Role of Stomatal Conductance in Modifying the Dose Response of Stress-Volatile Emissions in Methyl Jasmonate Treated Leaves of Cucumber (Cucumis Sativa)
Abstract
:1. Introduction
2. Results
2.1. Dose Response of Photosynthesis and Stomatal Conductance to MeJA Treatment
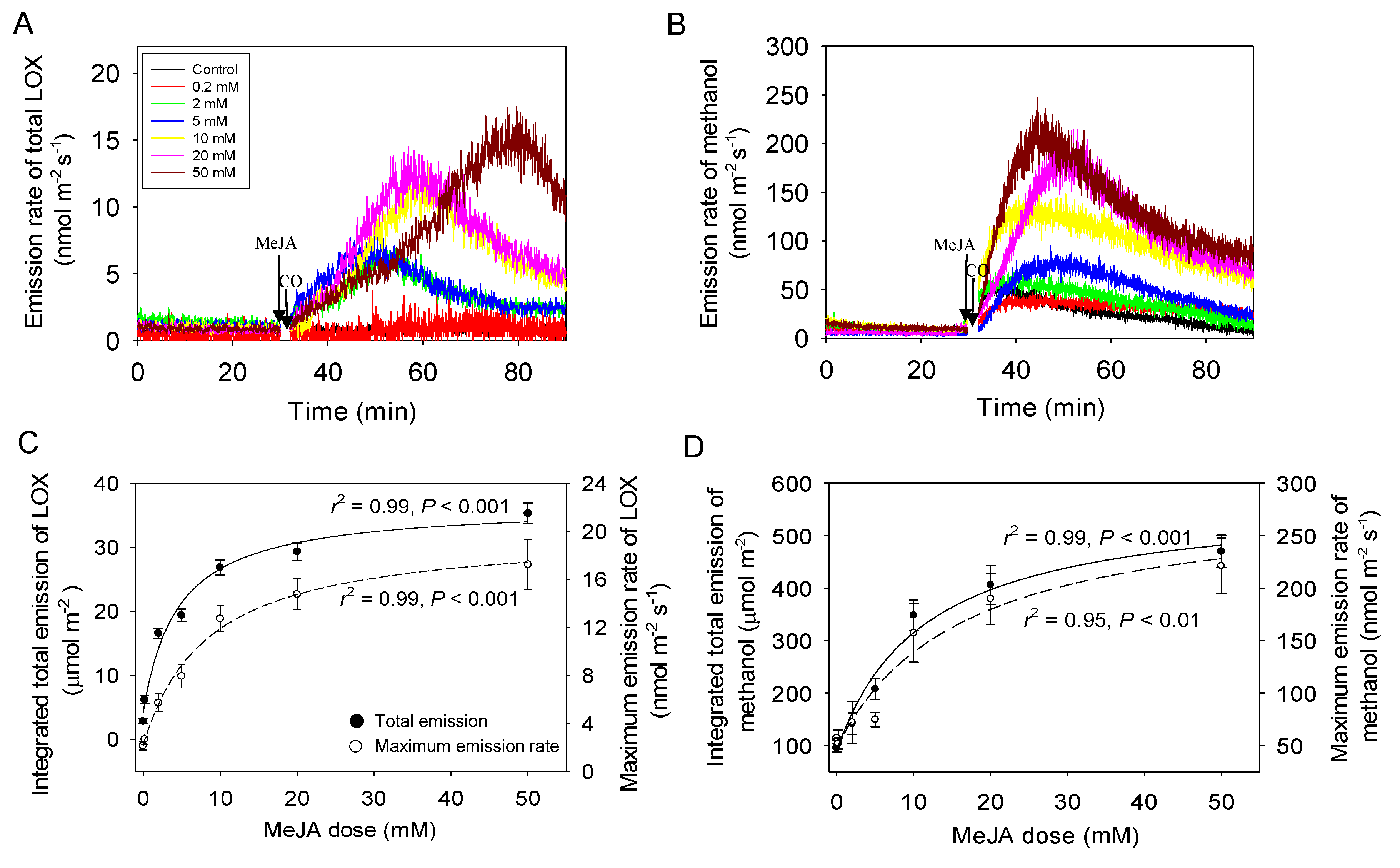
2.2. Dose Response of MeJA-Induced Volatile Emissions
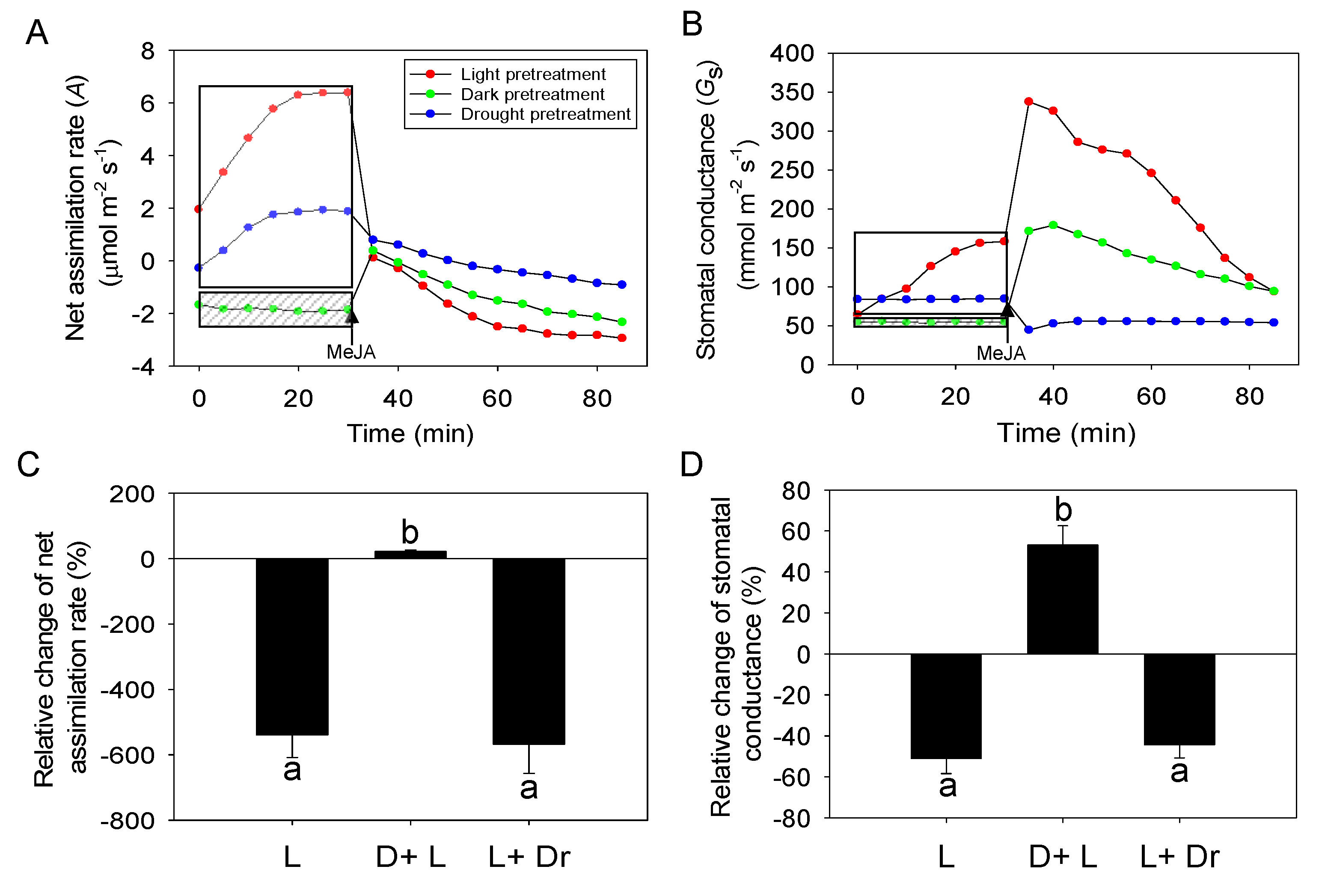
2.3. Effects of Dark and Drought Pretreatments on the Responses of Photosynthesis and Stomatal Conductance to MeJA Treatment
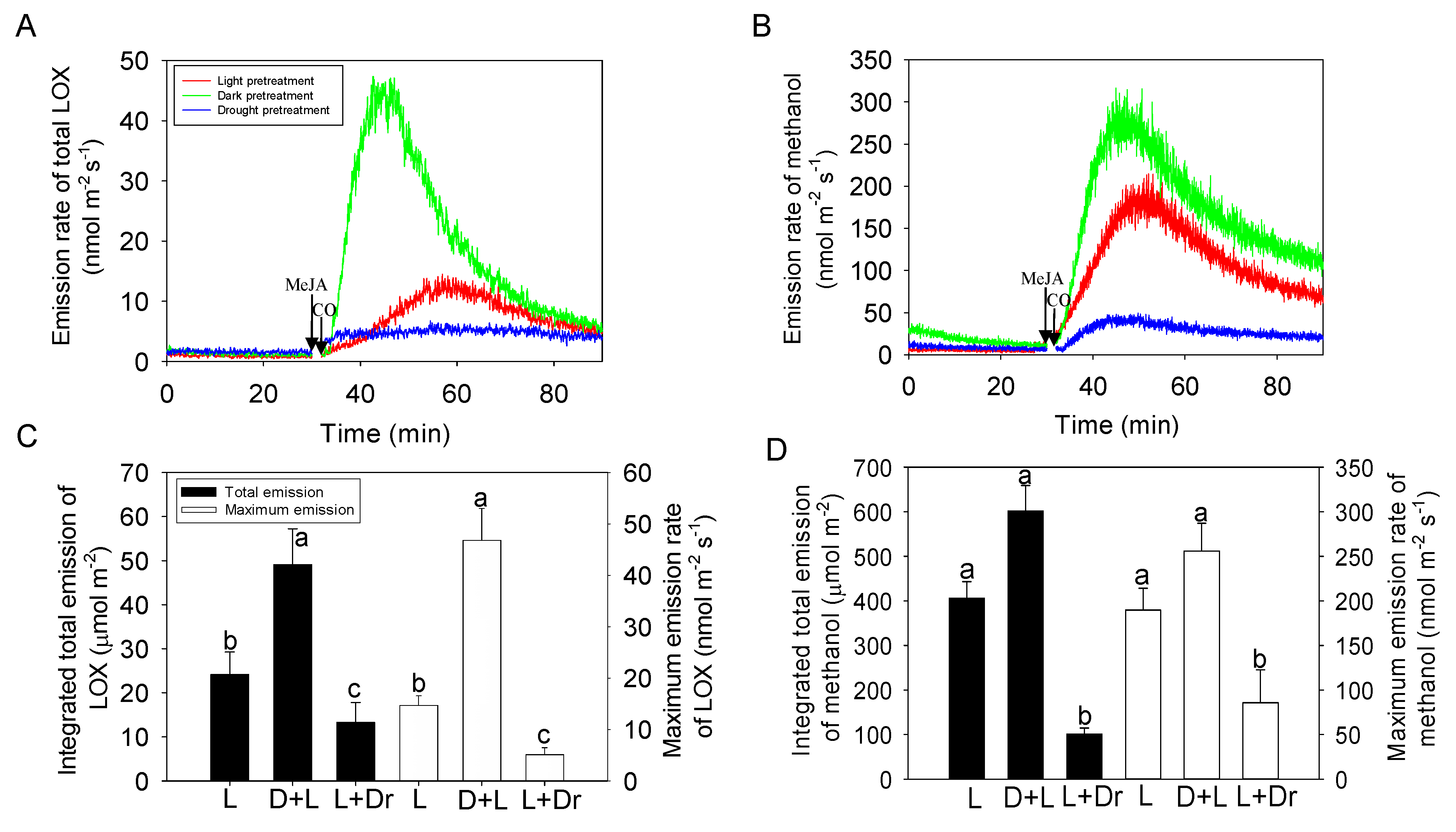
2.4. Effects of Dark and Drought Pretreatments on the Responses of Volatile Emissions to MeJA Treatment
3. Discussion
3.1. MeJA Dose-Dependent Changes in Foliage Photosynthetic Characteristics in Cucumber
3.2. MeJA Concentration-Dependent Emissions of LOX and Methanol
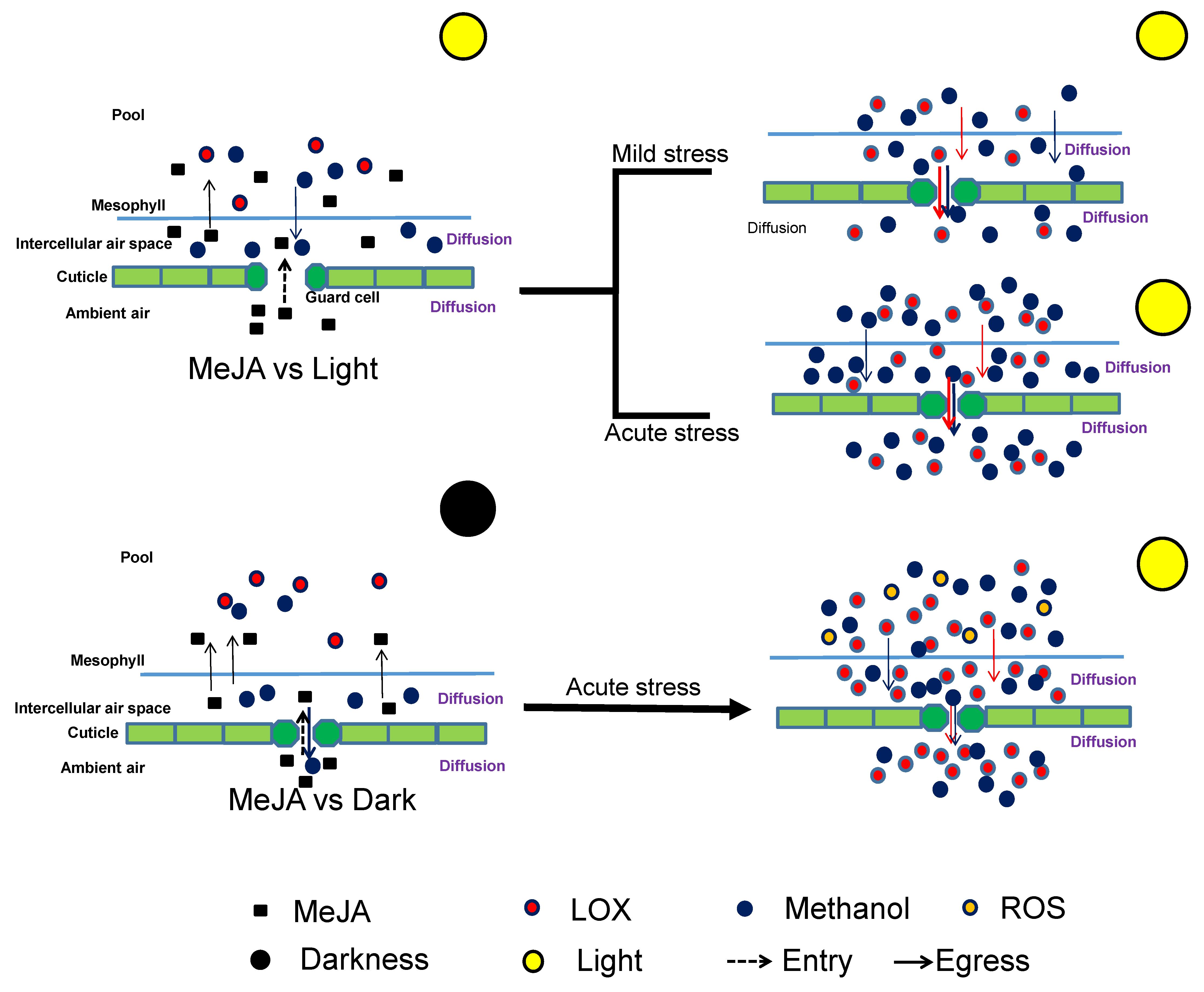
3.3. Impacts of Dark and Drought Pretreatments on MeJA Responses
4. Material and Methods
4.1. Plant Growth Conditions
4.2. Methyl Jasmonate (MeJA) Treatments
4.3. Interactions of MeJA Treatments with Abiotic Factors
4.4. Photosynthesis and Stomatal Conductance Measurements
4.5. Calculation of Relative Changes in Photosynthesis and Stomatal Conductance upon MeJA Treatment
4.6. Online Monitoring of the Kinetics of VOC (Volatile Organic Compound) Emission with PTR-TOF-MS Combined with Dynamic Head-Space Volatile Collection
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P.; Song, C.P. Guard cell signaling for hydrogen peroxide and abscisic acid. New Phytol. 2008, 178, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Israelsson, M.; Siegel, R.S.; Young, J.; Hashimoto, M.; Iba, K.; Schroeder, J.I. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 2006, 9, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, K.A.; Sibbernsen, E.D.; Shope, J.C. The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 2008, 31, 1299–1306. [Google Scholar] [CrossRef]
- Fan, L.M.; Zhao, Z.; Assmann, S.M. Guard cells: A dynamic signaling model. Curr. Opin. Plant Biol. 2004, 7, 537–546. [Google Scholar] [CrossRef]
- Acharya, B.; Assmann, S. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Zeng, W.; Melotto, M.; He, S.Y. Plant stomata: A checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, D.H.; Kopischke, M.; Robatzek, S. Gate control: Guard cell regulation by microbial stress. New Phytol. 2014, 203, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.R.; Gehring, C.A.; Parish, R.W. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc. Natl. Acad. Sci. USA 1992, 89, 1790–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, C.A.; Irving, H.R.; McConchie, R.; Parish, R.W. Jasmonates induce intracellular alkalinization and closure of Paphiopedilum guard cells. Ann. Bot. 1997, 80, 485–489. [Google Scholar] [CrossRef] [Green Version]
- Suhita, D.; Raghavendra, A.S.; Kwak, J.M.; Vavasseur, A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-Induced stomatal closure. Plant Physiol. 2004, 134, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Munemasa, S.; Oda, K.; Watanabe-Sugimoto, M.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. The coronatine-Insensitive mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells: Specific impairment of ion channel activation and second messenger production. Plant Physiol. 2007, 143, 1398–1407. [Google Scholar] [CrossRef] [Green Version]
- Akter, N.; Okuma, E.; Sobahan, M.A.; Uraji, M.; Munemasa, S.; Nakamura, Y.; Murata, Y. Negative regulation of methyl jasmonate-induced stomatal closure by glutathione in Arabidopsis. J. Plant Growth Regul. 2013, 32, 208–215. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Hamayun, M.; Kang, S.M.; Lee, I.J. Foliar application of methyl jasmonate induced physio-Hormonal changes in Pisum sativum under diverse temperature regimes. Plant Physiol. Biochem. 2015, 96, 406e416. [Google Scholar] [CrossRef]
- Zhu, M.; Dai, S.; Zhu, N.; Booy, A.; Simons, B.; Yi, S.; Chen, S. Methyl jasmonate responsive proteins in Brassica napus guard cells revealed by iTRAQ-Based quantitative proteomics. J. Proteome Res. 2012, 11, 3728–3742. [Google Scholar] [CrossRef]
- Song, Y.; Miao, Y.; Son, C.P. Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytologist. 2014, 201, 1121–1140. [Google Scholar] [CrossRef]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse Stomatal Signaling and the Signal Integration Mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.S.; Gao, J.; Pan, S.; Wang, G.X. Chlorella induces stomatal closure via NADPH oxidase-Dependent ROS production and its effects on instantaneous water use efficiency in Vicia faba. PLoS ONE 2014, 9, e93290. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.M.; Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol. 2010, 51, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of endogenous abscisic acid in methyl jasmonate-Induced stomatal closure in Arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; McLamore, E.S.; Dong, S.; Gao, H.; Taguchi, M.; Wang, N.; Zhang, T.; Su, X.; Shen, Y. The role of plasma membrane H+ -ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J. 2015, 83, 638–649. [Google Scholar] [CrossRef]
- Jung, S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. J. Plant Physiol. Biochem. 2004, 42, 231–255. [Google Scholar] [CrossRef]
- Attaran, E.; Major, I.; Cruz, J.; Rosa, B.; Koo, A.; Chen, J.; Kramer, D.; He, S.Y.; Howe, G. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiol. 2014, 165, 1302–1314. [Google Scholar] [CrossRef] [Green Version]
- Moldau, H.; Wong, S.C.; Osmond, C.B. Transient depression of photosynthesis in bean leaves during rapid water loss. Aust. J. Plant Physiol. 1993, 20, 45–54. [Google Scholar]
- Rasulov, B.; Talts, E.; Niinemets, Ü. A novel approach for real-Time monitoring of leaf wounding responses demonstrates unprecedently fast and high emissions of volatiles from cut leaves. Plant Sci. 2019, 283, 256–265. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Vislap, V.; Niinemets, Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. J. Chem. Ecol. 2011, 37, 18–28. [Google Scholar] [CrossRef]
- Gosset, V.; Harmel, N.; Göbel, C.; Francis, F.; Haubruge, E.; Wathelet, J.P.; du Jardin, P.; Feussner, I.; Fauconnier, M.L. Attacks by a piercing-Sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009, 60, 1231–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toome, M.; Randjärv, P.; Copolovici, L.; Niinemets, Ü.; Heinsoo, K.; Luik, A.; Noe, S.M. Leaf rust induced volatile organic compounds signaling in willow during the infection. Planta 2010, 232, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Arimura, G.I.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Starvaggi Cucuzza, L.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arimura, G.I.; Ozawa, R.; Maffei, M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011, 12, 3723–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, F.; Cellini, A.; Marchetti, L.; Mudigere, N.K.; Piovene, C. Emission and Function of Volatile Organic Compounds in Response to Abiotic Stress, in Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A.K., Venkateswarlu, B., Eds.; InTech: Rijeka, Croatia, 2011; pp. 367–394. [Google Scholar]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Niinemets, Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 2014, 100, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.F.; Ye, J.Y.; Veromann, L.L.; Niinemets, Ü. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiol. 2016, 36, 856–872. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.F.; Ye, J.Y.; Li, S.; Niinemets, Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber (Cucumis sativus): A high-resolution analysis of dose dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef]
- Copolovici, L.; Niinemets, Ü. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010, 33, 1582–1594. [Google Scholar] [CrossRef]
- Beauchamp, J.; Wisthaler, A.; Hansel, A.; Kleist, E.; Miebach, M.; Niinemets, Ü.; Schurr, U.; Wildt, J. Ozone induced emissions of biogenic VOC from tobacco: Relations between ozone uptake and emission of LOX products. Plant Cell Environ. 2005, 28, 1334–1343. [Google Scholar] [CrossRef]
- Heiden, A.C.; Kobel, K.; Langebartels, C.; Schuh-Thomas, G.; Wildt, J. Emissions of oxygenated volatile organic com-Pounds from plants, Part I: Emissions from lipoxygenase activity. J. Atmos. Chem. 2003, 45, 143–172. [Google Scholar] [CrossRef]
- Loreto, F.; Barta, C.; Brilli, F.; Nogues, I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006, 29, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Estrada, M.; Kazantsev, T.; Talts, E.; Tosens, T.; Niinemets, Ü. Emission timetable and quantitative patterns of wound-Induced volatiles across different damage treatments in aspen (Populus tremula). J. Chem. Ecol. 2015, 41, 1105–1117. [Google Scholar] [CrossRef]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-Induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.F.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, Ü. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage and emission of lox products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Saona, C.; Polashock, J.; Malo, E. Jasmonate-mediated induced volatiles in the American cranberry, Vaccinium macrocarpon: From gene expression to organismal interactions. Front Plant Sci. 2013, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü.; Loreto, F.; Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar] [CrossRef]
- Harley, P.C. The Roles of Stomatal Conductance and Compound Volatility in Controlling the Emission of Volatile Organic Compounds from Leaves. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 47–93. [Google Scholar]
- Niinemets, Ü.; Reichstein, M.; Staudt, M.; Seufert, G.; Tenhunen, J.D. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol. 2002, 130, 1371–1385. [Google Scholar] [CrossRef] [Green Version]
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press: San Diego, CA, USA, 2009. [Google Scholar]
- Niinemets, Ü.; Reichstein, M. Controls on the emission of plant volatiles through stomata: Sensitivity or insensitivity of the emission rates to stomatal closure explained. J. Geophys. Res. 2003, 108, 4208. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Verstappen, F.W.A.; Posthumus, M.A.; Dicke, M. Spider mite-Induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-Homoterpene biosynthesis. Plant Physiol. 1999, 121, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappers, I.F.; Verstappen, F.W.A.; Luckerhoff, L.L.P.; Bouwmeester, H.J.; Dicke, M. Genetic variation in jasmonic acid- and spider mite-Induced plant volatile emission of cucumber accessions and attraction of the predator Phytoseiulus persimilis. J. Chem. Ecol. 2010, 36, 500–512. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, H.; Legner, N. Localization of mechanisms involved in hydropassive and hydroactive stomatal responses of Sambucus nigra to dry air. Plant Physiol. 2007, 143, 1068–1077. [Google Scholar] [CrossRef] [Green Version]
- Raschke, K. Stomatal responses to pressure changes and interruptions in the water supply of detached leaves of Zea mays L. Plant Physiol. 1970, 45, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Bak, G.; Lee, E.J.; Lee, Y.; Kato, M.; Segami, S.; Sze, H.; Maeshima, M.; Hwang, J.U.; Lee, Y. Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5-bisphosphate. Plant Cell 2013, 25, 2202–2216. [Google Scholar] [CrossRef] [Green Version]
- Koo, A.J.K.; Gao, X.; Daniel, J.A.; Howe, G.A. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009, 59, 974–986. [Google Scholar] [CrossRef]
- Preston, C.A.; Laue, G.; Baldwin, I.T. Plant–Plant signaling: Application of trans- or cis-Methyl jasmonate equivalent to sagebrush releases does not elicit direct defenses in native tobacco. J. Chem. Ecol. 2004, 30, 2193–2214. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef]
- Wu, J.Q.; Baldwin, I.T. Herbivory-Induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tielas, C.; Graña, E.; Maffei, M.E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Plasma membrane depolarization precedes photosynthesis damage and long-Term leaf bleaching in (E)-Chalcone-Treated Arabidopsis shoots. J. Plant Physiol. 2017, 218, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Chen, L.; Liu, L.; Wan, X. Maintenance of mesophyll potassium and regulation of plasma membrane H+-ATPase are associated with physiological responses of tea plants to drought and subsequent rehydration. Crop J. 2018, 6, 611–620. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D. Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol. 2008, 49, 1092–1111. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Shapiguzov, A.; Vainonen, J.P.; Wrzaczek, M.; Kangasjärvi, J. ROS-Talk-How the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef] [Green Version]
- Repka, V. A bestatin primes grapevine cells for augmented elicitation of the hypersensitive like cell death, associated defense responses by methyl jasmonate. Vitis 2002, 41, 69–76. [Google Scholar]
- Repka, V.; Čarná, M.; Pavlovkin, J. Methyl jasmonate-Induced cell death in grapevine requires both lipoxygenase activity and functional octadecanoid biosynthetic pathway. Biologia 2013, 68, 896–903. [Google Scholar] [CrossRef]
- Popova, L.P.; Vaklinova, S.G. Effect of jasmonic acid on the synthesis of ribulose-1,5-Bisphosphate carboxylase/oxygenase in barley. J. Plant Physiol. 1988, 133, 210–215. [Google Scholar] [CrossRef]
- Popova, L.P.; Tsonev, T.D.; Vaklinova, S.G. Changes in some photosynthetic and photorespiratory properties in barley leaves after treatment with jasmonic acid. J. Plant Physiol. 1988, 132, 257–261. [Google Scholar] [CrossRef]
- Popova, L.; Ananieva, E.; Haristova, V.; Christov, K.; Georgiera, K.; Alexieva, E.; Stoinova, Z. Salicylic acid and methyl jasmonate-Induced protection on photosynthesis to paraquat oxidative stress. Bulg. J. Plant Physiol. 2003, Special Issue, 133–152. [Google Scholar]
- Hüve, K.; Bichele, I.; Rasulov, B.; Niinemets, Ü. When it is too hot for photosynthesis: Heat-Induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 2011, 34, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-Analysis. New Phytol. 2018, 220, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimot, Y. Reactive short-Chain leaf volatiles act as powerful inducers of abiotic stress-Related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-To-Plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, K.A.; Jensen, P.E.; Møller, I.M.; Schulz, A. Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H(2)DCFDA and confocal laser microscopy. Plant Physiol. 2009, 136, 369–383. [Google Scholar] [CrossRef]
- Proietti, S.; Falconieri, G.S.; Bertini, L.; Baccelli, I.; Paccosi, E.; Belardo, A.; Timperio, A.M.; Caruso, C. GLYI4 Plays A Role in Methylglyoxal Detoxification and Jasmonate-Mediated Stress Responses in Arabidopsis thaliana. Biomolecules 2019, 9, 635. [Google Scholar] [CrossRef] [Green Version]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef]
- Kanagendran, A.; Pazouki, L.; Li, S.; Liu, B.; Kännaste, A.; Niinemets, Ü. Ozone-Triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. J. Exp. Bot. 2017, 69, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Kask, K.; Kännaste, A.; Talts, E.; Copolovici, L.; Niinemets, Ü. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant Cell Environ. 2016, 39, 2027–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüve, K.; Christ, M.M.; Kleist, E.; Uerlings, R.; Niinemets, Ü.; Walter, A.; Wildt, J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. J. Exp. Bot. 2007, 58, 1783–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschbaum, M.U.F.; Küppers, M.; Schneider, H.; Giersch, C.; Noe, S. Modelling photosynthesis in fluctuating light with inclusion of stomatal conductance, biochemical activation and pools of key photosynthetic intermediates. Planta 1998, 204, 16–26. [Google Scholar] [CrossRef]
- Robinson, S.P.; Walker, D.A. The significance of light activation of enzymes during the induction phase of photosynthesis in isolated chloroplasts. Arch. Biochem. Biophys. 1980, 202, 617–623. [Google Scholar] [CrossRef]
- Sassenrath-Cole, G.F.; Pearcy, R.W.; Steinmaus, S. The role of enzyme activation state in limiting carbon assimilation under variable light conditions. Photosynth. Res. 1994, 41, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Woodrow, I.E.; Walker, D.A. Light-Mediated activation of stromal sedoheptulose bisphosphate. Biochem. J. 1980, 191, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Barber, J. Molecular basis of the vulnerability of photosystem II to damage by light. Aust. J. Plant Physiol. 1994, 22, 201–208. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar]
- Yadav, D.K.; Pospíšil, P. Evidence on the formation of singlet oxygen in the donor side photoinhibition of Photosystem II: EPR spin-Trapping study. PLoS ONE. 2012, 7, e45883. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Havaux, M.; Strasser, R.J.; Greppin, H. In vivo photoregulation of photochemical and non-Photochemical deactivation of photosystem II in intact plant leaves. Plant Physiol. Biochem. 1990, 28, 735–746. [Google Scholar]
- Johnson, G.N.; Young, A.J.; Horton, P. Activation of non-photochemical quenching in thylakoids and leaves. Planta 1994, 194, 550–556. [Google Scholar] [CrossRef]
- Mano, J.I.; Tokushige, K.; Mizoguchi, H.; Fujii, H.; Khorobrykh, S. Accumulation of lipid peroxide-Derived, toxic α,β-Unsaturated aldehydes (E)-2-pentenal, acrolein and (E)-2-Hexenal in leaves under photoinhibitory illumination. Plant Biotech. 2010, 27, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Choi, Y.B.; Suh, J.; Lee, J.; Assmann, S.M.; Joe, C.O.; Keller, J.F.; Crain, R.C. Abscisic acid-Induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol. 1996, 110, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, Z.Q.; Zhang, L.T.; Sun, M.M.; Lin, T.B. Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica 2014, 52, 377–385. [Google Scholar] [CrossRef]
- Abdelgawad, Z.A.; Khalafaallah, A.A.; Abdallah, M.M. Impact of methyl jasmonate on antioxidant activity and some biochemical aspects of maize plant grown under water stress condition. Agric. Sci. 2014, 5, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Tung, S.A.; Samad, R.A.; Wang, L.C.; Khan, I.; Rehman, N.U.; Shah, N.; Shahzad, B. Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol. Plant. 2016, 38, 25. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A.B.; et al. Estimations of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Ye, J.; Rasulov, B.; Niinemets, Ü. Role of Stomatal Conductance in Modifying the Dose Response of Stress-Volatile Emissions in Methyl Jasmonate Treated Leaves of Cucumber (Cucumis Sativa). Int. J. Mol. Sci. 2020, 21, 1018. https://doi.org/10.3390/ijms21031018
Jiang Y, Ye J, Rasulov B, Niinemets Ü. Role of Stomatal Conductance in Modifying the Dose Response of Stress-Volatile Emissions in Methyl Jasmonate Treated Leaves of Cucumber (Cucumis Sativa). International Journal of Molecular Sciences. 2020; 21(3):1018. https://doi.org/10.3390/ijms21031018
Chicago/Turabian StyleJiang, Yifan, Jiayan Ye, Bahtijor Rasulov, and Ülo Niinemets. 2020. "Role of Stomatal Conductance in Modifying the Dose Response of Stress-Volatile Emissions in Methyl Jasmonate Treated Leaves of Cucumber (Cucumis Sativa)" International Journal of Molecular Sciences 21, no. 3: 1018. https://doi.org/10.3390/ijms21031018
APA StyleJiang, Y., Ye, J., Rasulov, B., & Niinemets, Ü. (2020). Role of Stomatal Conductance in Modifying the Dose Response of Stress-Volatile Emissions in Methyl Jasmonate Treated Leaves of Cucumber (Cucumis Sativa). International Journal of Molecular Sciences, 21(3), 1018. https://doi.org/10.3390/ijms21031018




