) | 94.2 ± 22 | 97.7 ± 20.5 |
| BMI (Kg/m2) | 34.9 ± 6.1 | 35.1 ± 6.6 |
| Waist circumference (cm) | 112.6 ± 17.3 | 114 ± 15.2 |
| Hip circumference (cm) | 118.3 ± 13.5 | 120.5 ± 13.2 |
| Met-S (%) | 50% | 16% ** |
| IFG and/or DM (%) | 71.4% | 16% ** |
| Dyslipidemia (%) | 45.2% | 24% * |
| HT (%) | 59.5% | 32% * |
| Hormonal Status | Ni Allergy Confirmed (n = 42) | Ni Allergy Excluded (n = 25) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| GH (ng/mL) | 0.27 ± 0.21 | 0.34 ± 0.31 |
| IGF-1 (ng/mL) | 128.3 ± 42.4 | 177.3 ± 78 * |
| TSH (µUI/mL) | 2.2 ± 1.3 | 2.2 ± 1.2 |
| FT4 (ng/dL) | 1.2 ± 0.17 | 1.2 ± 0.18 |
| PRL (ng/mL) | 12.8 ± 6.2 | 13.7 ± 6.8 |
| ACTH (pg/mL) | 20.9 ± 11.7 | 32.6 ± 12.8 ** |
| Cortisol (nmol/L) | 130.2 ± 54.9 | 154.8 ± 47.8 ^ |
| Pituitary Morphological Parameters | Ni Allergy Confirmed | Ni AllergyExcluded | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Whole population (n) | 42 | 25 | |
| Pituitary Volume (mm2) | 389.5 ± 141.1 | 482.2 ± 124.9 | 0.004 |
| Pituitary Height (mm) | 4.3 ± 1.5 | 5.3 ± 1.9 | 0.02 |
| Pituitary Width (mm) | 14.6 ± 2.1 | 14.8 ± 2.2 | NS |
| Pituitary Length (mm) | 12.7 ± 1.9 | 12.6 ± 1.5 | NS |
| Pituitary Stalk Thickness (mm) | 2.44 ± 0.66 | 2.46 ± 0.71 | NS |
| T1W PTM intensity | 1.2 ± 0.16 | 1.40 ± 0.27 | NS |
| T2W PTM intensity | 4.9 ± 1.7 | 3.6 ± 1.18 | 0.005 |
| Patients with ES diagnosis excluded (n) | 23 | 18 | |
| Pituitary Volume (mm2) | 405.5 ± 151.8 | 500.5 ± 125.8 | 0.03 |
| Pituitary Height (mm) | 4.8 ± 1.6 | 5.7 ± 1.9 | NS |
| Pituitary Width (mm) | 14.3 ± 2.2 | 14.6 ± 1.9 | NS |
| Pituitary Length (mm) | 11.8 ± 1.5 | 12.1 ± 1.2 | NS |
| Pituitary Stalk Thickness (mm) | 2.60 ± 0.53 | 2.60 ± 0.69 | NS |
| T1W PTM intensity | 1.2 ± 0.16 | 1.38 ± 0.26 | NS |
| T2W PTM intensity | 4.9 ± 1.5 | 3.4 ± 1.3 | 0.02 |
| Ni A | BMI | GH | IGF1 | GH Peak | PV | T1WPTM | T2WPTM | WBC | L(n) | N(n) | CRP | ESR | Ferritin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni A | 1 | |||||||||||||
| BMI | −0.01 | 1 | ||||||||||||
| GH | −0.15 | −0.12 | 1 | |||||||||||
| IGF1 | −0.35 ** | 0.09 | −0.01 | 1 | ||||||||||
| GH peak | −0.22 | −0.15 | 0.05 | 0.24 | 1 | |||||||||
| PV | −0.23 ** | 0.19 | −0.04 | 0.21 | 0.08 | 1 | ||||||||
| T1WPTM | 0.13 | 0.01 | 0.04 | 0.16 | −0.08 | −0.2 | 1 | |||||||
| T2WPTM | 0.39 ** | 0.14 | 0.04 | 0.23 | 0.06 | −0.15 | 0.33 * | 1 | ||||||
| WBC | −0.01 | −0.03 | −0.34 * | 0.25 * | 0.02 | −0.09 | −0.02 | −0.04 | 1 | |||||
| L (n) | 0.04 | 0.13 | −0.31 * | 0.37 ** | 0.17 | 0.01 | 0.07 | −0.02 | 0.56 *** | 1 | ||||
| N (n) | −0.04 | −0.09 | −0.29 * | 0.16 | −0.1 | −0.09 | −0.05 | −0.06 | 0.94 *** | 0.26 * | 1 | |||
| CRP | 0.09 | −0.03 | −0.29 | −0.09 | −0.26 | −0.13 | 0.02 | −0.16 | 0.32 * | 0.12 | 0.32 * | 1 | ||
| ESR | −0.14 | −0.20 | −0.13 | 0.09 | −0.01 | −0.05 | −0.03 | −0.19 | 0.28 * | 0.13 | 0.28 * | 0.44 ** | 1 | |
| Ferritin | −0.14 | −0.06 | 0.12 | −0.15 | 0.21 | −0.17 | 0.03 | 0.03 | −0.01 | 0.18 | −0.09 | −0.07 | 0.12 | 1 |
| 6BMI−Adj. | Ni A | GH | IGF1 | GH Peak | PV | T1WPTM | T2WPTM | WBC | L(n) | N(n) | CRP | ESR | Ferritin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni A | 1 | ||||||||||||
| GH | −0.57 | 1 | |||||||||||
| IGF1 | −0.55 ^ | 0.48 | 1 | ||||||||||
| GH peak | −0.37 | 0.68 * | 0.47 | 1 | |||||||||
| PV | −0.38 | 0.33 | −0.21 | 0.46 | 1 | ||||||||
| T1WPTM | −0.01 | 0.37 | −0.01 | −0.12 | −0.19 | 1 | |||||||
| T2WPTM | −0.16 | 0.30 | 0.49 | 0.37 | 0.22 | −0.28 * | 1 | ||||||
| WBC | 0.59 * | −0.53 * | −0.07 | −0.45 | −0.41 | 0.13 | −0.07 | 1 | |||||
| L (n) | 0.53 ^ | −0.27 * | 0.24 | −0.37 | −0.43 | 0.20 | 0.21 | 0.81 *** | 1 | ||||
| N (n) | 0.53 ^ | −0.56 * | −0.14 | −0.47 | −0.28 | 0.05 | −0.14 | 0.97 *** | 0.56 * | 1 | |||
| CRP | 0.73 * | −0.40 | −0.71 * | −0.56 ^ | −0.28 | 0.32 | −0.49 | 0.27 | −0.04 | 0.32 | 1 | ||
| ESR | −0.24 | −0.11 | −0.18 | 0.36 | 0.63 | −0.51 | 0.04 | −0.16 | −0.27 | −0.05 | −0.38 | 1 | |
| Ferritin | −0.03 | 0.52 | 0.01 | 0.13 | −0.26 | 0.47 | −0.31 | −0.14 | 0.02 | −0.32 | 0.06 | −0.26 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risi, R.; Masieri, S.; Poggiogalle, E.; Watanabe, M.; Caputi, A.; Tozzi, R.; Gangitano, E.; Masi, D.; Mariani, S.; Gnessi, L.; et al. Nickel Sensitivity Is Associated with GH-IGF1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects. Int. J. Mol. Sci. 2020, 21, 9733. https://doi.org/10.3390/ijms21249733
Risi R, Masieri S, Poggiogalle E, Watanabe M, Caputi A, Tozzi R, Gangitano E, Masi D, Mariani S, Gnessi L, et al. Nickel Sensitivity Is Associated with GH-IGF1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects. International Journal of Molecular Sciences. 2020; 21(24):9733. https://doi.org/10.3390/ijms21249733
Chicago/Turabian StyleRisi, Renata, Simonetta Masieri, Eleonora Poggiogalle, Mikiko Watanabe, Alessandra Caputi, Rossella Tozzi, Elena Gangitano, Davide Masi, Stefania Mariani, Lucio Gnessi, and et al. 2020. "Nickel Sensitivity Is Associated with GH-IGF1 Axis Impairment and Pituitary Abnormalities on MRI in Overweight and Obese Subjects" International Journal of Molecular Sciences 21, no. 24: 9733. https://doi.org/10.3390/ijms21249733










