Endurance Exercise Mitigates Immunometabolic Adipose Tissue Disturbances in Cancer and Obesity
Abstract
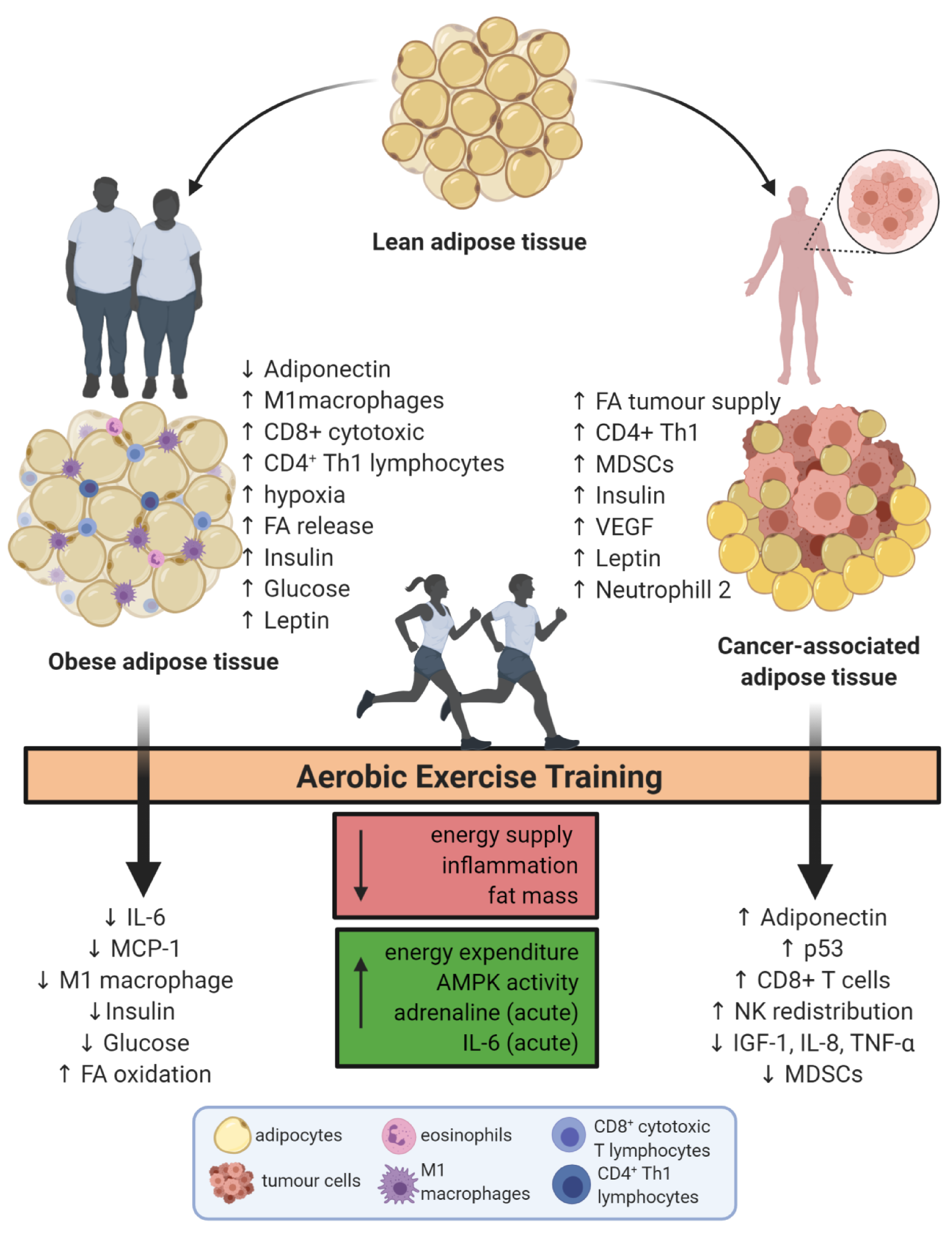
:1. Introduction
2. The Role of Aerobic Exercise Training in Adipose Tissue Immunometabolism
3. Obese Adipose Tissue Remodeling by Aerobic Exercise Training
4. Aerobic Exercise Training Causes Immunometabolic Adaptations That Mitigate the Disturbances Caused by Obesity
4.1. Findings in Rodent Models
4.2. Studies in Humans
5. Adipose Tissue and Cancer: Linked by Inflammation
6. Therapeutic Effects of Exercise in Cancer
6.1. Studies in Animals
6.2. Studies in Humans
7. Conclusions
Funding
Conflicts of Interest
References
- Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pond, C.M.; Mattacks, C.A. The anatomy of adipose tissue in captive Macaca monkeys and its implications for human biology. Folia Primatol. 1987, 48, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Schoettl, T.; Fischer, I.P.; Ussar, S. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 2018, 221, jeb162958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pond, C.M.; Mattacks, C.A. The effects of noradrenaline and insulin on lipolysis in adipocytes isolated from nine different adipose depots of guinea-pigs. Int. J. Obes. 1991, 15, 609–618. [Google Scholar] [PubMed]
- Khan, S.; Chan, Y.T.; Revelo, X.S.; Winer, D.A. The immune landscape of visceral adipose tissue during obesity and aging. Front. Endocrinol. 2020, 11, 267. [Google Scholar] [CrossRef]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Ma, X.; Su, K.; Qi, S.; Zhu, Y.; Lin, J.; Wang, C.; Yang, R.; Chen, X.; Wang, W.; et al. ChREBP-beta regulates thermogenesis in brown adipose tissue. J. Endocrinol. 2020, 245, 343–356. [Google Scholar] [CrossRef]
- Morroni, M.; Giordano, A.; Zingaretti, M.C.; Boiani, R.; De Matteis, R.; Kahn, B.B.; Nisoli, E.; Tonello, C.; Pisoschi, C.; Luchetti, M.M.; et al. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc. Natl. Acad. Sci. USA 2004, 101, 16801–16806. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, R.; Zingaretti, M.C.; Murano, I.; Vitali, A.; Frontini, A.; Giannulis, I.; Barbatelli, G.; Marcucci, F.; Bordicchia, M.; Sarzani, R.; et al. In vivo physiological transdifferentiation of adult adipose cells. Stem Cells 2009, 27, 2761–2768. [Google Scholar] [CrossRef]
- Cozzo, A.J.; Fuller, A.M.; Makowski, L. Contribution of adipose tissue to development of cancer. Compr. Physiol. 2017, 8, 237–282. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Gavaldà-Navarro, A.; Giralt, M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 2018, 27, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wu, H. T cells in adipose tissue: Critical players in immunometabolism. Front. Immunol. 2018, 9, 2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Grant, R.W.; Dixit, V.D. Adipose tissue as an immunological organ. Obesity 2015, 23, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Morgan-Bathke, M.E.; Jensen, M.D. Adipose tissue macrophage burden, systemic inflammation, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E254–E264. [Google Scholar] [CrossRef]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the aging immune system. Ageing Res. Rev. 2012, 11, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. The diseasome of physical inactivity-and the role of myokines in muscle-fat cross talk. J. Physiol. 2009, 587, 5559–5568. [Google Scholar] [CrossRef] [PubMed]
- Batatinha, H.A.P.; Biondo, L.A.; Lira, F.S.; Castell, L.M.; Rosa-Neto, J.C. Nutrients, immune system, and exercise: Where will it take us? Nutrition 2019, 61, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Lila, M.A.; Gillitt, N.D. Immunometabolism: A multi-omics approach to interpreting the influence of exercise and diet on the immune system. Annu. Rev. Food Sci. Technol. 2019, 10, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 221, jeb161570. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.I.; Middelbeek, R.J.; Goodyear, L.J. Exercise effects on white adipose tissue: Beiging and metabolic adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef] [Green Version]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Riis, S.; Christensen, B.; Nellemann, B.; Moller, A.B.; Husted, A.S.; Pedersen, S.B.; Schwartz, T.W.; Jorgensen, J.O.L.; Jessen, N. Molecular adaptations in human subcutaneous adipose tissue after ten weeks of endurance exercise training in healthy males. J. Appl. Physiol. 2019, 126, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Vosselman, M.J.; Hoeks, J.; Brans, B.; Pallubinsky, H.; Nascimento, E.B.; van der Lans, A.A.; Broeders, E.P.; Mottaghy, F.M.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. 2015, 39, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Oh-ishi, S.; Kizaki, T.; Toshinai, K.; Haga, S.; Fukuda, K.; Nagata, N.; Ohno, H. Swimming training improves brown-adipose-tissue activity in young and old mice. Mech. Ageing Dev. 1996, 89, 67–78. [Google Scholar] [CrossRef]
- Yoshioka, K.; Yoshida, T.; Wakabayashi, Y.; Nishioka, H.; Kondo, M. Effects of exercise training on brown adipose tissue thermogenesis in ovariectomized obese rats. Endocrinol. Jpn. 1989, 36, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.V.; Bikopoulos, G.; Hung, S.; Ceddia, R.B. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: Impact on whole-body energy expenditure. J. Biol. Chem. 2014, 289, 34129–34140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollisch, K.S.; Brandauer, J.; Jessen, N.; Toyoda, T.; Nayer, A.; Hirshman, M.F.; Goodyear, L.J. Effects of exercise training on subcutaneous and visceral adipose tissue in normal-and high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E495–E504. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Yamashita, A.S.; Koyama, C.H.; Batista, M.L., Jr.; Seelaender, M. Endurance training induces depot-specific changes in IL-10/TNF-alpha ratio in rat adipose tissue. Cytokine 2009, 45, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Castellani, L.; Root-Mccaig, J.; Frendo-Cumbo, S.; Beaudoin, M.S.; Wright, D.C. Exercise training protects against an acute inflammatory insult in mouse epididymal adipose tissue. J. Appl. Physiol. 2014, 116, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Silveira, L.S.; Batatinha, H.A.P.; Castoldi, A.; Camara, N.O.S.; Festuccia, W.T.; Souza, C.O.; Rosa Neto, J.C.; Lira, F.S. Exercise rescues the immune response fine-tuned impaired by peroxisome proliferator-activated receptors gamma deletion in macrophages. J. Cell Physiol. 2019, 234, 5241–5251. [Google Scholar] [CrossRef]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Tarini, V.A.; Arida, R.M.; Faloppa, F.; Alves, E.S.; do Nascimento, C.O.; Oyama, L.M.; Seelaender, M.; et al. Inflammation and adipose tissue: Effects of progressive load training in rats. Lipids Health Dis. 2010, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Dam, V.; Sikder, T.; Santosa, S. From neutrophils to macrophages: Differences in regional adipose tissue depots. Obes. Rev. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- Qiao, N.; Lin, Y.; Wang, Z.; Chen, J.Y.; Ge, Y.Y.; Yao, S.L.; Gong, J. Maresin1 promotes M2 macrophage polarization through peroxisome proliferator-activated receptor-gamma activation to expedite resolution of acute lung injury. J. Surg. Res. 2020, 256, 584–594. [Google Scholar] [CrossRef]
- Silveira, L.S.; Biondo, L.A.; de Souza Teixeira, A.A.; de Lima Junior, E.A.; Castoldi, A.; Camara, N.O.S.; Festuccia, W.T.; Rosa-Neto, J.C.; Lira, F.S. Macrophage immunophenotype but not anti-inflammatory profile is modulated by peroxisome proliferator-activated receptor gamma (PPARgamma) in exercised obese mice. Exerc. Immunol. Rev. 2020, 26, 10–22. [Google Scholar]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgibbons, T.P. Effect of weight gain on skeletal muscle and adipose tissue perfusion: Human fat goes with the flow. Arter. Thromb. Vasc. Biol. 2020, 40, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Gealekman, O.; Guseva, N.; Hartigan, C.; Apotheker, S.; Gorgoglione, M.; Gurav, K.; Tran, K.V.; Straubhaar, J.; Nicoloro, S.; Czech, M.P.; et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 2011, 123, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Sethi, J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Zorena, K.; Jachimowicz-Duda, O.; Slezak, D.; Robakowska, M.; Mrugacz, M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: A review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- May, F.J.; Baer, L.A.; Lehnig, A.C.; So, K.; Chen, E.Y.; Gao, F.; Narain, N.R.; Gushchina, L.; Rose, A.; Doseff, A.I.; et al. Lipidomic adaptations in white and brown adipose tissue in response to exercise demonstrate molecular species-specific remodeling. Cell Rep. 2017, 18, 1558–1572. [Google Scholar] [CrossRef]
- Garcia-Martinez, I.; Shaker, M.E.; Mehal, W.Z. Therapeutic opportunities in damage-associated molecular pattern-driven metabolic diseases. Antioxid. Redox Signal. 2015, 23, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Mardare, C.; Kruger, K.; Liebisch, G.; Seimetz, M.; Couturier, A.; Ringseis, R.; Wilhelm, J.; Weissmann, N.; Eder, K.; Mooren, F.C. Endurance and resistance training affect high fat diet-induced increase of ceramides, inflammasome expression, and systemic inflammation in mice. J. Diabetes Res. 2016, 2016, 4536470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, A.S.; Lira, F.S.; Rosa, J.C.; Paulino, E.C.; Brum, P.C.; Negrao, C.E.; dos Santos, R.V.; Batista, M.L., Jr.; do Nascimento, C.O.; Oyama, L.M.; et al. Depot-specific modulation of adipokine levels in rat adipose tissue by diet-induced obesity: The effect of aerobic training and energy restriction. Cytokine 2010, 52, 168–174. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Simpson, K.A.; Singh, M.A. Effects of exercise on adiponectin: A systematic review. Obesity 2008, 16, 241–256. [Google Scholar] [CrossRef]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Geng, L.; Liao, B.; Jin, L.; Huang, Z.; Triggle, C.R.; Ding, H.; Zhang, J.; Huang, Y.; Lin, Z.; Xu, A. Exercise alleviates obesity-induced metabolic dysfunction via enhancing FGF21 sensitivity in adipose tissues. Cell Rep. 2019, 26, 2738–2752.e4. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Zhou, F.; Chen, J.; Han, Q.; Huang, M.; Tan, X.; Liu, Q.; et al. Exercise ameliorates the FGF21-adiponectin axis impairment in diet-induced obese mice. Endocr. Connect. 2019, 8, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta 2014, 1842, 446–462. [Google Scholar] [CrossRef] [Green Version]
- Hassnain Waqas, S.F.; Noble, A.; Hoang, A.C.; Ampem, G.; Popp, M.; Strauss, S.; Guille, M.; Roszer, T. Adipose tissue macrophages develop from bone marrow-independent progenitors in Xenopus laevis and mouse. J. Leukoc. Biol. 2017, 102, 845–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, S.; Merlin, J.; Lee, M.K.S.; Murphy, A.J.; Guinamard, R.R. Biology and function of adipose tissue macrophages, dendritic cells and B cells. Atherosclerosis 2018, 271, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wouters, K.; Gaens, K.; Bijnen, M.; Verboven, K.; Jocken, J.; Wetzels, S.; Wijnands, E.; Hansen, D.; van Greevenbroek, M.; Duijvestijn, A.; et al. Circulating classical monocytes are associated with CD11c(+) macrophages in human visceral adipose tissue. Sci. Rep. 2017, 7, 42665. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010, 16, 105–118. [Google Scholar] [PubMed]
- Caslin, H.L.; Bhanot, M.; Bolus, W.R.; Hasty, A.H. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol. Rev. 2020, 295, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Red Eagle, A.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013, 45, 1684–1693. [Google Scholar] [CrossRef]
- Kawanishi, N.; Niihara, H.; Mizokami, T.; Yada, K.; Suzuki, K. Exercise training attenuates neutrophil infiltration and elastase expression in adipose tissue of high-fat-diet-induced obese mice. Physiol. Rep. 2015, 3, e12534. [Google Scholar] [CrossRef]
- Lee, S.; Norheim, F.; Langleite, T.M.; Gulseth, H.L.; Birkeland, K.I.; Drevon, C.A. Effects of long-term exercise on plasma adipokine levels and inflammation-related gene expression in subcutaneous adipose tissue in sedentary dysglycaemic, overweight men and sedentary normoglycaemic men of healthy weight. Diabetologia 2019, 62, 1048–1064. [Google Scholar] [CrossRef] [Green Version]
- Nono Nankam, P.A.; Mendham, A.E.; De Smidt, M.F.; Keswell, D.; Olsson, T.; Bluher, M.; Goedecke, J.H. Changes in systemic and subcutaneous adipose tissue inflammation and oxidative stress in response to exercise training in obese black African women. J. Physiol. 2020, 598, 503–515. [Google Scholar] [CrossRef]
- Nono Nankam, P.A.; Bluher, M.; Kehr, S.; Kloting, N.; Krohn, K.; Adams, K.; Stadler, P.F.; Mendham, A.E.; Goedecke, J.H. Distinct abdominal and gluteal adipose tissue transcriptome signatures are altered by exercise training in African women with obesity. Sci. Rep. 2020, 10, 10240. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Exercise metabolism: Fuels for the fire. Cold Spring Harb. Perspect. Med. 2018, 8, a029744. [Google Scholar] [CrossRef] [PubMed]
- Verboven, K.; Stinkens, R.; Hansen, D.; Wens, I.; Frederix, I.; Eijnde, B.O.; Jocken, J.W.E.; Goossens, G.H.; Blaak, E.E. Adrenergically and non-adrenergically mediated human adipose tissue lipolysis during acute exercise and exercise training. Clin. Sci. 2018, 132, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Yin, Y.L.; Guo, W.Z.; Ma, Y.Q.; Wang, Y.B.; Shu, C.; Dong, L.Q. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Hiscock, N.; Sacchetti, M.; Fischer, C.P.; Pedersen, B.K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 2004, 53, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Petersen, E.W.; Carey, A.L.; Sacchetti, M.; Steinberg, G.R.; Macaulay, S.L.; Febbraio, M.A.; Pedersen, B.K. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E155–E162. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Zacho, M.; Asp, S.; Pedersen, B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998, 508, 949–953. [Google Scholar] [CrossRef]
- Christensen, R.H.; Lehrskov, L.L.; Wedell-Neergaard, A.S.; Legaard, G.E.; Ried-Larsen, M.; Karstoft, K.; Krogh-Madsen, R.; Pedersen, B.K.; Ellingsgaard, H.; Rosenmeier, J.B. Aerobic exercise induces cardiac fat loss and alters cardiac muscle mass through an interleukin-6 receptor-dependent mechanism: Cardiac analysis of a double-blind randomized controlled clinical trial in abdominally obese humans. Circulation 2019, 140, 1684–1686. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: A randomized controlled trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef]
- Rao, S.; Pandey, A.; Garg, S.; Park, B.; Mayo, H.; Despres, J.P.; Kumbhani, D.; de Lemos, J.A.; Neeland, I.J. Effect of exercise and pharmacological interventions on visceral adiposity: A systematic review and meta-analysis of long-term randomized controlled trials. Mayo Clin. Proc. 2019, 94, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallone, F.; Deudon, R.; Muller, C.; Vaysse, C. Breast cancer, obesity and adipose tissue: A high-risk combination. Med. Sci. 2018, 34, 1079–1086. [Google Scholar] [CrossRef]
- Fairfield, K.M.; Willett, W.C.; Rosner, B.A.; Manson, J.E.; Speizer, F.E.; Hankinson, S.E. Obesity, weight gain, and ovarian cancer. Obs. Gynecol. 2002, 100, 288–296. [Google Scholar] [CrossRef]
- Berstein, L.M. Insulinemia, heterogeneity of obesity and the risk of different types of endometrial cancer: Existing evidence. Expert Rev. Endocrinol. Metab. 2016, 11, 51–64. [Google Scholar] [CrossRef]
- Ribeiro, R.; Monteiro, C.; Catalan, V.; Hu, P.; Cunha, V.; Rodriguez, A.; Gomez-Ambrosi, J.; Fraga, A.; Principe, P.; Lobato, C.; et al. Obesity and prostate cancer: Gene expression signature of human periprostatic adipose tissue. BMC Med. 2012, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Useros, J.; Garcia-Foncillas, J. Obesity and colorectal cancer: Molecular features of adipose tissue. J. Transl. Med. 2016, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Murphy, N.; Jenab, M.; Gunter, M.J. Adiposity and gastrointestinal cancers: Epidemiology, mechanisms and future directions. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 659–670. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Graziano, M.; Sciacca, L.; Baratta, R.; Frittitta, L. Adipose tissue, obesity and adiponectin: Role in endocrine cancer risk. Int. J. Mol. Sci. 2019, 20, 2863. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Samdal Steinskog, E.S.; Wiig, H. Adipose tissue macrophages: The inflammatory link between obesity and cancer? Expert Opin. Targets 2015, 19, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Locasale, J.W.; Swanson, K.D.; Sharfi, H.; Heffron, G.J.; Amador-Noguez, D.; Christofk, H.R.; Wagner, G.; Rabinowitz, J.D.; Asara, J.M.; et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 2010, 329, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Butler, L.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Ching, M.M.; Reader, J.; Fulton, A.M. Eicosanoids in Cancer: Prostaglandin E2 receptor 4 in cancer therapeutics and immunotherapy. Front. Pharm. 2020, 11, 819. [Google Scholar] [CrossRef]
- Hidalgo-Estevez, A.M.; Stamatakis, K.; Jimenez-Martinez, M.; Lopez-Perez, R.; Fresno, M. Cyclooxygenase 2-regulated genes an alternative avenue to the development of new therapeutic drugs for colorectal cancer. Front. Pharm. 2020, 11, 533. [Google Scholar] [CrossRef]
- Nour Eldin, E.E.M.; Nour Eldein, M.M.; El-Readi, M.Z.; Mirza, A.A.; Fatani, S.H.; Al-Amodi, H.S.; Althubiti, M.A.; Al-Ezzi, E.M.; Eid, S.Y.; Kamel, H.F.M. Evaluation of the diagnostic and predicative values of 8-Iso-prostaglandin F2alpha as a biomarker of breast cancer. Oncol. Res. Treat. 2020, 43, 1–9. [Google Scholar] [CrossRef]
- Yin, J.; Kim, S.S.; Choi, E.; Oh, Y.T.; Lin, W.; Kim, T.H.; Sa, J.K.; Hong, J.H.; Park, S.H.; Kwon, H.J.; et al. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat. Commun. 2020, 11, 2978. [Google Scholar] [CrossRef]
- Yoshitake, R.; Saeki, K.; Eto, S.; Shinada, M.; Nakano, R.; Sugiya, H.; Endo, Y.; Fujita, N.; Nishimura, R.; Nakagawa, T. Aberrant expression of the COX2/PGE2 axis is induced by activation of the RAF/MEK/ERK pathway in BRAF(V595E) canine urothelial carcinoma. Sci Rep. 2020, 10, 7826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhu, N.; Zhang, C.J.; Wang, Y.K.; Wu, H.T.; Li, Q.; Du, K.; Liao, D.F.; Qin, L. Friend or foe: Multiple roles of adipose tissue in cancer formation and progression. J. Cell Physiol. 2019, 234, 21436–21449. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogure, A.; Naito, Y.; Yamamoto, Y.; Yashiro, M.; Kiyono, T.; Yanagihara, K.; Hirakawa, K.; Ochiya, T. Cancer cells with high-metastatic potential promote a glycolytic shift in activated fibroblasts. PLoS ONE 2020, 15, e0234613. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, M.; Roychoudhuri, R.; Restifo, N.P. Nutrient competition: A new axis of tumor immunosuppression. Cell 2015, 162, 1206–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea-Maier, R.T.; Smit, J.W.A.; Netea, M.G. Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Lett. 2018, 413, 102–109. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Sun, X.; Ingman, W.V. Cytokine networks that mediate epithelial cell-macrophage crosstalk in the mammary gland: Implications for development and cancer. J. Mammary Gland Biol. Neoplasia 2014, 19, 191–201. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Atoum, M.F.; Alzoughool, F.; Al-Hourani, H. Linkage between obesity leptin and breast cancer. Breast Cancer 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Jequier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef]
- Bjorbak, C.; Lavery, H.J.; Bates, S.H.; Olson, R.K.; Davis, S.M.; Flier, J.S.; Myers, M.G., Jr. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000, 275, 40649–40657. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef] [Green Version]
- Bain, G.H.; Collie-Duguid, E.; Murray, G.I.; Gilbert, F.J.; Denison, A.; McKiddie, F.; Ahearn, T.; Fleming, I.; Leeds, J.; Phull, P.; et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br. J. Cancer 2014, 110, 1525–1534. [Google Scholar] [CrossRef]
- Niu, J.; Jiang, L.; Guo, W.; Shao, L.; Liu, Y.; Wang, L. The association between leptin level and breast cancer: A meta-analysis. PLoS ONE 2013, 8, e67349. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, Y.; Funahashi, T.; Tanaka, S.; Taguchi, T.; Tamaki, Y.; Shimomura, I.; Noguchi, S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer 2006, 118, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Artac, M.; Bozcuk, H.; Kiyici, A.; Eren, O.O.; Boruban, M.C.; Ozdogan, M. Serum leptin level and waist-to-hip ratio (WHR) predict the overall survival of metastatic breast cancer (MBC) patients treated with aromatase inhibitors (AIs). Breast Cancer 2013, 20, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Phillips, F.C.; Getzin, S.C.; Jacobson, T.L.; Jacobson, M.K.; Christensen, T.A.; Juneja, S.C.; Grande, J.P.; Maihle, N.J. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res. Treat. 2003, 77, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Juneja, S.C.; Phillips, F.C.; Hu, X.; Grande, J.P.; Maihle, N.J. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp. Biol. Med. 2004, 229, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Balsan, G.A.; Vieira, J.L.; Oliveira, A.M.; Portal, V.L. Relationship between adiponectin, obesity and insulin resistance. Rev. Assoc. Med. Bras. 2015, 61, 72–80. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Arditi, J.D.; Venihaki, M.; Karalis, K.P.; Chrousos, G.P. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: A potential hormonal link between obesity and cancer. Horm. Metab. Res. 2007, 39, 9–13. [Google Scholar] [CrossRef]
- Katira, A.; Tan, P.H. Adiponectin and its receptor signaling: An anti-cancer therapeutic target and its implications for anti-tumor immunity. Expert Opin. Targets 2015, 19, 1105–1125. [Google Scholar] [CrossRef]
- Grisouard, J.; Dembinski, K.; Mayer, D.; Keller, U.; Muller, B.; Christ-Crain, M. Targeting AMP-activated protein kinase in adipocytes to modulate obesity-related adipokine production associated with insulin resistance and breast cancer cell proliferation. Diabetol. Metab. Syndr. 2011, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Nepal, S.; Kim, M.J.; Chang, J.H.; Kim, S.H.; Jeong, G.S.; Jeong, C.H.; Park, G.H.; Jung, S.; Lim, J.; et al. Critical role of AMPK/FoxO3A axis in globular adiponectin-induced cell cycle arrest and apoptosis in cancer cells. J. Cell Physiol. 2016, 231, 357–369. [Google Scholar] [CrossRef]
- Mauro, L.; Naimo, G.D.; Gelsomino, L.; Malivindi, R.; Bruno, L.; Pellegrino, M.; Tarallo, R.; Memoli, D.; Weisz, A.; Panno, M.L.; et al. Uncoupling effects of estrogen receptor alpha on LKB1/AMPK interaction upon adiponectin exposure in breast cancer. FASEB J. 2018, 32, 4343–4355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naimo, G.D.; Gelsomino, L.; Catalano, S.; Mauro, L.; Ando, S. Interfering role of ERalpha on adiponectin action in breast cancer. Front. Endocrinol. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam. Repub. Iran 2019, 33, 56. [Google Scholar] [CrossRef] [PubMed]
- Surmacz, E. Function of the IGF-I receptor in breast cancer. J. Mammary Gland Biol. Neoplasia 2000, 5, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.H.; Heyn, G.S.; Magalhaes, K.G. The Impact of the adipose organ plasticity on inflammation and cancer progression. Cells 2019, 8, 662. [Google Scholar] [CrossRef] [Green Version]
- Apostoli, A.J.; Skelhorne-Gross, G.E.; Rubino, R.E.; Peterson, N.T.; Di Lena, M.A.; Schneider, M.M.; SenGupta, S.K.; Nicol, C.J. Loss of PPARgamma expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment. Int. J. Cancer 2014, 134, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Boughanem, H.; Cabrera-Mulero, A.; Hernandez-Alonso, P.; Bandera-Merchan, B.; Tinahones, A.; Tinahones, F.J.; Morcillo, S.; Macias-Gonzalez, M. The expression/methylation profile of adipogenic and inflammatory transcription factors in adipose tissue are linked to obesity-related colorectal cancer. Cancers 2019, 11, 1629. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.; Bos, S.A.; Gill, C.M.; Torriani, M.; Bredella, M.A. Brown adipose tissue and cancer progression. Skelet. Radiol. 2020, 49, 635–639. [Google Scholar] [CrossRef]
- Beluzi, M.; Peres, S.B.; Henriques, F.S.; Sertie, R.A.; Franco, F.O.; Santos, K.B.; Knobl, P.; Andreotti, S.; Shida, C.S.; Neves, R.X.; et al. Pioglitazone treatment increases survival and prevents body weight loss in tumor-bearing animals: Possible anti-cachectic effect. PLoS ONE 2015, 10, e0122660. [Google Scholar] [CrossRef] [Green Version]
- Lira, F.S.; Neto, J.C.; Seelaender, M. Exercise training as treatment in cancer cachexia. Appl. Physiol. Nutr. Metab. 2014, 39, 679–686. [Google Scholar] [CrossRef]
- Teixeira, A.A.; Lira, F.S.; Pimentel, G.D.; Oliveira de Souza, C.; Batatinha, H.; Biondo, L.A.; Yamashita, A.S.; Junior, E.A.; Neto, J.C. Aerobic exercise modulates the free fatty acids and inflammatory response during obesity and cancer cachexia. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Echavez, J.F.; Gonzalez-Jimenez, E.; Ramirez-Velez, R. Supervised exercise reduces cancer-related fatigue: A systematic review. J. Physiother. 2015, 61, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramanandam, V.S.; Dunn, V. Exercise for the management of cancer-related fatigue in lung cancer: A systematic review. Eur. J. Cancer Care 2015, 24, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.M. Can exercise reduce the risk of cancer? Phys. Sportsmed. 1986, 14, 170–178. [Google Scholar] [CrossRef]
- Winningham, M.L.; MacVicar, M.G.; Burke, C.A. Exercise for cancer patients: Guidelines and precautions. Phys. Sportsmed. 1986, 14, 125–134. [Google Scholar] [CrossRef]
- Dimeo, F.; Rumberger, B.G.; Keul, J. Aerobic exercise as therapy for cancer fatigue. Med. Sci. Sports Exerc. 1998, 30, 475–478. [Google Scholar] [CrossRef]
- Courneya, K.S.; Friedenreich, C.M. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann. Behav. Med. 1999, 21, 171–179. [Google Scholar] [CrossRef]
- Daneryd, P.L.; Hafstrom, L.R.; Karlberg, I.H. Effects of spontaneous physical exercise on experimental cancer anorexia and cachexia. Eur. J. Cancer 1990, 26, 1083–1088. [Google Scholar] [CrossRef]
- Cavalheri, V.; Granger, C.L. Exercise training as part of lung cancer therapy. Respirology 2020. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Warner, A.B.; Jones, L.W.; Dewhirst, M.W. Exercise as adjunct therapy in cancer. Semin. Radiat. Oncol. 2019, 29, 16–24. [Google Scholar] [CrossRef]
- Campbell, K.L.; Zadravec, K.; Bland, K.A.; Chesley, E.; Wolf, F.; Janelsins, M.C. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: Systematic review of randomized controlled trials. Phys. Ther. 2020, 100, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvao, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.E.; Mirza, N.N.; Lustgarten, J. Immunity, cancer and aging: Lessons from mouse models. Aging Dis. 2011, 2, 512–523. [Google Scholar] [PubMed]
- Nilsson, M.I.; Bourgeois, J.M.; Nederveen, J.P.; Leite, M.R.; Hettinga, B.P.; Bujak, A.L.; May, L.; Lin, E.; Crozier, M.; Rusiecki, D.R.; et al. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS ONE 2019, 14, e0210863. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; Endicott, E.; Ladiges, W.C. Pre-tumor exercise decreases breast cancer in old mice in a distance-dependent manner. Am. J. Cancer Res. 2014, 4, 378–384. [Google Scholar] [PubMed]
- Kim, M.K.; Kim, Y.; Park, S.; Kim, E.; Kim, J.H. Effects of steady low-intensity exercise on high-fat diet stimulated breast cancer progression via the alteration of macrophage polarization. Integr. Cancer 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Murphy, E.A.; Davis, J.M.; Barrilleaux, T.L.; McClellan, J.L.; Steiner, J.L.; Carmichael, M.D.; Pena, M.M.; Hebert, J.R.; Green, J.E. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine 2011, 55, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; King, B.; Ewert, E.; Su, X.; Mardiyati, N.; Zhao, Z.; Wang, W. Exercise activates p53 and negatively regulates IGF-1 pathway in epidermis within a skin cancer model. PLoS ONE 2016, 11, e0160939. [Google Scholar] [CrossRef] [Green Version]
- Theriau, C.F.; Shpilberg, Y.; Riddell, M.C.; Connor, M.K. Voluntary physical activity abolishes the proliferative tumor growth microenvironment created by adipose tissue in animals fed a high fat diet. J. Appl. Physiol. 2016, 121, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Emmons, R.; Xu, G.; Hernandez-Saavedra, D.; Kriska, A.; Pan, Y.X.; Chen, H.; De Lisio, M. Effects of obesity and exercise on colon cancer induction and hematopoiesis in mice. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E210–E220. [Google Scholar] [CrossRef]
- Garritson, J.; Krynski, L.; Haverbeck, L.; Haughian, J.M.; Pullen, N.A.; Hayward, R. Physical activity delays accumulation of immunosuppressive myeloid-derived suppressor cells. PLoS ONE 2020, 15, e0234548. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, W.R.; Bernards, N.T.; de Rooij, M.H.; Koppeschaar, H.P. Dynamic exercise discloses different time-related responses in stress hormones. Psychosom. Med. 2000, 62, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Schjerling, P.; Pedersen, B.K. Physical activity and plasma interleukin-6 in humans–Effect of intensity of exercise. Eur. J. Appl. Physiol. 2000, 83, 512–515. [Google Scholar] [CrossRef]
- Shalamzari, S.A.; Agha-Alinejad, H.; Alizadeh, S.; Shahbazi, S.; Khatib, Z.K.; Kazemi, A.; Saei, M.A.; Minayi, N. The effect of exercise training on the level of tissue IL-6 and vascular endothelial growth factor in breast cancer bearing mice. Iran. J. Basic Med. Sci. 2014, 17, 231–258. [Google Scholar]
- Lu, C.C.; Kuo, H.C.; Wang, F.S.; Jou, M.H.; Lee, K.C.; Chuang, J.H. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int. J. Mol. Sci. 2014, 16, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Carson, J.A.; Baltgalvis, K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc. Sport Sci. Rev. 2010, 38, 168–176. [Google Scholar] [CrossRef]
- Lee, B.; Chung, W. Effects of aerobic exercise on cytokine expression in a breast cancer mouse model. Iran. J. Public Health 2020, 49, 14–20. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Davis, M.K. Exercise prevention of cardiovascular disease in breast cancer survivors. J. Oncol. 2015, 2015, 917606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedenreich, C.M.; Cust, A.E. Physical activity and breast cancer risk: Impact of timing, type and dose of activity and population subgroup effects. Br. J. Sports Med. 2008, 42, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Kenfield, S.A.; Jimenez, A. Exercise-induced biochemical changes and their potential influence on cancer: A scientific review. Br. J. Sports Med. 2017, 51, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Augsten, M.; Stromberg, A.; Rullman, E.; Mijwel, S.; Kharaziha, P.; Panaretakis, T.; Gustafsson, T.; Ostman, A. Effect of acute exercise on prostate cancer cell growth. PLoS ONE 2013, 8, e67579. [Google Scholar] [CrossRef]
- Fairey, A.S.; Courneya, K.S.; Field, C.J.; Bell, G.J.; Jones, L.W.; Mackey, J.R. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: A randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2003, 12, 721–727. [Google Scholar]
- Cao, Y.; Nimptsch, K.; Shui, I.M.; Platz, E.A.; Wu, K.; Pollak, M.N.; Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.L. Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer. Int. J. Cancer 2015, 136, 2418–2426. [Google Scholar] [CrossRef] [Green Version]
- Hensley, P.J.; Cao, Z.; Pu, H.; Dicken, H.; He, D.; Zhou, Z.; Wang, C.; Koochekpour, S.; Kyprianou, N. Predictive and targeting value of IGFBP-3 in therapeutically resistant prostate cancer. Am. J. Clin. Exp. Urol. 2019, 7, 188–202. [Google Scholar]
- Hou, Y.L.; Luo, P.; Ji, G.Y.; Chen, H. Clinical significance of serum IGFBP-3 in colorectal cancer. J. Clin. Lab. Anal. 2019, 33, e22912. [Google Scholar] [CrossRef]
- Song, G.; Liu, K.; Zhu, X.; Yang, X.; Shen, Y.; Wang, W.; Shi, G.; Li, Q.; Duan, Y.; Zhao, Y.; et al. The low IGFBP-3 level is associated with esophageal cancer patients: A meta-analysis. World J. Surg. Oncol. 2016, 14, 307. [Google Scholar] [CrossRef] [Green Version]
- Rohrmann, S.; Linseisen, J.; Becker, S.; Allen, N.; Schlehofer, B.; Overvad, K.; Olsen, A.; Tjonneland, A.; Melin, B.S.; Lund, E.; et al. Concentrations of IGF-I and IGFBP-3 and brain tumor risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, L. AMPK as a metabolic intersection between diet and physical exercise. Yakugaku Zasshi 2018, 138, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Biondo, L.A.; Silveira, L.S.; Teixeira, A.A.d.S.; Neto, J.C.R. White adipose tissue and cancer: Impacts of doxorubicin and potential co-therapies. Immunometabolism 2020, 2, e200030. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Parmentier, J.H.; Sami, N.; Lee, K.; Spicer, D.; Mack, W.J.; Sattler, F.; Mittelman, S.D. Adipose tissue inflammation in breast cancer survivors: Effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res. Treat. 2018, 168, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Fairey, A.S.; Courneya, K.S.; Field, C.J.; Bell, G.J.; Jones, L.W.; Martin, B.S.; Mackey, J.R. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: A randomized controlled trial. Brain Behav. Immun. 2005, 19, 381–388. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Winters-Stone, K. Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Phys. Sportsmed. 2009, 37, 62–67. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.; Chen, W.Y. Clinical implications of low skeletal muscle mass in early-stage breast and colorectal cancer. Proc. Nutr. Soc. 2018, 77, 382–387. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A clinical practice guideline. Curr. Oncol 2017, 24, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Kontos, D.; Schnall, M.D.; Wu, S.; Schmitz, K.H. The dose-response effects of aerobic exercise on body composition and breast tissue among women at high risk for breast cancer: A randomized trial. Cancer Prev. Res. 2016, 9, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Sturgeon, K.; Digiovanni, L.; Good, J.; Salvatore, D.; Fenderson, D.; Domchek, S.; Stopfer, J.; Galantino, M.L.; Bryan, C.; Hwang, W.T.; et al. Exercise-Induced dose-response alterations in adiponectin and leptin levels are dependent on body fat changes in women at risk for breast cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, K.H.; Williams, N.I.; Kontos, D.; Domchek, S.; Morales, K.H.; Hwang, W.T.; Grant, L.L.; DiGiovanni, L.; Salvatore, D.; Fenderson, D.; et al. Dose-response effects of aerobic exercise on estrogen among women at high risk for breast cancer: A randomized controlled trial. Breast Cancer Res. Treat. 2015, 154, 309–318. [Google Scholar] [CrossRef]
- Haley, J.S.; Hibler, E.A.; Zhou, S.; Schmitz, K.H.; Sturgeon, K.M. Dose-dependent effect of aerobic exercise on inflammatory biomarkers in a randomized controlled trial of women at high risk of breast cancer. Cancer 2020, 126, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Rickels, M.R.; Troxel, A.B.; Zemel, B.S.; Damjanov, N.; Ky, B.; Rhim, A.D.; Rustgi, A.K.; Courneya, K.S.; Schmitz, K.H. Dose-response effects of exercise on insulin among colon cancer survivors. Endocr. Relat. Cancer 2018, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ruan, Y.; Gao, X.; Sun, J. Systematic review and meta-analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Horm. Metab. Res. 2017, 49, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuri, R.; Moghaddasi, M.; Darvishi, H.; Izadpanah, A. Effect of aerobic exercise on leptin and ghrelin in patients with colorectal cancer. J. Cancer Res. 2016, 12, 169–174. [Google Scholar] [CrossRef]
- Swisher, A.K.; Abraham, J.; Bonner, D.; Gilleland, D.; Hobbs, G.; Kurian, S.; Yanosik, M.A.; Vona-Davis, L. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: Effects on body fat, physical function, quality of life, and adipokine profile. Support. Care Cancer 2015, 23, 2995–3003. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa-Neto, J.C.; Silveira, L.S. Endurance Exercise Mitigates Immunometabolic Adipose Tissue Disturbances in Cancer and Obesity. Int. J. Mol. Sci. 2020, 21, 9745. https://doi.org/10.3390/ijms21249745
Rosa-Neto JC, Silveira LS. Endurance Exercise Mitigates Immunometabolic Adipose Tissue Disturbances in Cancer and Obesity. International Journal of Molecular Sciences. 2020; 21(24):9745. https://doi.org/10.3390/ijms21249745
Chicago/Turabian StyleRosa-Neto, José Cesar, and Loreana Sanches Silveira. 2020. "Endurance Exercise Mitigates Immunometabolic Adipose Tissue Disturbances in Cancer and Obesity" International Journal of Molecular Sciences 21, no. 24: 9745. https://doi.org/10.3390/ijms21249745
APA StyleRosa-Neto, J. C., & Silveira, L. S. (2020). Endurance Exercise Mitigates Immunometabolic Adipose Tissue Disturbances in Cancer and Obesity. International Journal of Molecular Sciences, 21(24), 9745. https://doi.org/10.3390/ijms21249745





