Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects
Abstract
:1. Introduction
2. Results
2.1. CB1R and CB2R Expression in Liver, Cortex, and Spleen
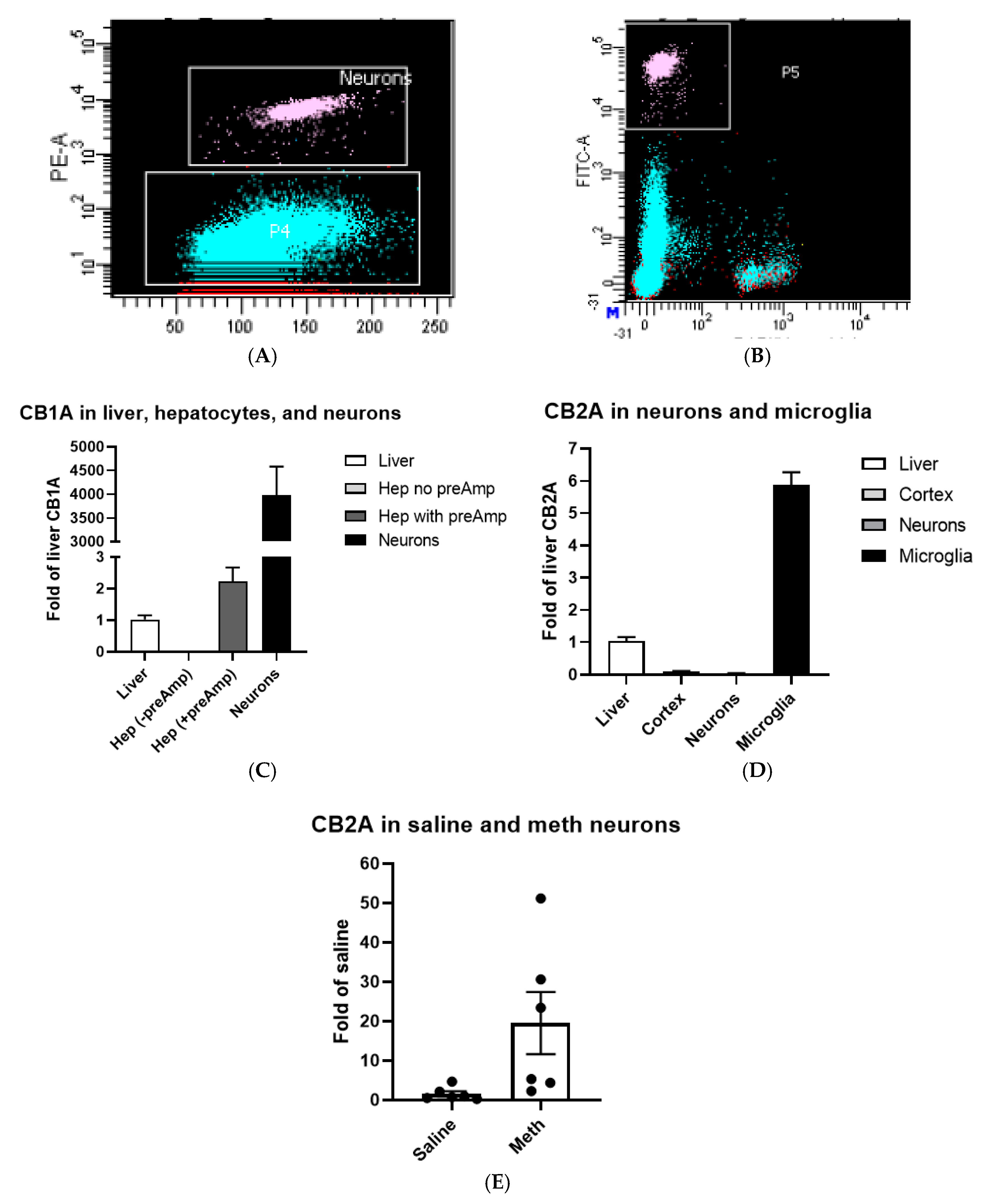
2.2. CB1R and CB2R Expression in Hepatocytes, Neurons, and Microglia
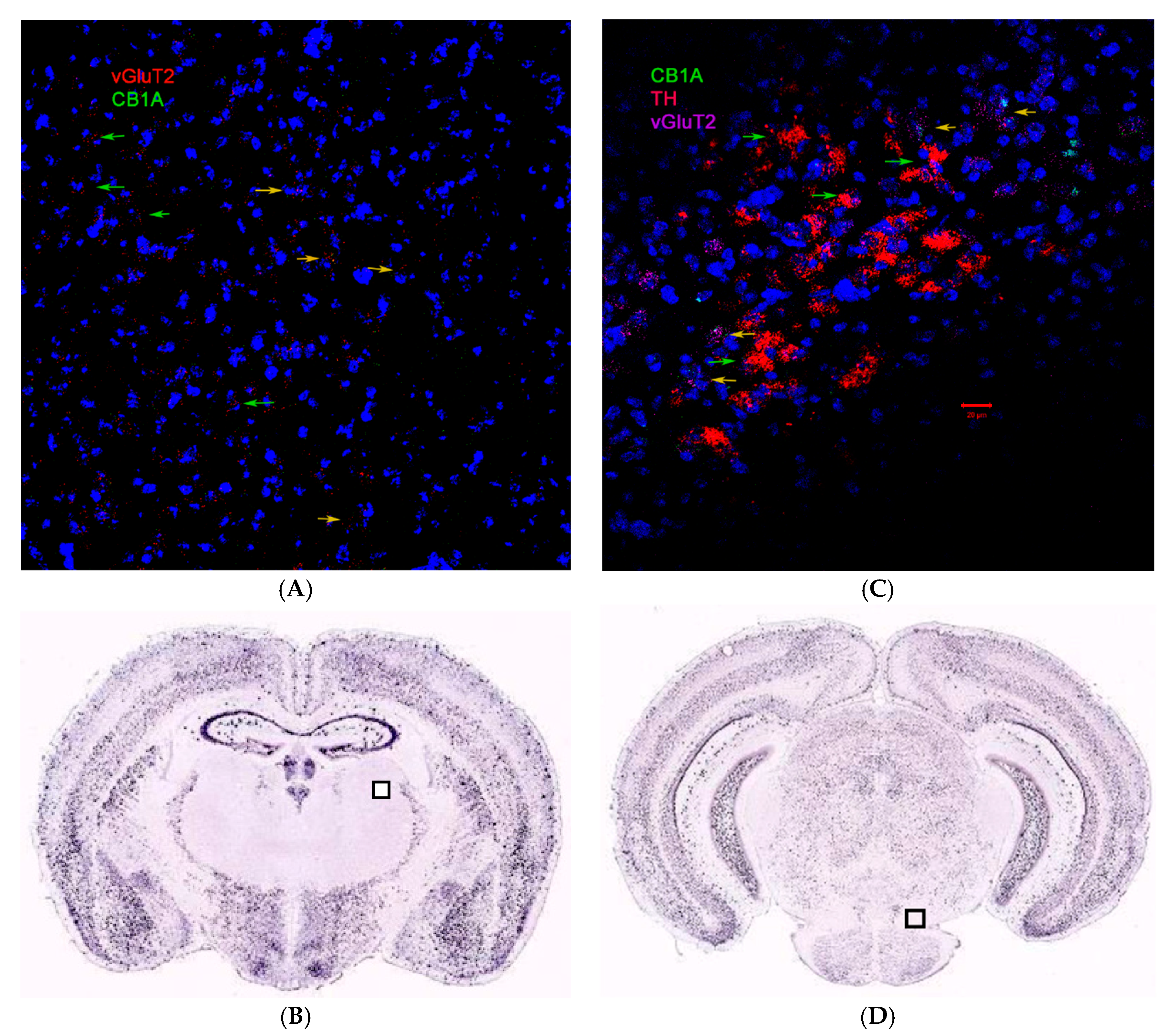
2.3. CB1R and CB2R Cannabinoidergic Neurons
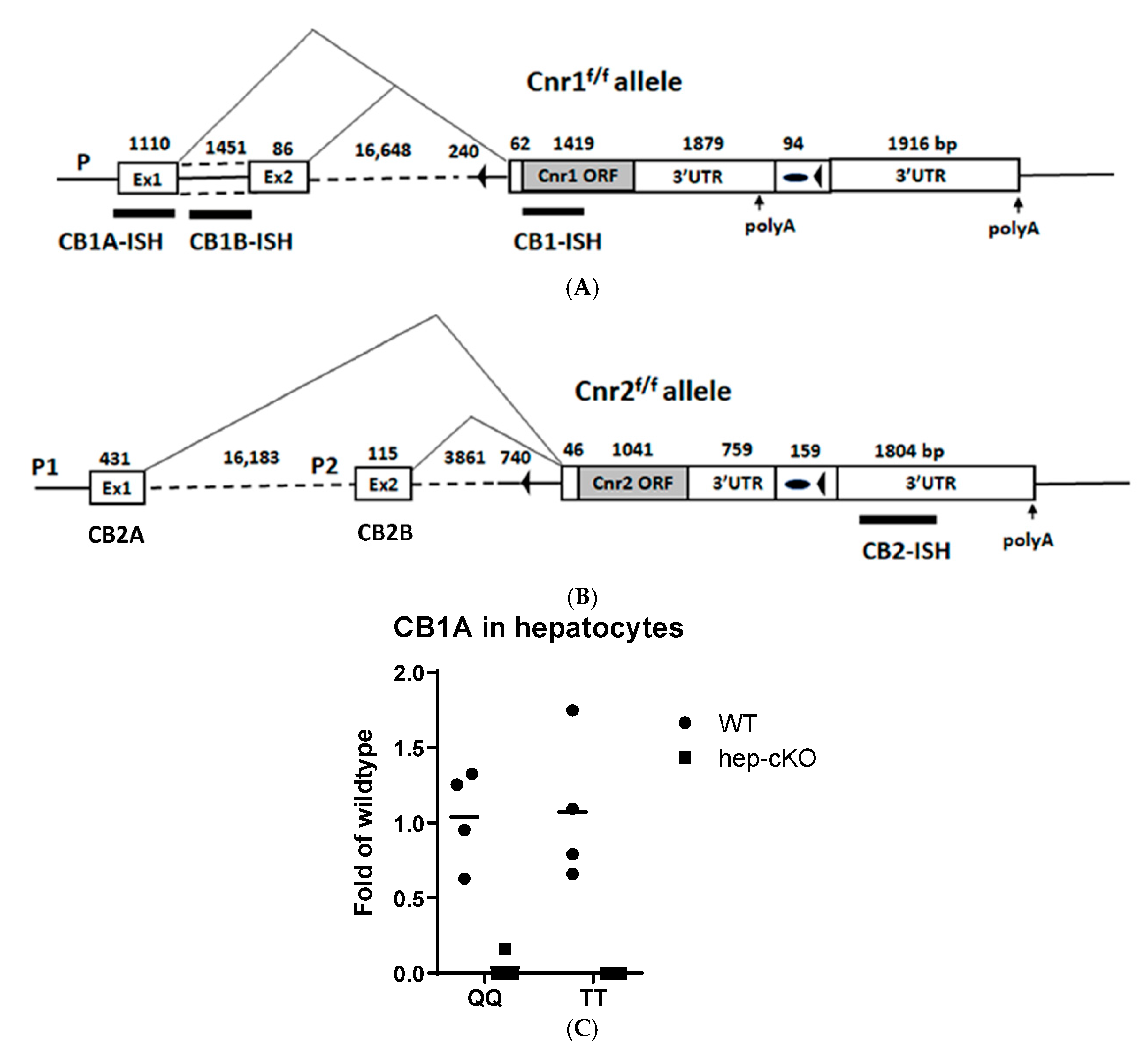
2.4. Generation of Cnr1f/f, Cnr2f/f, Abl-Cnr1Δ, and Dat-Cnr2Δ Mouse Strains
2.5. Tetrad and Alcohol Effects Following Deletion of CB2Rs in Dopamine Neurons and Microglia
3. Discussion
4. Materials and Methods
4.1. Animals and Isolation of Hepatocytes, Neurons, and Microglia
4.2. RNA Isolation and TaqMan RT-qPCR, RT-preAmp-qPCR Assays
4.3. RNAscope In Situ Hybridization (ISH)
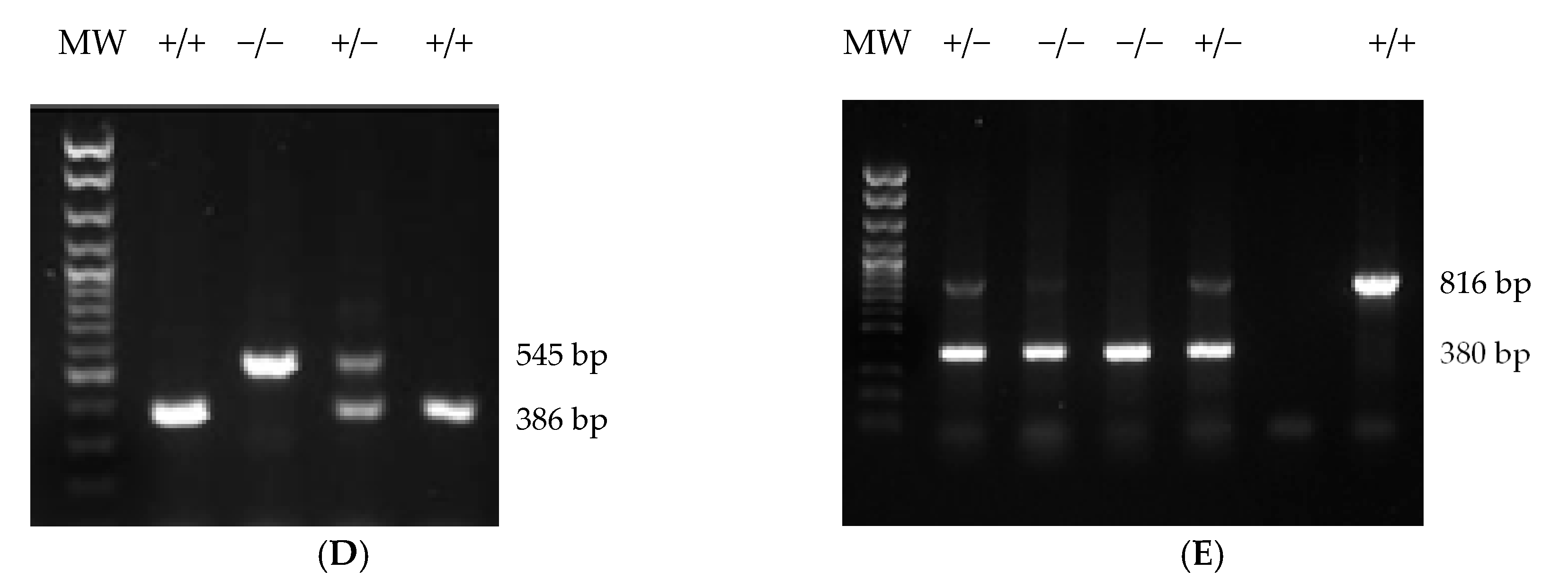
4.4. Generation Cnr1f/f and Cnr2f/f Mouse Strains
4.5. Generation of Abl-Cnr1Δ, Dat-Cnr2Δ, and Cx3cr1-Cnr2Δ Recombinant Strains
4.6. Behavioral Assays
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEA | Aarachidonyl 2′ chloroethylamide |
| ANOVA | Analysis of variance |
| CB1R | Cannabinoid type 1 receptor |
| CB2R | Cannabinoid type 2 receptor |
| Cnr1 | Mouse cannabinoid receptor 1 gene symbol |
| Cnr2 | Mouse cannabinoid receptor 2 gene symbol |
| cKO | Conditional knockout |
| Cnr1ff | Cannabinoid receptor 1 floxed |
| Cnr2ff | Cannabinoid receptor 2z floxed |
| CPP | Conditioned place preference |
| Ct | Cycle threshold |
| Cx3cr1 | Chemokine receptor1 |
| DAT | Dopamine transporter |
| DSI | Depolarization-induced suppression of inhibition |
| DSE | Depolarization-induced suppression of excitation |
| DMSO | Dimethyl sulfoxide |
| ECS | Endocannabinoid system |
| eGFP | Enhanced green fluorescence protein |
| ESCs | Embryonic stem cells |
| FACS | Fluorescence-activated cell sorting |
| FITC | Fluorescein isothiocyanate |
| gKO | Germline knockout |
| GFP | Green fluorescence protein |
| GPCR | G-protein coupled receptor |
| Hep-cKO | Hepatocyte conditional knockout |
| HFHS | High-fat high sugar |
| ISH | In situ hybridization |
| NIS | Sodium iodide transporter |
| ORF | Open reading frame |
| PE | Phycoerythrin |
| RT–PCR | Real-time polymerase chain reaction |
| SYN-Cnr2 | CB2R deletion from synapse |
| Δ9-THC | Delta-9-tetrahydrocannabinol |
| TH | Tyrosine hydroxylase |
| UTR | Untranslated region |
| VGLUT2 | Vesicular glutamate transporter |
| VTA | Ventral tegmental area |
| WT | Wild type |
References
- Little, P.J.; Compton, D.R.; Johnson, M.R.; Melvin, L.S.; Martin, B.R. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J. Pharmacol. Exp. Ther. 1988, 247, 1046–1051. [Google Scholar] [PubMed]
- Liu, Q.R.; Canseco-Alba, A.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [PubMed]
- Liu, Q.R.; Huang, N.S.; Qu, H.; O’Connell, J.F.; Gonzalez-Mariscal, I.; Santa-Cruz-Calvo, S.; Doyle, M.E.; Xi, Z.X.; Wang, Y.; Onaivi, E.S.; et al. Identification of novel mouse and rat CB1R isoforms and in silico modeling of human CB1R for peripheral cannabinoid therapeutics. Acta Pharmacol. Sin. 2019, 40, 387–397. [Google Scholar] [PubMed] [Green Version]
- Zhang, H.Y.; Bi, G.H.; Li, X.; Li, J.; Qu, H.; Zhang, S.J.; Li, C.Y.; Onaivi, E.S.; Gardner, E.L.; Xi, Z.X.; et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 2015, 40, 1037–1051. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar]
- Cinar, R.; Iyer, M.R.; Kunos, G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol. Ther. 2020, 208, 107477. [Google Scholar]
- Onaivi, E.S.; Ishiguro, H.; Gu, S.; Liu, Q.R. CNS effects of CB2 cannabinoid receptors: Beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 2012, 26, 92–103. [Google Scholar]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar]
- Zhang, H.Y.; Shen, H.; Jordan, C.J.; Liu, Q.R.; Gardner, E.L.; Bonci, A.; Xi, Z.X. CB2 receptor antibody signal specificity: Correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharmacol. Sin. 2019, 40, 398–409. [Google Scholar]
- McCoy, K.L.; Matveyeva, M.; Carlisle, S.J.; Cabral, G.A. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: Evidence for CB2 receptor participation. J. Pharmacol. Exp. Ther. 1999, 289, 1620–1625. [Google Scholar]
- Griffin, G.; Wray, E.J.; Tao, Q.; McAllister, S.D.; Rorrer, W.K.; Aung, M.M.; Martin, B.R.; Abood, M.E. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: Further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur. J. Pharmacol. 1999, 377, 117–125. [Google Scholar]
- Lopez, A.; Aparicio, N.; Pazos, M.R.; Grande, M.T.; Barreda-Manso, M.A.; Benito-Cuesta, I.; Vazquez, C.; Amores, M.; Ruiz-Perez, G.; Garcia-Garcia, E.; et al. Cannabinoid CB2 receptors in the mouse brain: Relevance for Alzheimer’s disease. J. Neuroinflammation 2018, 15, 158. [Google Scholar] [PubMed] [Green Version]
- Schmole, A.C.; Lundt, R.; Gennequin, B.; Schrage, H.; Beins, E.; Kramer, A.; Zimmer, T.; Limmer, A.; Zimmer, A.; Otte, D.M. Expression Analysis of CB2-GFP BAC Transgenic Mice. PLoS ONE 2015, 10, e0138986. [Google Scholar]
- Aracil-Fernandez, A.; Trigo, J.M.; Garcia-Gutierrez, M.S.; Ortega-Alvaro, A.; Ternianov, A.; Navarro, D.; Robledo, P.; Berbel, P.; Maldonado, R.; Manzanares, J. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacology 2012, 37, 1749–1763. [Google Scholar] [PubMed] [Green Version]
- Franco, R.; Villa, M.; Morales, P.; Reyes-Resina, I.; Gutierrez-Rodriguez, A.; Jimenez, J.; Jagerovic, N.; Martinez-Orgado, J.; Navarro, G. Increased expression of cannabinoid CB2 and serotonin 5-HT1A heteroreceptor complexes in a model of newborn hypoxic-ischemic brain damage. Neuropharmacology 2019, 152, 58–66. [Google Scholar] [PubMed]
- Garcia-Gutierrez, M.S.; Ortega-Alvaro, A.; Busquets-Garcia, A.; Perez-Ortiz, J.M.; Caltana, L.; Ricatti, M.J.; Brusco, A.; Maldonado, R.; Manzanares, J. Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology 2013, 73, 388–396. [Google Scholar] [PubMed]
- Lanciego, J.L.; Barroso-Chinea, P.; Rico, A.J.; Conte-Perales, L.; Callen, L.; Roda, E.; Gomez-Bautista, V.; Lopez, I.P.; Lluis, C.; Labandeira-Garcia, J.L.; et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J. Psychopharmacol. 2011, 25, 97–104. [Google Scholar]
- Martin-Sanchez, A.; Warnault, V.; Montagud-Romero, S.; Pastor, A.; Mondragon, N.; De La Torre, R.; Valverde, O. Alcohol-induced conditioned place preference is modulated by CB2 cannabinoid receptors and modifies levels of endocannabinoids in the mesocorticolimbic system. Pharmacol. Biochem. Behav. 2019, 183, 22–31. [Google Scholar]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. Adv. Exp. Med. Biol. 2019, 1162, 1–12. [Google Scholar]
- Zimmer, A.; Zimmer, A.M.; Hohmann, A.G.; Herkenham, M.; Bonner, T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 5780–5785. [Google Scholar]
- Buckley, N.E.; McCoy, K.L.; Mezey, E.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar] [CrossRef]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshaarawy, O.; Kurjan, E.; Truong, N.; Olson, L.K. Diet-Induced Obesity in Cannabinoid-2 Receptor Knockout Mice and Cannabinoid Receptor 1/2 Double-Knockout Mice. Obesity 2019, 27, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutierrez, S.O.; van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanki, H.; Suzuki, H.; Itohara, S. High-efficiency CAG-FLPe deleter mice in C57BL/6J background. Exp. Anim. 2006, 55, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dymecki, S.M. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 6191–6196. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A. Cre recombinase: The universal reagent for genome tailoring. Genesis 2000, 26, 99–109. [Google Scholar] [CrossRef]
- Musella, A.; Sepman, H.; Mandolesi, G.; Gentile, A.; Fresegna, D.; Haji, N.; Conrad, A.; Lutz, B.; Maccarrone, M.; Centonze, D. Pre- and postsynaptic type-1 cannabinoid receptors control the alterations of glutamate transmission in experimental autoimmune encephalomyelitis. Neuropharmacology 2014, 79, 567–572. [Google Scholar] [CrossRef]
- Monory, K.; Massa, F.; Egertova, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Deis, S.; Srivastava, R.K.; Ruiz de Azua, I.; Bindila, L.; Baraghithy, S.; Lutz, B.; Bab, I.; Tam, J. Age-related regulation of bone formation by the sympathetic cannabinoid CB1 receptor. Bone 2018, 108, 34–42. [Google Scholar] [CrossRef]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez-Rodriguez, A.; Bonilla-Del Rio, I.; Puente, N.; Gomez-Urquijo, S.M.; Fontaine, C.J.; Egana-Huguet, J.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Robin, L.M.; et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 2018, 66, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz de Azua, I.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Investig. 2017, 127, 4148–4162. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, T.; Park, J.K.; Varga, Z.V.; Paloczi, J.; Coffey, N.J.; Rosenberg, A.Z.; Godlewski, G.; Cinar, R.; Mackie, K.; Pacher, P.; et al. Cannabinoid-1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes Obes. Metab. 2018, 20, 698–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffal, E.; Cron, M.; Glodde, N.; Bald, T.; Kuner, R.; Zimmer, A.; Lutz, B.; Tuting, T. Cannabinoid 1 receptors in keratinocytes modulate proinflammatory chemokine secretion and attenuate contact allergic inflammation. J. Immunol. 2013, 190, 4929–4936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osei-Hyiaman, D.; Liu, J.; Zhou, L.; Godlewski, G.; Harvey-White, J.; Jeong, W.I.; Batkai, S.; Marsicano, G.; Lutz, B.; Buettner, C.; et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008, 118, 3160–3169. [Google Scholar] [CrossRef]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Ozdogan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Racz, I.; Ponomarenko, A.; et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef] [Green Version]
- Stumpf, A.; Parthier, D.; Sammons, R.P.; Stempel, A.V.; Breustedt, J.; Rost, B.R.; Schmitz, D. Cannabinoid type 2 receptors mediate a cell type-specific self-inhibition in cortical neurons. Neuropharmacology 2018, 139, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Cabanero, D.; Ramirez-Lopez, A.; Drews, E.; Schmole, A.; Otte, D.M.; Wawrzczak-Bargiela, A.; Huerga Encabo, H.; Kummer, S.; Ferrer-Montiel, A.; Przewlocki, R.; et al. Protective role of neuronal and lymphoid cannabinoid CB2 receptors in neuropathic pain. Elife 2020, 9, 9. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Romero, J.; Velasco, G.; Tolon, R.M.; Ramos, J.A.; Guzman, M. Cannabinoid CB2 receptor: A new target for controlling neural cell survival? Trends Pharmacol. Sci. 2007, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ranganathan, M.; Shu, C.; Liang, X.; Ganesh, S.; Osafo-Addo, A.; Yan, C.; Zhang, X.; Aouizerat, B.E.; Krystal, J.H.; et al. Single-cell Transcriptome Mapping Identifies Common and Cell-type Specific Genes Affected by Acute Delta9-tetrahydrocannabinol in Humans. Sci. Rep. 2020, 10, 3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Xiao, F.; Yuan, Q.; Zhang, J.; Zhan, J.; Zhang, Z. Cannabinoid receptor 2: A potential novel therapeutic target for sepsis? Acta Clin. Belg. 2019, 74, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; de Petrocellis, L.; Ullmer, C.; Rothenhausler, B.; Perret, C.; van Gils, N.; et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017, 8, 13958. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, T.; Djaouti, L.; Demizieux, L.; Gresti, J.; Verges, B.; Degrace, P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 2010, 59, 926–934. [Google Scholar] [CrossRef] [Green Version]
- De Biase, L.M.; Schuebel, K.E.; Fusfeld, Z.H.; Jair, K.; Hawes, I.A.; Cimbro, R.; Zhang, H.Y.; Liu, Q.R.; Shen, H.; Xi, Z.X.; et al. Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia. Neuron 2017, 95, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.R.; Rubio, F.J.; Bossert, J.M.; Marchant, N.J.; Fanous, S.; Hou, X.; Shaham, Y.; Hope, B.T. Detection of molecular alterations in methamphetamine-activated Fos-expressing neurons from a single rat dorsal striatum using fluorescence-activated cell sorting (FACS). J. Neurochem. 2014, 128, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Canseco-Alba, A.; Schanz, N.; Ishiguro, H.; Liu, Q.R.; Onaivi, E.S. Behavioral Evaluation of Seeking and Preference of Alcohol in Mice Subjected to Stress. Bio. Protoc. 2018, 8, e3061. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Xiong, K.; Godlewski, G.; Mukhopadhyay, B.; Tam, J.; Yin, S.; Gao, P.; Shan, X.; Pickel, J.; et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 2012, 142, 1218–1228.e1. [Google Scholar] [CrossRef] [Green Version]
- De Gottardi, A.; Spahr, L.; Ravier-Dall’Antonia, F.; Hadengue, A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int. 2010, 30, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.B.; Zheng, Y.X.; Li, N.; Cai, S.L.; Huang, Y.; Wang, J.; Hu, X.W.; Wang, Y.; Wu, J.; Fan, X.G. Protective effects of specific cannabinoid receptor 2 agonist GW405833 on concanavalin A-induced acute liver injury in mice. Acta Pharmacol. Sin. 2019, 40, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Wright, O.; Zhang, L.; Liu, Y.; Yoshimi, T.; Zheng, Y.; Tunnacliffe, A. Critique of the use of fluorescence-based reporters in Escherichia coli as a screening tool for the identification of peptide inhibitors of Abeta42 aggregation. J. Pept. Sci. 2013, 19, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, J.M.; Hoermann, B.; Schlimbach, T.; Teleman, A.A. Changes in global translation elongation or initiation rates shape the proteome via the Kozak sequence. Sci. Rep. 2018, 8, 4018. [Google Scholar] [CrossRef] [Green Version]
- Borowska-Fielding, J.; Murataeva, N.; Smith, B.; Szczesniak, A.M.; Leishman, E.; Daily, L.; Toguri, J.T.; Hillard, C.J.; Romero, J.; Bradshaw, H.; et al. Revisiting cannabinoid receptor 2 expression and function in murine retina. Neuropharmacology 2018, 141, 21–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Stefanovic, B. LARP6 Meets Collagen mRNA: Specific Regulation of Type I Collagen Expression. Int. J. Mol. Sci. 2016, 17, 419. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Garcia, A.; Bernal-Chico, A.; Colomer, T.; Rodriguez-Antiguedad, A.; Matute, C.; Mato, S. Gene Expression Analysis of Astrocyte and Microglia Endocannabinoid Signaling during Autoimmune Demyelination. Biomolecules 2020, 10, 1228. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.Z.; Persidsky, Y. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J. Leukoc. Biol. 2013, 93, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Gao, M.; Shen, H.; Bi, G.H.; Yang, H.J.; Liu, Q.R.; Wu, J.; Gardner, E.L.; Bonci, A.; Xi, Z.X. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict. Biol. 2017, 22, 752–765. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H. The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2018, 148, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Reiner, D.; Shen, H.; Wu, K.J.; Liu, Q.R.; Wang, Y. Time-Dependent Protection of CB2 Receptor Agonist in Stroke. PLoS ONE 2015, 10, e0132487. [Google Scholar] [CrossRef]
- Palacios, J.M.; Mengod, G. Receptor visualization and the atomic bomb. A historical account of the development of the chemical neuroanatomy of receptors for neurotransmitters and drugs during the Cold War. J. Chem. Neuroanat. 2018, 88, 76–112. [Google Scholar] [CrossRef]
- Barker, D.J.; Root, D.H.; Zhang, S.; Morales, M. Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J. Chem. Neuroanat. 2016, 73, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Ye, A.Y.; Liu, Q.R.; Li, C.Y.; Zhao, M.; Qu, H. Human transporter database: Comprehensive knowledge and discovery tools in the human transporter genes. PLoS ONE 2014, 9, e88883. [Google Scholar] [CrossRef]
- Xi, Z.-X.; Peng, X.-Q.; Li, X.; Song, R.; Zhang, H.-Y.; Liu, Q.-R.; Yang, H.-J.; Bi, G.-H.; Li, J.; Gardner, E.L. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat. Neurosci. 2011, 14, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Gao, F.; Larsen, B.; Gao, M.; Luo, Z.; Chen, D.; Ma, X.; Qiu, S.; Zhou, Y.; Xie, J.; et al. Mechanisms of cannabinoid CB2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. EBioMedicine 2019, 42, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenadl, C.; Klingel, K.; Mertsching, J.; Hofschneider, P.H.; Kandolf, R. Strand-specific detection of enteroviral RNA in myocardial tissue by in situ hybridization. Mol. Cell. Probes 1991, 5, 11–20. [Google Scholar] [CrossRef]
- Flagella, M.; Bui, S.; Zheng, Z.; Nguyen, C.T.; Zhang, A.; Pastor, L.; Ma, Y.; Yang, W.; Crawford, K.L.; McMaster, G.K.; et al. A multiplex branched DNA assay for parallel quantitative gene expression profiling. Anal. Biochem. 2006, 352, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Luquin, N.; Rico, A.J.; Gomez-Bautista, V.; Roda, E.; Dopeso-Reyes, I.G.; Vazquez, A.; Martinez-Pinilla, E.; Labandeira-Garcia, J.L.; Franco, R.; et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: Changes following experimental parkinsonism. Brain Struct. Funct. 2015, 220, 2721–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.W.; Kim, J.I.; Tawfik, V.L.; Lalchandani, R.R.; Scherrer, G.; Ding, J.B. Input- and cell-type-specific endocannabinoid-dependent LTD in the striatum. Cell Rep. 2015, 10, 75–87. [Google Scholar] [CrossRef]
- Diana, M.A.; Marty, A. Endocannabinoid-mediated short-term synaptic plasticity: Depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br. J. Pharmacol. 2004, 142, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hishimoto, A.; Pletnikova, O.; Lang, D.L.; Troncoso, J.C.; Egan, J.M.; Liu, Q.R. Neurexin 3 transmembrane and soluble isoform expression and splicing haplotype are associated with neuron inflammasome and Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duff, G.; Argaw, A.; Cecyre, B.; Cherif, H.; Tea, N.; Zabouri, N.; Casanova, C.; Ptito, M.; Bouchard, J.F. Cannabinoid receptor CB2 modulates axon guidance. PLoS ONE 2013, 8, e70849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Zhang, M.; Liu, X.; Zhang, J.; Wang, H. CB2R orchestrates neuronal autophagy through regulation of the mTOR signaling pathway in the hippocampus of developing rats with status epilepticus. Int. J. Mol. Med. 2020, 45, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Smanik, P.A.; Liu, Q.; Furminger, T.L.; Ryu, K.; Xing, S.; Mazzaferri, E.L.; Jhiang, S.M. Cloning of the human sodium lodide symporter. Biochem. Biophys. Res. Commun. 1996, 226, 339–345. [Google Scholar] [CrossRef]
- Tazebay, U.H.; Wapnir, I.L.; Levy, O.; Dohan, O.; Zuckier, L.S.; Zhao, Q.H.; Deng, H.F.; Amenta, P.S.; Fineberg, S.; Pestell, R.G.; et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 2000, 6, 871–878. [Google Scholar] [CrossRef]
- Gonzalez-Mariscal, I.; Montoro, R.A.; Doyle, M.E.; Liu, Q.R.; Rouse, M.; O’Connell, J.F.; Santa-Cruz Calvo, S.; Krzysik-Walker, S.M.; Ghosh, S.; Carlson, O.D.; et al. Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia 2018, 61, 1470–1483. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Mariscal, I.; Montoro, R.A.; O’Connell, J.F.; Kim, Y.; Gonzalez-Freire, M.; Liu, Q.R.; Alfaras, I.; Carlson, O.D.; Lehrmann, E.; Zhang, Y.; et al. Muscle cannabinoid 1 receptor regulates Il-6 and myostatin expression, governing physical performance and whole-body metabolism. FASEB J. 2019, 33, 5850–5863. [Google Scholar] [CrossRef]
- Backman, C.M.; Malik, N.; Zhang, Y.; Shan, L.; Grinberg, A.; Hoffer, B.J.; Westphal, H.; Tomac, A.C. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis 2006, 44, 383–390. [Google Scholar] [CrossRef]
- Canseco-Alba, A.; Schanz, N.; Sanabria, B.; Zhao, J.; Lin, Z.; Liu, Q.R.; Onaivi, E.S. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav. Brain Res. 2019, 360, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Jiang, M.; Zhang, B.; Gokce, O.; Sudhof, T.C. Conditional Deletion of All Neurexins Defines Diversity of Essential Synaptic Organizer Functions for Neurexins. Neuron 2017, 94, 611–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seigneur, E.; Sudhof, T.C. Genetic Ablation of All Cerebellins Reveals Synapse Organizer Functions in Multiple Regions Throughout the Brain. J. Neurosci. 2018, 38, 4774–4790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.Y.; Kuo, H.Y.; Liu, F.C. Stereotaxic Surgery for Genetic Manipulation in Striatal Cells of Neonatal Mouse Brains. J. Vis. Exp. 2018, 137, e57270. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Lee, D.K.; Jo, Y.H. Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Mol. Metab. 2017, 6, 306–312. [Google Scholar] [CrossRef]
- Chen, D.; Ren, K.; Liu, H.; Mao, H.; Li, Z.; Mo, H.; Xie, S.; Shi, Y.; Chen, Q.; Wang, W. A Whole-Brain Cell-Type-Specific Sparse Neuron Labeling Method and Its Application in a Shank3 Autistic Mouse Model. Front. Cell. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef]
- Wykes, A.D.; Ma, S.; Bathgate, R.A.D.; Gundlach, A.L. Targeted viral vector transduction of relaxin-3 neurons in the rat nucleus incertus using a novel cell-type specific promoter. IBRO Rep. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Sharma, V. Cannabis for COVID-19: Can cannabinoids quell the cytokine storm? Future Sci. OA 2020, 6, FSO625. [Google Scholar] [CrossRef]
- Toelzer, C.; Gupta, K.; Yadav, S.K.N.; Borucu, U.; Davidson, A.D.; Kavanagh Williamson, M.; Shoemark, D.K.; Garzoni, F.; Staufer, O.; Milligan, R.; et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 2020, 370, 725–730. [Google Scholar] [CrossRef]
- Inui, A. Transgenic study of energy homeostasis equation: Implications and confounding influences. FASEB J. 2000, 14, 2158–2170. [Google Scholar] [CrossRef] [Green Version]
- Benard, G.; Massa, F.; Puente, N.; Lourenco, J.; Bellocchio, L.; Soria-Gomez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Bilkei-Gorzo, A.; Nozaki, C.; Togo, A.; Nakamura, K.; Ohta, K.; Zimmer, A.; Asahi, T. Age-dependent Alteration in Mitochondrial Dynamics and Autophagy in Hippocampal Neuron of Cannabinoid CB1 Receptor-deficient Mice. Brain Res. Bull. 2020, 160, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rouse, M.; Gonzalez-Mariscal, I.; Egan, J.M.; O’Connell, J.F. Dietary curcumin enhances insulin clearance in diet-induced obese mice via regulation of hepatic PI3K-AKT axis and IDE, and preservation of islet integrity. Nutr. Metab 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Salem, E.S.B.; Murakami, K.; Takahashi, T.; Bernhard, E.; Borra, V.; Bethi, M.; Nakamura, T. Isolation of Primary Mouse Hepatocytes for Nascent Protein Synthesis Analysis by Non-radioactive L-azidohomoalanine Labeling Method. J. Vis. Exp. 2018, 140, e58323. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.J.; Li, X.; Liu, Q.R.; Cimbro, R.; Hope, B.T. Fluorescence Activated Cell Sorting (FACS) and Gene Expression Analysis of Fos-expressing Neurons from Fresh and Frozen Rat Brain Tissue. J. Vis. Exp. 2016, 114, e54358. [Google Scholar] [CrossRef]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef]

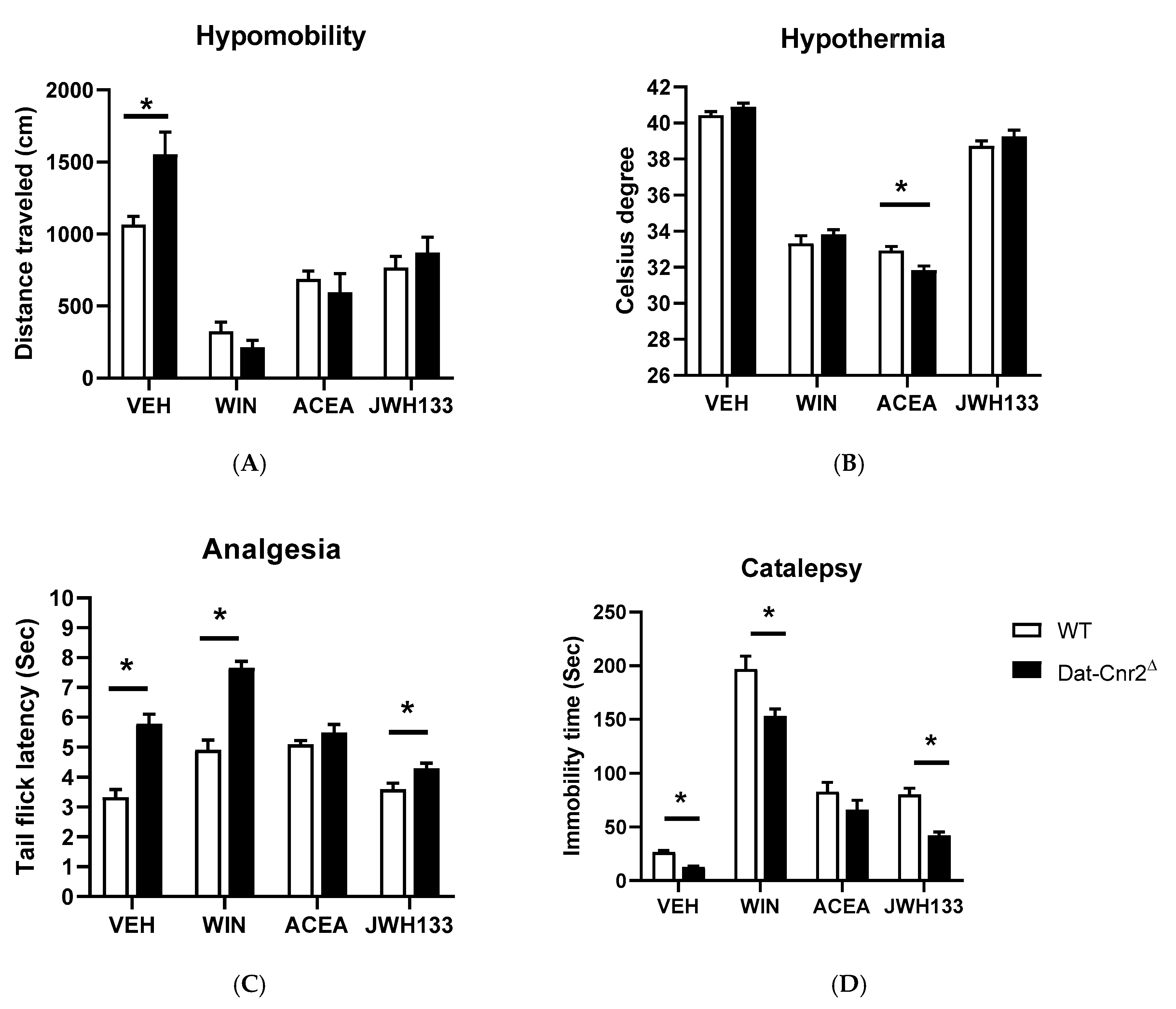
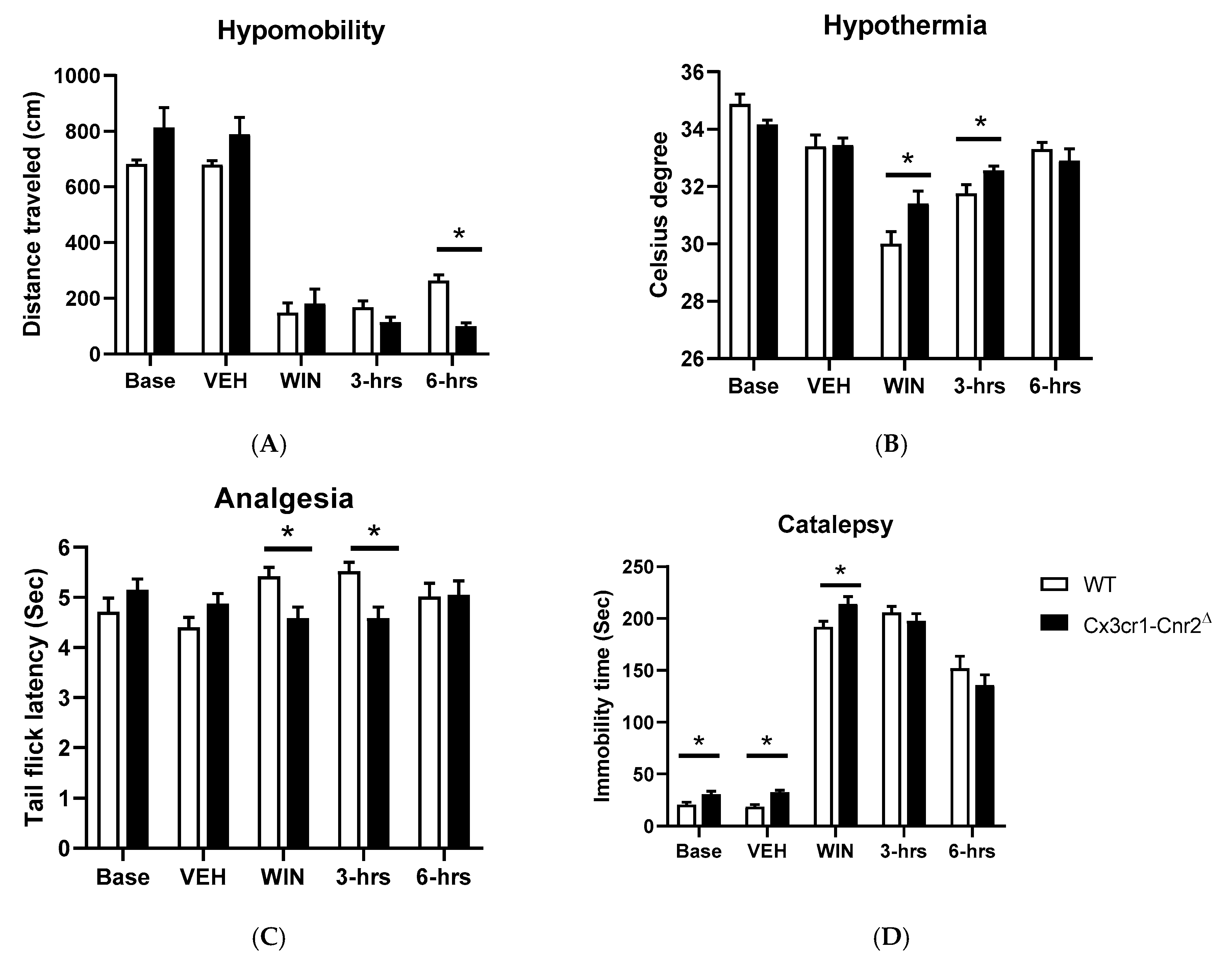

| Dat-Cnr2Δ | Main Effect of Drugs | Main Effect of Genotypes | Interaction |
|---|---|---|---|
| a Locomotion | F (1, 14) = 2.583 p = 0.1303 | F (2.217, 31.04) = 39.01 p < 0.0001 | F (3, 42) = 4.017 p = 0.0134 |
| a Temperature | F (1, 14) = 0.2189 p = 0.6471 | F (2.481, 34.73) = 435.1 p < 0.0001 | F (3, 42) = 4.240 p = 0.0105 |
| a Nociception | F (1, 14) = 62.10 p < 0.0001 | F (1.820, 25.48) = 34.80 p < 0.0001 | F (3, 42) = 12.34 p < 0.0001 |
| a Catalepsy | F (1, 14) = 31.49 p < 0.0001 | F (2.151, 30.12) = 181.8 p < 0.0001 | F (3, 42) = 2.382 p = 0.0830 |
| a Ethanol 8% | F (1, 18) = 27.88 p < 0.0001 | F (1, 18) = 143.7 p < 0.0001 | F (1, 18) = 114.7 p < 0.0001 |
| Cx3cr1-Cnr2Δ | |||
| a Locomotion | F (1, 14) = 0.09896 p = 0.7577 | F (2.058, 28.81) = 191.3 p < 0.0001 | F (4, 56) = 7.032 p = 0.0001 |
| a Temperature | F (1, 14) = 2.516 p = 0.1350 | F (2.567, 35.94) = 33.74 p < 0.0001 | F (4, 56) = 3.120 p = 0.0218 |
| b Nociception | F (1, 69) = 1.196 p > 0.05 | F (2.221, 38.31) = 380.4 p < 0.0001 | F (4, 69) = 2.999 p < 0.05 |
| b Catalepsy | F (1, 14) = 0.6054 p > 0.05 | F (2.387, 32.22) = 1.676 p > 0.05 | F (4, 54) = 6.708 p < 0.05 |
| a Ethanol 8% | F (1, 18) = 3.369 p = 0.0830 | F (1, 18) = 317.4 p < 0.0001 | F (1, 18) = 11.35 p = 0.0034 |
| Alleles | TaqMan Probe or PCR Fragment | Forward Primer | Reverse Primer |
|---|---|---|---|
| Cnr1-wt | CATCTGTTGGTGATTTCT(FAM) | CCTAAGAACTGCATGGCATGAAG | GCTGGGAACCCCAAATGGT |
| Cnr1-flox | CTAGCATCTGTTGGAGTGTAC(VIC) | CCTAAGAACTGCATGGCATGAAG | GGAACTTCGCTAGACTAGTACGC |
| Cnr2-wt | AGTCTTCAGAGAACTCT(FAM) | GCTGGGTTCACTGGAGGTACA | ACACAGCAAAATGTCACAAGGAA |
| Cnr2-flox | AGTCTTCAATTGCGTACGTT(VIC) | GCTGGGTTCACTGGAGGTACA | CGCGACACGGACACAATC |
| Cnr2-wt | 386 bp PCR FRAGMENT | GGTCAAGAATTATGATGCCCTAAGGACC | CCCAACTCCTTCTGCTTATCCTTCAGG |
| Cnr2-flox | 545 bp PCR FRAGMENT | GGTCAAGAATTATGATGCCCTAAGGACC | CCCAACTCCTTCTGCTTATCCTTCAGG |
| Abl-wt | CCTGTCATGCCCACACAAATCTCTCC(FAM) | GCTGTCATCTCTTGTGGGCTGT | ACTCATGGGAGCTGCTGGTTC |
| Abl-Cre | CTATCAACCCCGGGATCC(VIC) | AGCGAGTCTTTCTGCACACA | GCTGCAGGTCGACTCTAGATC |
| Dat-wt | AGATCACAAAGGAAACC(FAM) | GCCAGCTGGGCCATCTC | AAGTGGCCCTCCTTTCTTGAC |
| Dat-Cre | CCCCCCTAACGTTACT(VIC) | GTTGGTGTAAAGTGGAAGGAGACA | CGCACACCGGCCTTATTC |
| Cx3cr1-wt | 380 bp PCR FRAGMENT | AGCTCACGACTGCCTTCTTC | GCAGGGAAATCTGATGCAAG |
| Cx3cr1-Cre | 816 bp PCR FRAGMENT | GACATTTGCCTTGCTGGAC | GCAGGGAAATCTGATGCAAG |
| Cre | GGTTAGCACCGCAGG(VIC) | TTAATCCATATTGGCAGAACGAAAACG | CAGGCTAAGTGCCTTCTCTACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.-R.; Canseco-Alba, A.; Liang, Y.; Ishiguro, H.; Onaivi, E.S. Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects. Int. J. Mol. Sci. 2020, 21, 9763. https://doi.org/10.3390/ijms21249763
Liu Q-R, Canseco-Alba A, Liang Y, Ishiguro H, Onaivi ES. Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects. International Journal of Molecular Sciences. 2020; 21(24):9763. https://doi.org/10.3390/ijms21249763
Chicago/Turabian StyleLiu, Qing-Rong, Ana Canseco-Alba, Ying Liang, Hiroki Ishiguro, and Emmanuel S. Onaivi. 2020. "Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects" International Journal of Molecular Sciences 21, no. 24: 9763. https://doi.org/10.3390/ijms21249763
APA StyleLiu, Q.-R., Canseco-Alba, A., Liang, Y., Ishiguro, H., & Onaivi, E. S. (2020). Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects. International Journal of Molecular Sciences, 21(24), 9763. https://doi.org/10.3390/ijms21249763





