Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases?
Abstract
:1. Introduction
2. Probiotics
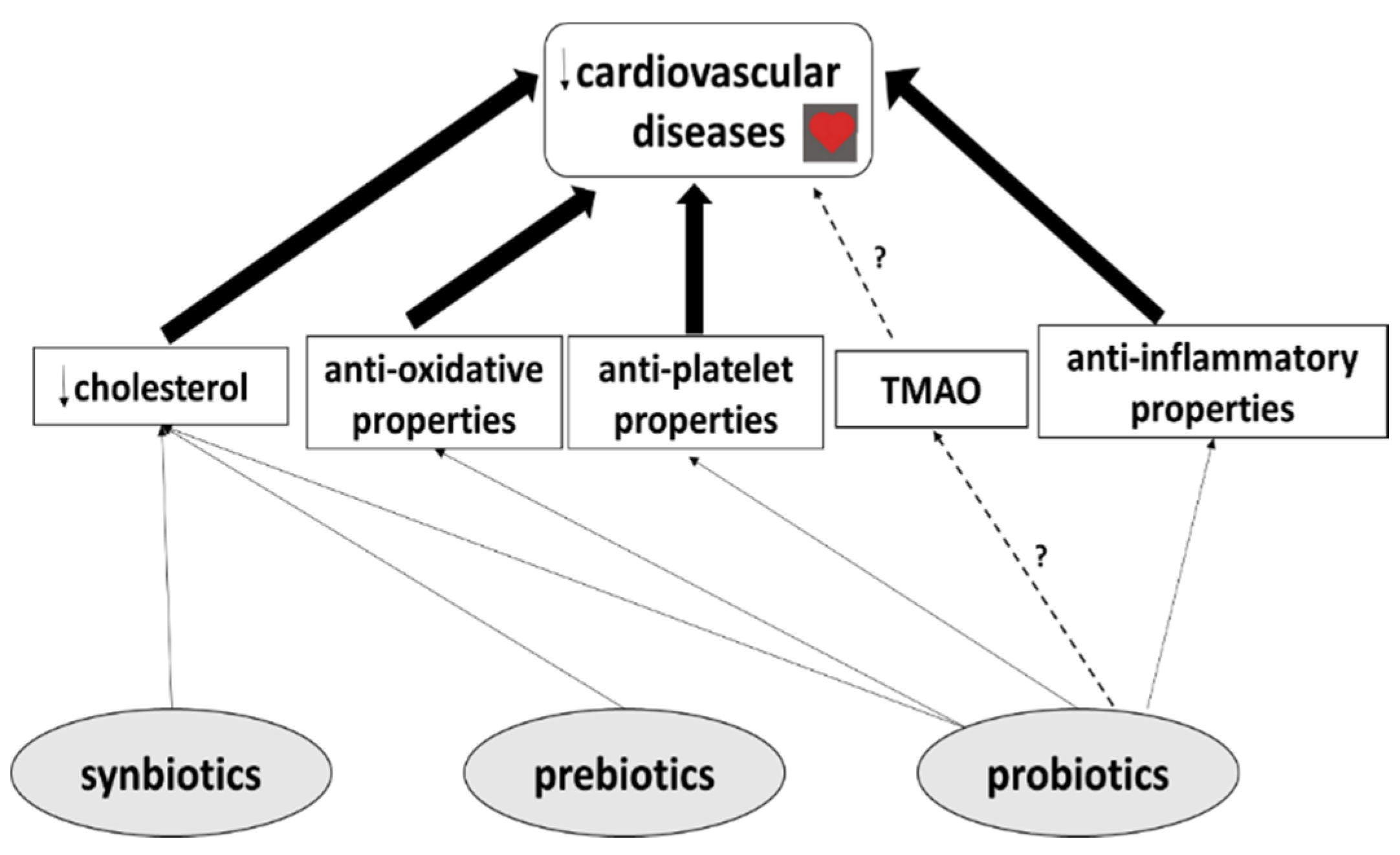
Probiotics and CVDs
3. Prebiotics
Prebiotics and CVDs
4. Synbiotics
Synbiotics and CVDs
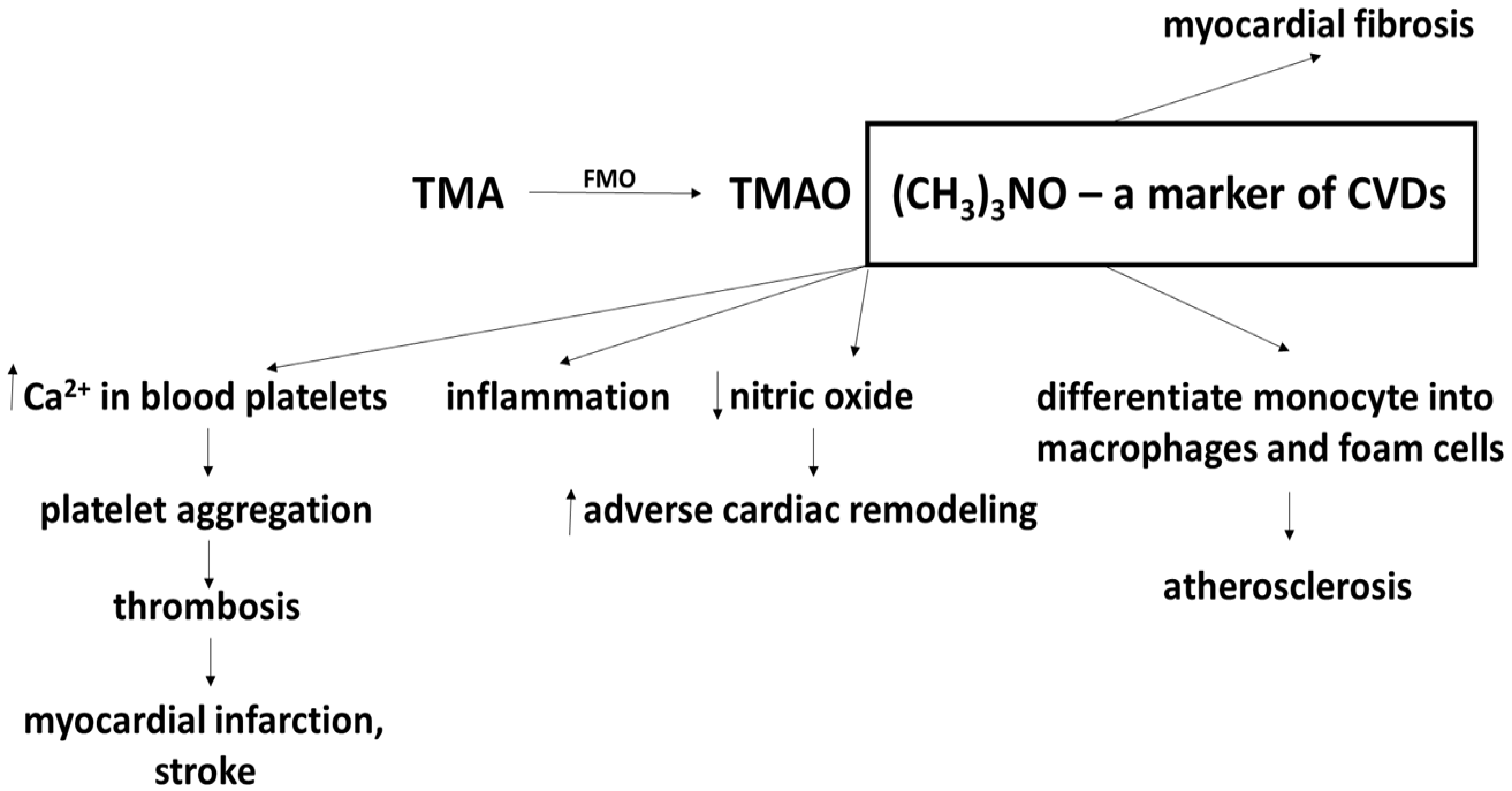
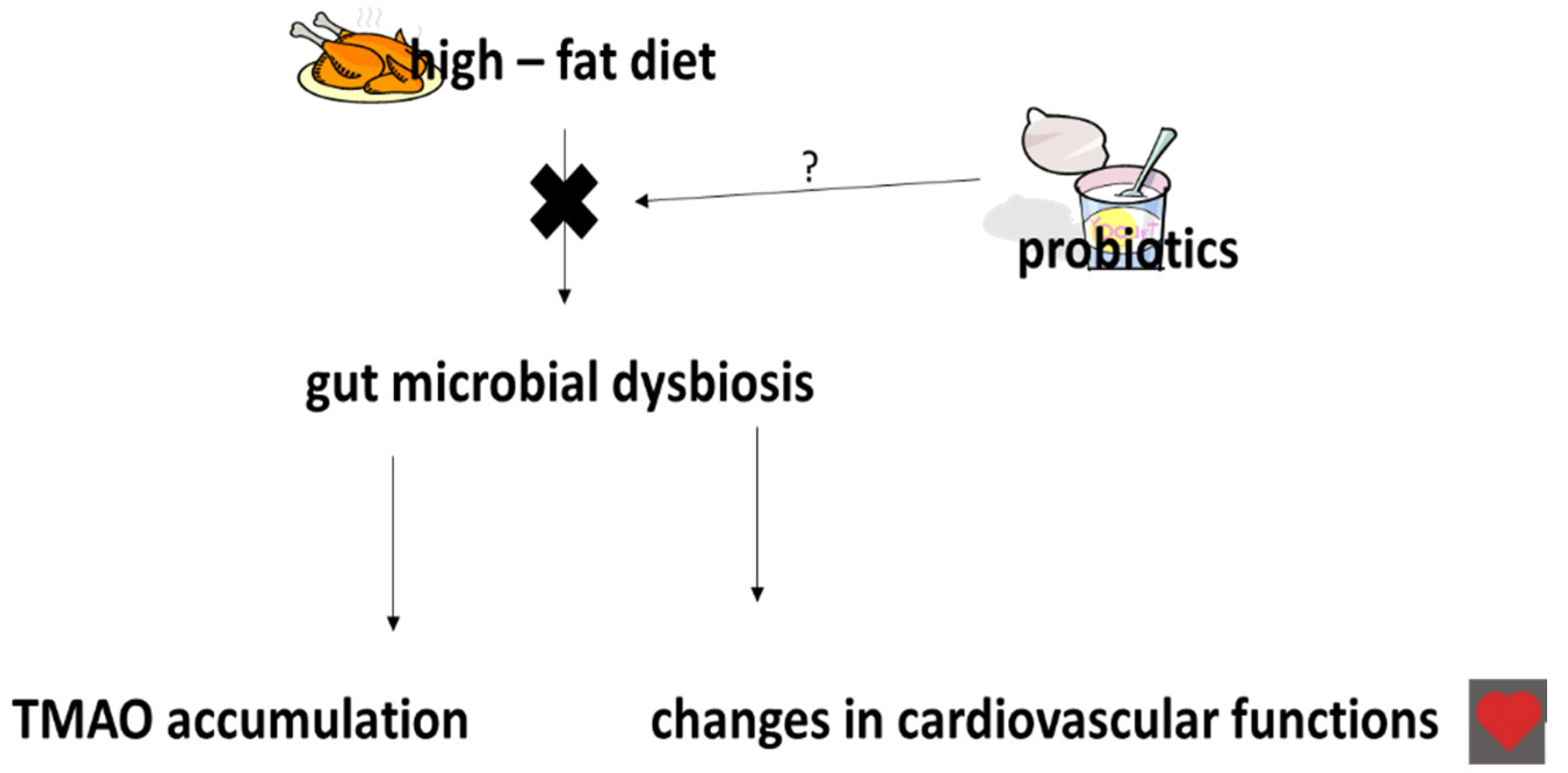
5. The Effects of Probiotics, Prebiotics and Synbiotics on TMAO Levels
6. The Effects of Probiotics, Prebiotics and Synbiotics on Uric Acid Levels
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Shanthi, M.; Pekka, P.; Bo, N. Global Atlas on Cardiovascular Diseases Prevention and Control; World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization: Geneva, Switzerland, 2011; pp. 3–18. [Google Scholar]
- GBD. Mortality and Causes of Death Collaborators. 2013. Available online: https://doi.org/10.1016/S0140-6736(14)61682-2 (accessed on 17 December 2014).
- Katsi, V.; Didagelos, M.; Skevofilax, S.; Armenis, I.; Kartalis, A.; Vlachopoulos, C.; Karvounis, H.; Tousoulis, D. Gut microbiota—Gut dysbiosis—Arterial hypertension: New horizons. Curr. Hyperten. Rev. 2018, 12, 1–14. [Google Scholar]
- Pimenta, F.S.; Luaces-Regueira, M.; Ton, A.M.M.; Campagnaro, B.P.; Campos-Toimil, M.; Pereira, T.M.C.; Vasquez, E.C. Mechanisms of action of kefir in chronic cardiovascular and metabolic diseases. Cell. Physiol. Biochem. 2018, 48, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Herrmann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Weis, M. Impact of the gut microbiome in cardiovascular and autoimmune diseases. Clin. Sci. 2018, 132, 2387–2389. [Google Scholar] [CrossRef]
- O’Morain, V.L.; Ramji, D.P. The potential of probiotics in the prevention and treatment of atherosclerosis. Mol. Nutr. Food Res. 2020, 64, 1–15. [Google Scholar] [CrossRef]
- Deng, X.; Ma, J.; Song, M.; Jin, Y.; Ge, W.; Guo, C. Effects of products designed to modulate the gut microbiota on hyperlipidaemia. Eur. J. Nutr. 2018, 1, 1–15. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Pereira, T.M.C.; Peotta, V.A.; Baldo, M.P.; Campos-Toimil, M. Probiotics as beneficial dietary supplements to prevent and treat cardiovascular diseases: Uncovering their impact on oxidative stress. Oxid. Med. Cell. Longev. 2019, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2010, 52, 7577–7587. [Google Scholar] [CrossRef]
- Guildelines for the Evaluation of Probiotics in Food; Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food: London, ON, Canada, 2002.
- Metchnikoff, E. Sur la lute des cellules de l’organisme centre l’invasion des microbes. Ann. Inst. Pasteur. 1887, 1, 321. [Google Scholar]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williview, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Oberreuther-Moschner, D.L.; Jahreis, G.; Rechkemmer, G.; Pool-Zobel, B.L. Dietary intervention with the probiotics Lactobacillus acidophilus 145 and Bifidobacterium longum 913 modulates the potential of human faecal water to induce damage in HT29clone19A cells. Br. J. Nutr. 2004, 91, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, A.; Paliwoda, A.; Blasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanism and therapeutic perspectives. Crit. Rev. Food. Sci. Nutr. 2018, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.R.; Chauhan, P.; Dixit, K.; Babu, S.; Jamil, K. Probiotics as complementary therapy for hypercholesterolemia. Biol. Med. 2009, 1, 1–13. [Google Scholar]
- Bronzato, S.; Durante, A. Dietary supplements and cardiovascular diseases. Inter. J. Cardiovas. Dis. 2018, 9, 1–10. [Google Scholar]
- Gadelha, C.J.M.U.; Bezerra, A.N. Effects of probiotics on the lipid profile: Systematic review. J. Vasc. Bras. 2019, 9, 1–18. [Google Scholar]
- Mo, R.; Zhang, X.; Yang, Y. Effect of probiotics on lipid profiles in hypercholesterolaemic adults: A meta-analysis of randomized controlled trials. Med. Clin. 2018, 19, 1–12. [Google Scholar]
- Niamah, A.K.; Sahi, A.A.; Al-Sharifi, A.S.N. Effect of feeding soy milk fermented by probiotic bacteria on some blood criteria and weight of experimental animals. Prob. Antimicrob. Proteins 2017, 9, 284–291. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in med with stable coronary artery disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Lew, L.C.; Choi, S.B.; Khoo, B.Y.; Sreenivasan, S.; Ong, K.L.; Liong, M.T. Lactobacillus plantarum DR7 reduces cholesterol via phosphorylation of AMPK that down-regulated the mRNA expression of HMG-CoA reductase. Kor. J. Food Sci. Animal Res. 2018, 38, 350–361. [Google Scholar]
- Nguyen, T.D.T.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Inter. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, B.; Zhou, X.; Wang, Y.; Wang, H.; Jia, S.; Zhang, Z.; Chu, C.; Mu, J. Combined lowering effects of rosuvastatin and L. acidophilus on cholesterol levels in rat. J. Microbiol. Biotechnol. 2019, 29, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Pinto, R.; Pietropaoli, D.; Monaco, A.; Desideri, G.; Ferri, C.; Grassi, D. Non-pharmacological strategies against systemic inflammation: Molecular basis and clinical evidence. Curr. Pharm. Des. 2020, 3, 1–20. [Google Scholar]
- Lin, M.Y.; Chang, F.J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- El-Adawi, H.I.; Khalil, M.A.; El-Sheekh, M.M.; El-Deeb, N.M.; Hussein, M.Z. Cytotoxicity assay and antioxidant activities of the lactic acid bacterial strains. Afric. J. Microbiol. Res. 2012, 6, 1700–1712. [Google Scholar]
- Zhong, C.; Qu, C.; Wang, B.; Liang, B.S.; Zeng, B. Probiotics for preventing and treating intestinal bacterial overgrowth: A meta-analysis and systematic review of current evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
- Yadav, R.; Khan, S.H.; Mada, S.B.; Meena, S.; Kapila, R.; Kaila, S. Consumption of probiotic Lactobacillus fermentum MTCC: 5898-fermented milk attenuates dyslipidemia, oxidative stress, and inflammation in male rats fed on cholesterol-enriched diet. Probiotics Antimicro. Protein 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Tenorio-Jimenez, C.; Martinez-Rmirez, M.J.; Tercero-Lozano, M.; Arraiza-Irigoyen, C.; Del Castillo-Codes, I.; Olza, J.; Plaza-Diaz, J.; Fontana, L.; Migueles, J.H.; Olivares, M.; et al. Evaluation of the effect of Lactobacillus reuteri V3401 on biomarkers of inflammation, cardiovascular risk and liver steatosis in obese adults with metabolic syndrome: A randomized clinical trial (PROSIR). BMC Compl. Alter. Med. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Szulinska, M.; Loniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdanska, P. Multispecies probiotic supplementation favorably affects vascular function and reduces arterial stiffness in obese postmenopausal women- A 12-week placebo-controlled and randomized clinical study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef] [Green Version]
- Agerholm-Larsen, L.; Raben, A.; Haulvik, N.; Hansen, A.S.; Manders, M.; Astrup, A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur. J. Clin. Nutr. 2000, 54, 288–297. [Google Scholar] [CrossRef]
- Mizushima, S.; Ohshige, K.; Watanabe, J.; Kimura, M.; Kodowski, T.; Nakamurs, Y.; Tochikubo, O.; Ueshima, H. Randomized controlled trial of sour milk on blood pressure in bordeline hypertensive men. Am. J. Hypertens 2004, 17, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Aoyagi, Y.; Park, S.; Matsubara, S.; Honda, Y.; Amamoto, R.; Kushiro, A.; Miyazaki, K.; Shephard, R.J. Habitual intake of fermented milk products containing Lactobacillus casei strain Shirota and a reduced risk of hypertension in older people. Benef. Microbes 2017, 8, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Johansson, M.L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Microbiome 2017, 5, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T. Potential benefits of garlic and other dietary supplements for the management of hypertension. Review. Exp. Ther. Med. 2020, 19, 1479–1484. [Google Scholar]
- Olas, B. Dietary supplements with antiplatelet activity: A solution for everyone? Adv. Nutr. 2018, 9, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olas, B. Anti-aggregatory potential of selected vegetables—Promising dietary components for the prevention and treatment of cardiovascular disease. Adv. Nutr. 2019, 10, 280–290. [Google Scholar] [CrossRef]
- Schreiber, O.; Petersson, J.; Phillipson, M.; Perry, M.; Ross, S.; Holm, L. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G534–G542. [Google Scholar] [CrossRef] [Green Version]
- Haro, C.; Medina, M. Lactobacillus casei CRL 431 improves endothelial and platelet functionality in a pneumococcal infection model. Benef. Microbes 2019, 10, 533–541. [Google Scholar] [CrossRef]
- Zhou, J.S.; Rutherfurd, K.J.; Gill, H.S. Inability of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019 to induce human platelet aggregation I vitro. J. Food Protect. 2005, 68, 2459–2464. [Google Scholar] [CrossRef]
- Libby, P. History of discovery: Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Majewska, K.; Kregielska-Narożna, M.; Jakubowski, K.; Szulinska, M.; Bogdanski, P. The multispecies probiotic effectively reduces homocysteine concentration in obese women: A randomized double-blind placebo-controlled study. J. Clin. Med. 2020, 4, 1–11. [Google Scholar]
- Kuo, S.M. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv. Nutr. 2013, 4, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidaka, H.; Eida, T.; Takizawa, T.; Tokunage, T.; Tashiro, Y. Effects of fructooligosacharides on intestinal flora and human health. Bifidobact. Microflora 1986, 5, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, H.; Tashiro, Y.; Eida, T. Proliferation of bifidobacteria by oligosaccharides and their useful effects on human health. Bifidobact. Microflora 1991, 10, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.K.; Liao, J.W.; Chung, Y.C.; Hsieh, C.P.M.; Chan, Y.C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004, 134, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Cherry, P.; Vadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Probiotics from seaweeeds: An ocean of opportunity? Marine Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnell, J.A.; Reimer, R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: A dose-response study in JCR:LA-cp rats. Br. J. Nutr. 2010, 103, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.A.; Reamer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- Hume, M.P.; Nicolucci, A.C.; Reimer, R.A. Prebiotic supplementation improves appetite control in children with overweight and obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 790–799. [Google Scholar] [CrossRef] [Green Version]
- Nicolucci, A.C.; Hume, M.P.; Martinez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Sot-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Choi, H.N.; Yim, J.E. Effect of diet on the gut microbiota associated with obesity. J. Obesity Metab. Synd. 2019, 28, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffari, S.; Roshanravan, N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020, 128, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T. Probiotics: A critical review of their potential role as antihypertenstives, immune modulators, hypocholesterolemics, and perimenopausal treatments. Nutr. Rev. 2007, 65, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T.; Dunshea, F.R.; Shah, N.P. Effects of a symbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolemic pigs on high- and low-fat diets. Br. J. Nutr. 2007, 98, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighat, N.; Mohammadshahi, M.; Shayanpour, S.; Haghighizadeh, M.H. Effect of symbiotic and probiotic supplementation on serum levels of endothelial cell adhesion molecules in hemodialysis patients: A randomized control study. Probiotics Antimicro. Proteins 2018, 1, 1–9. [Google Scholar]
- Mofid, V.; Izadi, A.; Majtehedi, S.Y.; Khemoat, L. Therapeutic and nutritional effects of synbiotic yogurts in children and adults: A clinical review. Probiotics Antimicrob. Proteins 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Din, A.U.; Hassan, A.; Zhu, Y.; Yin, T.; Gregersen, H.; Wang, G. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl. Microbiol. Biotechnol. 2019, 103, 9217–9228. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramirez, M.J.; Milago, F.I.; Martinez, J.A.; Solas, M. Implication of trimethylamine N-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Wilson, T.W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Eng. J. Med. 2013, 368, 1575–1584. [Google Scholar]
- Moludi, J.; Alizadeh, M.; Yagin, N.L.; Pasdar, Y.; Nachvak, S.M.; Abdollahzad, H.; Tabaei, A.S. New insights on atherosclerosis: Endcananabinoid systems with gut microbiota. J. Cardiovasc. Thorac. Res. 2018, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S. New Research on Red Meat and Heart Disease; The Diane Rehm Show (Transcript); WAMU 88.5 American University Radio: Washington, DC, USA, 2013. [Google Scholar]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis 2006, 244, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-oxide: A carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Canad. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H. A gut feeling about thrombosis. N. Eng. J. Med. 2016, 374, 2494–2496. [Google Scholar] [CrossRef] [Green Version]
- Kanitsoraphan, C.; Rattanawong, P.; Charensri, S.; Vichai, S. Trimethylamine N-oxide and risk of cardiovascular disease and mortality. Curr. Nutr. Rep. 2018, 7, 2017–2213. [Google Scholar] [CrossRef] [PubMed]
- Hardin, S.J.; Homme, R.; George, A.K.; Tyagi, S.C. Diet-induced chronic syndrome, metabolically transformed trimethylamine-N-oxide, and the cardiovascular functions. Rev. Cardiovasc. Med. 2019, 20, 121–128. [Google Scholar] [PubMed]
- Leustean, A.M.; Ciocoiu, M.; Sava, A.; Costea, C.F.; Floria, M.; Tarniceriu, C.C.; Tanase, D.M. Implication of the intestinal microbiota in dignosisng the progression of diabetes and the presence of cardiovascular disease. J. Diab. Res. 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tripolt, N.J.; Leber, B.; Triebl, A.; Kofeler, H.; Stadlbauer, V.; Sourij, H. Effect of Lactobacillus casei Shirota supplementation on trimethylamine-N-oxide levels in patients with metabolic syndrome: An open-label, randomized study. Atherosclerosis 2015, 242, 141–144. [Google Scholar] [CrossRef]
- Boutagy, N.E.; Neilson, A.P.; Ostergerg, K.L.; Smithson, A.T.; England, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 2015, 23, 2357–2363. [Google Scholar] [CrossRef]
- Qui, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar]
- Tenore, G.C.; Caruso, D.; Buonamo, G.; D’Avino, M.; Ciampaglia, R.; Maisto, M.; Schsano, C.; Bochino, B.; Novellino, E. Lactofermented annurca apple puree as a functional food indicated for the control of plasma lipid an oxidative amine levels: Results from a randomized clinical trial. Nutrients 2019, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.C.; Yang, M.C.; Wu, Y.Y.; Chen, P.H.; Hsu, C.M.; Chen, L.W. Lactobacillus plantarum revers diabetes-induced Fmo3 and iCAM expression in mice through enteric dysbiosis-related c-Jun NH2-terminal kinase pathways. PLoS ONE 2018, 13, e0196511. [Google Scholar]
- Matsumoto, M.; Kitada, Y.; Shimomura, Y.; Naito, Y. Bifidobacterium animalis subsp. lactis LKM512 reduces levels of intestinal trimethylamine produced by intestinal microbiota in healthy volunteers: A double-blind, placebo-controlled study. J. Funct. Food 2017, 36, 94–101. [Google Scholar] [CrossRef]
- Bogiatzi, C.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Wong, R.G.; Urquhart, B.L.; Dinculescu, V.; Reutz, K.N.; Velenosi, T.J.; Pignanelli, M.; et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018, 273, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, C.; De Nicolo, A.; D’Ettorre, G.; D’Ascenzo, F.; Lazzaro, A.; Fettoni, M.; D’Avolio, A.; Bonora, S.; Celani, L.; Di Perri, G.; et al. Serum trimethylamine-N-oxide concentrations in people living with HIV and the effect of probiotic supplementation. Int. J. Antimicrob. Agents 2020, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vadivielso, J.M.; Rodriquez-Puyol, D.; Pascual, J.; Barrios, C.; Bermudez-Lopez, M.; Sanchez-Nino, M.D.; Perez-Fernandez, M.; Ortiz, A. Atherosclerosis in chronic kidney disease: More, less, or just different? Arterioscler. Thromb. Vasc. Biol. 2019, 10, 1938–1966. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: A review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.; Coombes, J.S.; Forbes, J.M.; McWhinney, B.C.; Ungerer, J.P.J.; Dimeski, G.; Campbell, K.L. SYNbiotics easing renal failure by improving gut microbiology (SYNERGY): A protocol of placebo-controlled randomized cross-over trial. BMC Neprol. 2014, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Hazen, S.L. Metaorganismal nutrient metabolism as a basis of cardiovascular disease. Curr. Opin. Lipidol. 2014, 25, 48–53. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.S.; Khalek, A.B.A.E.; Mohammed, S.E. Role of probiotic mixture with and without green tea extract in prevention of hepatorenal syndrome in rat model. Pak. J. Biol. Sci. 2019, 22, 21–27. [Google Scholar]
- Wang, H.; Mei, L.; Deng, Y.; Liu, Y.; Wei, X.; Liu, M.; Zhou, J.; Ma, H.; Zheng, P.; Yuan, J.; et al. Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition 2019, 62, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Tao, S.; Cheng, Y.; Liu, J.; Ma, L.; Fu, P. Effects of probiotic supplements on the progression of chronic kidney disease: A meta-analysis. Nephrology 2019, 24, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Taniguchi, A.; Tsubai, H.; Kano, H.; Asami, Y. Hypouricaemic effects of yoghurt containing Lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomized, double-blind, placebo-controlled study. Mod. Rhematol. 2019, 29, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arrayo, F.E.; Ganzaga, G.; Munoz-Jimenez, I.; Blas-Marron, M.G.; Silverio, O.; Tapia, E.; Soto, V.; Ranganathan, N.; Ranganathan, P.; Vyas, U.; et al. Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS ONE 2018, 24, e0202901. [Google Scholar]
- Firouzi, S.; Haghighatdoost, F. The effects of prebiotic, probiotic, and synbiotic supplementation on blood parameters of renal function: A systematic review and meta-analysis of clinical trials. Nutrition 2018, 51, 104–113. [Google Scholar] [CrossRef]
- Chi, C.; Li, C.; Wu, D.; Buys, N.; Wang, W.; Fan, H.; Sun, J. Effects of probiotics on patients with hypertension: A systematic review and meta-analysis. Curr. Hypertens Rep. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mraz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef]
| Probiotic/Prebiotic/Synbiotic Consumption | Dose (Per Day) | Days/Weeks | Subjects | Risk Factors of Cardiovascular Diseases | Ref. |
|---|---|---|---|---|---|
| Probiotic | |||||
| L. plantarum 299v | 20 billion cfu | 6 weeks | Patients with stable coronary artery disease (people) | Reducing inflammatory biomarkers. No effect on trimethylamine-N-oxide (TMAO) level | [21] |
| L. plantarum PH04 | 107 cfu | 14 days | Male mice fed a high-cholesterol diet containing 10% w/v skim milk and 10% w/v cream | Lowering total cholesterol and triglycerides | [23] |
| L. reuteri V3401 | 5 × 109 cfu | 12 weeks | Obese adults (people) with metabolic syndrome | Reducing inflammation | [30] |
| Multispecies probiotic Ecologic® Barrier | 2.5 × 109 cfu; 1 × 1010 cfu | 12 weeks | Obese postmenopausal Caucasian women | Decreasing systolic blood pressure and inflammation | [31] |
| Multispecies probiotic Ecologic® Barrier | 2.5 × 109 cfu | 12 weeks | Obese women | Decreasing homocysteine | [43] |
| L. acidophilus | 109 cfu/mL | 4 weeks | Hypercholesterolemic rats | Lowering total cholesterol and low-density lipoprotein (LDL) | [24] |
| L. fermentum MTCC:5898-fermented milk | 2 × 109 cfu | 90 days | Reducing dyslipidemia, oxidative stress and inflammation | [29] | |
| L. rhamnosus LRH11 and L. plantarum SGL07 | 3 × 108 cfu | 16 weeks | People with CVDs risks | Reducing plasma trimethylamine-N-oxide (TMAO) level | [75] |
| B. animalis subsp. lactis LKM512 | No described | 12 weeks | Healthy people | Reducing plasma trimethylamine-N-oxide (TMAO) level | [77] |
| L. casei Shirota | 6.5 × 109 cfu | 12 weeks | Subjects with metabolic syndrome | No effect on trimethylamine-N-oxide (TMAO) level | [72] |
| S. thermophilis, L. acidophilus LA-5 and B. bifidum BG-12 | No described (3 mL/day) | 4 weeks | Healthy animals | Lowering cholesterol and triglyceride | [20] |
| Prebiotic | |||||
| Prebiotic fiber | 10 or 20% | 10 weeks | Obese hyperlipidemic rats | Lowering total serum cholesterol and triacylglycerol | [50] |
| Oligofructose | 8 g/day | 16 weeks | Overweight and obese children aged 7–12 years | Decreasing body weight | [53] |
| Oligofructose | 21 g/day | 12 weeks | Overweight and obese adults | Decreasing body weight | [51] |
| Synbiotic | |||||
| Prebiotic (three different fiber types: 5 g fructooligosaccharides, 5 g galactooligosaccharides and 5 g inulin) and probiotic powder (Bioflora®) containing L. acidophilus strain T16, B. bifidum strain BIA-6, B. lactis strain BIA-6, and B. longum strain LAF-5 | 15 g of prebiotic and 5 g probiotic in sachet | 12 weeks | Hemodialysis patients | Reducing the concentration of intracellular adhesion molecule type 1 | [59] |
| Synbiotic containing L. acidophilus ATCC 4962, and three commercially available prebiotics (mannitol, fructooligosacharides and inulin) | L. acidophilus ATCC 4962 (1 g/pig per day), mannitol (1.56 g/pig per day), fructooligosacharides (1.25 g/pig per day) and inulin (2.2 g/pig per day) | 8 weeks | Hypercholesterolemic pigs | Reducing total cholesterol, triacylglycerol, low-density lipoprotein (LDL)-cholesterol | [57,58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B. Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. https://doi.org/10.3390/ijms21249737
Olas B. Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? International Journal of Molecular Sciences. 2020; 21(24):9737. https://doi.org/10.3390/ijms21249737
Chicago/Turabian StyleOlas, Beata. 2020. "Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases?" International Journal of Molecular Sciences 21, no. 24: 9737. https://doi.org/10.3390/ijms21249737
APA StyleOlas, B. (2020). Probiotics, Prebiotics and Synbiotics—A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? International Journal of Molecular Sciences, 21(24), 9737. https://doi.org/10.3390/ijms21249737





