Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages
Abstract
1. Introduction
2. Results
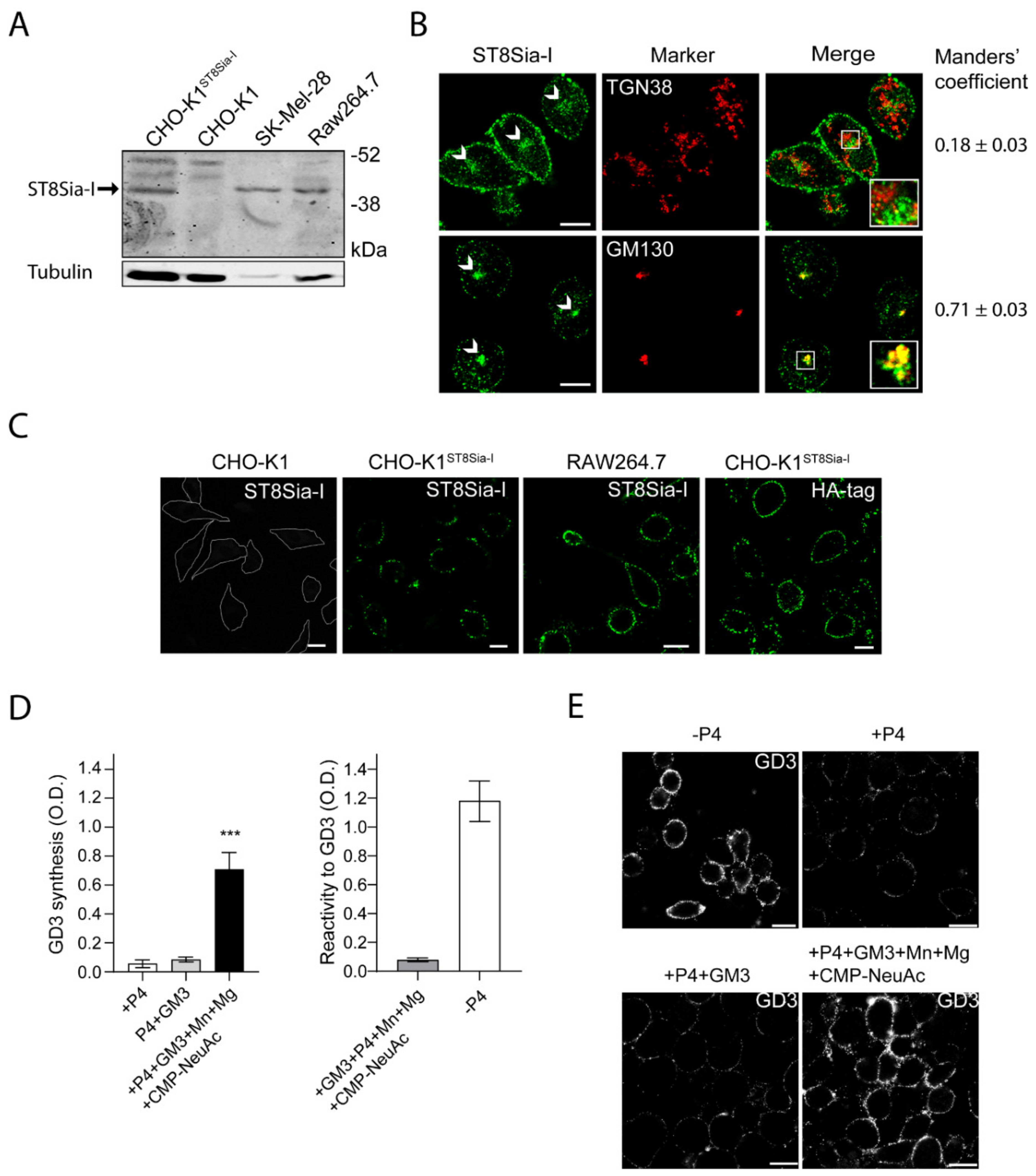
2.1. Endogenous ST8Sia-I Subcellular Localization in Macrophages
2.2. Expression and Cis-activity of Ecto-ST8Sia-I in Macrophages Stimulated by LPS
2.3. The Alteration in ST8Sia-I Expression Correlates with a Reduction of GD3 and GM1 and with an Increment of GD1a at the Plasma Membrane
2.4. Relative Levels of NEU1, NEU3 and ST3Gal-II Transcripts Analyzed by RT-qPCR
2.5. NO Formation is Involved in the Expression/Localization of ST8Sia-I at the Plasma Membrane
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Stimulation
4.2. Assay of NO Production
4.3. Antibodies
4.4. GD3 Detection by ELISA
4.5. Determination of ecto-ST8Sia-I Activity at the Cell Surface
4.6. Confocal Immunofluorescence Microscopy
4.7. Electrophoresis and Immunoblotting
4.8. Lipid Labeling, Extraction, and Chromatography
4.9. RNA Isolation and cDNA Synthesis
4.10. qPCR (Quantitative Real-time PCR)
4.11. Statistical Analyses
4.12. Image Processing
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniotti, J.L.; Iglesias-Bartolome, R. Metabolic pathways and intracellular trafficking of gangliosides. IUBMB Life 2011, 63, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, G. Ganglioside/glycosphingolipid turnover: New concepts. Glycoconj. J. 2004, 20, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Miljan, E.A.; Bremer, E.G. Regulation of growth factor receptors by gangliosides. Sci. Signal. 2002, 2002, RE15. [Google Scholar] [CrossRef]
- Proia, R.L. Glycosphingolipid functions: Insights from engineered mouse models. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Regina Todeschini, A.; Hakomori, S.I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Zurita, A.R.; Maccioni, H.J.; Daniotti, J.L. Modulation of epidermal growth factor receptor phosphorylation by endogenously expressed gangliosides. Biochem. J. 2001, 355 Pt. 2, 465–472. [Google Scholar] [CrossRef]
- Yo, S.; Hamamura, K.; Mishima, Y.; Hamajima, K.; Mori, H.; Furukawa, K.; Kondo, H.; Tanaka, K.; Sato, T.; Miyazawa, K.; et al. Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging. Int. J. Mol. Sci. 2019, 20, 2825. [Google Scholar] [CrossRef]
- Inokuchi, J.; Nagafuku, M.; Ohno, I.; Suzuki, A. Distinct selectivity of gangliosides required for CD4+ T and CD8+ T cell activation. Biochim. Biophys. Acta 2015, 1851, 98–106. [Google Scholar] [CrossRef]
- Hamamura, K.; Furukawa, K.; Hayashi, T.; Hattori, T.; Nakano, J.; Nakashima, H.; Okuda, T.; Mizutani, H.; Hattori, H.; Ueda, M.; et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11041–11046. [Google Scholar] [CrossRef]
- Hashimoto, N.; Hamamura, K.; Kotani, N.; Furukawa, K.; Kaneko, K.; Honke, K.; Furukawa, K. Proteomic analysis of ganglioside-associated membrane molecules: Substantial basis for molecular clustering. Proteomics 2012, 12, 3154–3163. [Google Scholar] [CrossRef]
- Daniotti, J.L.; Lardone, R.D.; Vilcaes, A.A. Dysregulated Expression of Glycolipids in Tumor Cells: From Negative Modulator of Anti-tumor Immunity to Promising Targets for Developing Therapeutic Agents. Front. Oncol. 2015, 5, 300. [Google Scholar] [CrossRef]
- Chung, T.W.; Choi, H.J.; Park, M.J.; Choi, H.J.; Lee, S.O.; Kim, K.J.; Kim, C.H.; Hong, C.; Kim, K.H.; Joo, M.; et al. The function of cancer-shed gangliosides in macrophage phenotype: Involvement with angiogenesis. Oncotarget 2017, 8, 4436–4448. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwak, C.H.; Ha, S.H.; Kwon, K.M.; Abekura, F.; Cho, S.H.; Chang, Y.C.; Lee, Y.C.; Ha, K.T.; Chung, T.W.; et al. Ganglioside GM3 Suppresses Lipopolysaccharide-Induced Inflammatory Responses In RAW 264.7 Macrophage Cells Through NF-κB, AP-1 And MAPKs Signaling. J. Cell Biochem. 2018, 119, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Cao, F.; Qin, Y.; Li, W.; Zhang, J. Ganglioside GD1a suppresses LPS-induced pro-inflammatory cytokines in RAW264.7 macrophages by reducing MAPKs and NF-κB signaling pathways through TLR4. Int. Immunopharmacol. 2015, 28, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Pyo, H.K.; Yang, M.S.; Ji, K.A.; Jou, I.; Joe, E.H. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia 2004, 48, 197–206. [Google Scholar] [CrossRef]
- Yohe, H.C.; Ryan, J.L. Ganglioside expression in macrophages from endotoxin responder and nonresponder mice. J. Immunol. 1986, 137, 3921–3927. [Google Scholar]
- Yohe, H.C.; Ye, S.; Reinhold, B.B.; Reinhold, V.N. Structural characterization of the disialogangliosides of murine peritoneal macrophages. Glycobiology 1997, 7, 1215–1227. [Google Scholar] [CrossRef][Green Version]
- Maccioni, H.J. Glycosylation of glycolipids in the Golgi complex. J. Neurochem. 2007, 103 (Suppl. 1), 81–90. [Google Scholar] [CrossRef]
- Crespo, P.M.; Iglesias-Bartolome, R.; Daniotti, J.L. Ganglioside GD3 traffics from the trans-Golgi network to plasma membrane by a Rab11-independent and brefeldin A-insensitive exocytic pathway. J. Biol. Chem. 2004, 279, 47610–47618. [Google Scholar] [CrossRef]
- De Matteis, M.A.; Luini, A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 2008, 9, 273–284. [Google Scholar] [CrossRef]
- Van Meer, G.; Holthuis, J.C. Sphingolipid transport in eukaryotic cells. Biochim. Biophys. Acta 2000, 1486, 145–170. [Google Scholar] [CrossRef]
- Crespo, P.M.; Demichelis, V.T.; Daniotti, J.L. Neobiosynthesis of glycosphingolipids by plasma membrane-associated glycosyltransferases. J. Biol. Chem. 2010, 285, 29179–29190. [Google Scholar] [CrossRef] [PubMed]
- Vilcaes, A.A.; Demichelis, V.T.; Daniotti, J.L. Trans-activity of plasma membrane-associated ganglioside sialyltransferase in mammalian cells. J. Biol. Chem. 2011, 286, 31437–31446. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Nasirikenari, M.; Manhardt, C.T.; Ashline, D.J.; Hanneman, A.J.; Reinhold, V.N.; Lau, J.T. Platelets support extracellular sialylation by supplying the sugar donor substrate. J. Biol. Chem. 2014, 289, 8742–8748. [Google Scholar] [CrossRef]
- Stamatos, N.M.; Carubelli, I.; van de Vlekkert, D.; Bonten, E.J.; Papini, N.; Feng, C.; Venerando, B.; d’Azzo, A.; Cross, A.S.; Wang, L.X.; et al. LPS-induced cytokine production in human dendritic cells is regulated by sialidase activity. J. Leukoc. Biol. 2010, 88, 1227–1239. [Google Scholar] [CrossRef]
- Wu, Y.; Lan, C.; Ren, D.; Chen, G.Y. Induction of Siglec-1 by Endotoxin Tolerance Suppresses the Innate Immune Response by Promoting TGF-β1 Production. J. Biol. Chem. 2016, 291, 12370–12382. [Google Scholar] [CrossRef]
- Shell, S.A.; Hesse, C.; Morris, S.M., Jr.; Milcarek, C. Elevated levels of the 64-kDa cleavage stimulatory factor (CstF-64) in lipopolysaccharide-stimulated macrophages influence gene expression and induce alternative poly(A) site selection. J. Biol. Chem. 2005, 280, 39950–39961. [Google Scholar] [CrossRef]
- Karmakar, J.; Roy, S.; Mandal, C. Modulation of TLR4 Sialylation Mediated by a Sialidase Neu1 and Impairment of Its Signaling in. Front. Immunol. 2019, 10, 2360. [Google Scholar] [CrossRef]
- Okada, M.; Furukawa, K.; Yamashiro, S.; Yamada, Y.; Haraguchi, M.; Horibe, K.; Kato, K.; Tsuji, Y.; Furukawa, K. High expression of ganglioside alpha-2,8-sialyltransferase (GD3 synthase) gene in adult T-cell leukemia cells unrelated to the gene expression of human T-lymphotropic virus type I. Cancer Res. 1996, 56, 2844–2848. [Google Scholar]
- Daniotti, J.L.; Martina, J.A.; Giraudo, C.G.; Zurita, A.R.; Maccioni, H.J. GM3 alpha2,8-sialyltransferase (GD3 synthase): Protein characterization and sub-golgi location in CHO-K1 cells. J. Neurochem. 2000, 74, 1711–1720. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Chen, M.; Li, H.; Bi, W. The activation of macrophage and upregulation of CD40 costimulatory molecule in lipopolysaccharide-induced acute lung injury. J. Biomed. Biotechnol. 2008, 2008, 852571. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wilson, C.A.; Roberts, K.L.; Baker, B.J.; Zhao, X.; Benveniste, E.N. LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood 2005, 106, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, F.M.; Vilcaes, A.A.; Iglesias-Bartolomé, R.; Daniotti, J.L. Critical role of evolutionarily conserved glycosylation at Asn211 in the intracellular trafficking and activity of sialyltransferase ST3Gal-II. Biochem. J. 2015, 469, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Sieve, I.; Ricke-Hoch, M.; Kasten, M.; Battmer, K.; Stapel, B.; Falk, C.S.; Leisegang, M.S.; Haverich, A.; Scherr, M.; Hilfiker-Kleiner, D. A positive feedback loop between IL-1β, LPS and NEU1 may promote atherosclerosis by enhancing a pro-inflammatory state in monocytes and macrophages. Vasc. Pharmacol. 2018, 103–105, 16–28. [Google Scholar] [CrossRef]
- Jou, I.; Lee, J.H.; Park, S.Y.; Yoon, H.J.; Joe, E.H.; Park, E.J. Gangliosides Trigger Inflammatory Responses via TLR4 in Brain Glia. Am. J. Pathol. 2006, 168, 1619–1630. [Google Scholar] [CrossRef]
- Nikolaeva, S.; Bayunova, L.; Sokolova, T.; Vlasova, Y.; Bachteeva, V.; Avrova, N.; Parnova, R. GM1 and GD1a gangliosides modulate toxic and inflammatory effects of E. coli lipopolysaccharide by preventing TLR4 translocation into lipid rafts. Biochim. Biophys. Acta 2015, 1851, 239–247. [Google Scholar] [CrossRef]
- Pyo, H. Gangliosides activate cultured rat brain microglia. J. Biol. Chem. 1999, 274, 34584–34589. [Google Scholar] [CrossRef]
- Bharti, A.C.; Singh, S.M. Inhibition of macrophage nitric oxide production by gangliosides derived from a spontaneous T cell lymphoma: The involved mechanisms. Nitric Oxide 2003, 8, 75–82. [Google Scholar] [CrossRef]
- Giraudo, C.G.; Maccioni, H.J. Ganglioside glycosyltransferases organize in distinct multienzyme complexes in CHO-K1 cells. J. Biol. Chem. 2003, 278, 40262–40271. [Google Scholar] [CrossRef]
- Nakayama, J. Expression cloning of a human GT3 synthase. GD3 AND GT3 are synthesized by a single enzyme. J. Biol. Chem. 1996, 271, 3684–3691. [Google Scholar] [CrossRef]
- Crespo, P.M.; Zurita, A.R.; Daniotti, J.L. Effect of gangliosides on the distribution of a glycosylphosphatidylinositol-anchored protein in plasma membrane from Chinese hamster ovary-K1 cells. J. Biol. Chem. 2002, 277, 44731–44739. [Google Scholar] [CrossRef]
- Ruggiero, F.M.; Vilcaes, A.A.; Yuki, N.; Daniotti, J.L. Membrane binding, endocytic trafficking and intracellular fate of high-affinity antibodies to gangliosides GD1a and GM1. Biochim. Biophys. Acta Biomembr. 2017, 1859, 80–93. [Google Scholar] [CrossRef]
- Pannu, R. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: Implications for neuroinflammatory diseases. J. Neurosci. 2004, 24, 5942–5954. [Google Scholar] [CrossRef]
- Giraudo, C.G.; Daniotti, J.L.; Maccioni, H.J. Physical and functional association of glycolipid N-acetyl-galactosaminyl and galactosyl transferases in the Golgi apparatus. Proc. Natl. Acad. Sci. USA 2001, 98, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Kouno, T. Specific enzyme complex of beta-1,4-galactosyltransferase-II and glucuronyltransferase-P facilitates biosynthesis of N-linked human natural killer-1 (HNK-1) carbohydrate. J. Biol. Chem. 2011, 286, 31337–31346. [Google Scholar] [CrossRef] [PubMed]
- Seko, A.; Yamashita, K. Characterization of a novel galactose beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8): The complex formation of beta3Gn-T2 and beta3Gn-T8 enhances enzymatic activity. Glycobiology 2005, 15, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J. Biol. Chem. 1999, 274, 5004–5011. [Google Scholar] [CrossRef]
- Pshezhetsky, A.V. Cloning, expression and chromosomal mapping of human lysosomal sialidase and characterization of mutations in sialidosis. Nat. Genet. 1997, 15, 316–320. [Google Scholar] [CrossRef]
- Tringali, C. Properties of recombinant human cytosolic sialidase HsNEU2. The enzyme hydrolyzes monomerically dispersed GM1 ganglioside molecules. J. Biol. Chem. 2004, 279, 3169–3179. [Google Scholar] [CrossRef]
- Yamaguchi, K. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem. J. 2005, 390 Pt. 1, 85–93. [Google Scholar] [CrossRef]
- Nan, X.; Carubelli, I.; Stamatos, N.M. Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J. Leukoc. Biol. 2007, 81, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Walker, M.; Daniotti, J.L. Human Sialidase Neu3 is S-Acylated and Behaves Like an Integral Membrane Protein. Sci. Rep. 2017, 7, 4167. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T. Aberrant expression of sialidase and cancer progression. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Amith, S.R. Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj. J. 2009, 26, 1197–1212. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Schwarzmann, G.; Chatterjee, S. GD3 recruits reactive oxygen species to induce cell proliferation and apoptosis in human aortic smooth muscle cells. J. Biol. Chem. 2002, 277, 16396–16402. [Google Scholar] [CrossRef]
- Preti, A. Occurrence of sialyltransferase activity in the synaptosomal membranes prepared from calf brain cortex. J. Neurochem. 1980, 35, 281–296. [Google Scholar] [CrossRef]
- Matsui, Y. Surface glycosyltransferase activities during development of neuronal cell cultures. J. Neurochem. 1986, 46, 144–150. [Google Scholar] [CrossRef]
- Kim, J.K. GD3 accumulation in cell surface lipid rafts prior to mitochondrial targeting contributes to amyloid-beta-induced apoptosis. J. Korean Med. Sci. 2010, 25, 1492–1498. [Google Scholar] [CrossRef]
- Rifat, S. Expression of sialyltransferase activity on intact human neutrophils. J. Leukoc. Biol. 2008, 84, 1075–1081. [Google Scholar] [CrossRef]
- Ohnishi, T.; Muroi, M.; Tanamoto, K. N-linked glycosylations at Asn(26) and Asn(114) of human MD-2 are required for toll-like receptor 4-mediated activation of NF-kappaB by lipopolysaccharide. J. Immunol. 2001, 167, 3354–3359. [Google Scholar] [CrossRef]
- Trottein, F. Glycosyltransferase and sulfotransferase gene expression profiles in human monocytes, dendritic cells and macrophages. Glycoconj. J. 2009, 26, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.G.; Fritz, V.M.R.; Maccioni, H.J. GA2/GM2/GD2 synthase localizes to the trans-golgi network of CHO-K1 cells. Biochem. J. 1999, 342 Pt. 3, 633–640. [Google Scholar] [CrossRef]
- Tveit, H. A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells. Traffic 2009, 10, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Saraste, J. Emerging new roles of the pre-Golgi intermediate compartment in biosynthetic-secretory trafficking. FEBS Lett. 2009, 583, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart, J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yan, L.J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J. Biochem. Pharmacol. Res. 2013, 1, 15–26. [Google Scholar]
- Pfeiffer, S. Protein tyrosine nitration in mouse peritoneal macrophages activated in vitro and in vivo: Evidence against an essential role of peroxynitrite. FASEB J. 2001, 15, 2355–2364. [Google Scholar] [CrossRef]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef]
- Souza, J.M.; Choi, I.; Chen, Q.; Weisse, M.; Daikhin, E.; Yudkoff, M.; Obin, M.; Ara, J.; Horwitz, J.; Ischiropoulos, H. Proteolytic degradation of tyrosine nitrated proteins. Arch. Biochem. Biophys. 2000, 380, 360–366. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, J.; Ma, Q.; Gao, X.; Ren, J.; Xue, Y. GPS-YNO2: Computational prediction of tyrosine nitration sites in proteins. Mol. Biosyst. 2011, 7, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, X.; Wen, L.S.; Wu, L.Y.; Deng, N.Y.; Chou, K.C. iNitro-Tyr: Prediction of nitrotyrosine sites in proteins with general pseudo amino acid composition. PLoS ONE 2014, 9, e105018. [Google Scholar] [CrossRef]
- Rodriguez-Walker, M.; Vilcaes, A.A.; Garbarino-Pico, E.; Daniotti, J.L. Role of plasma membrane-bound sialidase NEU3 in clathrin-mediated endocytosis. Biochem. J. 2015, 470, 131–144. [Google Scholar] [CrossRef]
- Garbarino-Pico, E.; Niu, S.; Rollag, M.D.; Strayer, C.A.; Besharse, J.C.; Green, C.B. Immediate early response of the circadian polyA ribonuclease nocturnin to two extracellular stimuli. RNA 2007, 13, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilcaes, A.A.; Garbarino-Pico, E.; Torres Demichelis, V.; Daniotti, J.L. Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages. Int. J. Mol. Sci. 2020, 21, 1063. https://doi.org/10.3390/ijms21031063
Vilcaes AA, Garbarino-Pico E, Torres Demichelis V, Daniotti JL. Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages. International Journal of Molecular Sciences. 2020; 21(3):1063. https://doi.org/10.3390/ijms21031063
Chicago/Turabian StyleVilcaes, Aldo A., Eduardo Garbarino-Pico, Vanina Torres Demichelis, and Jose L. Daniotti. 2020. "Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages" International Journal of Molecular Sciences 21, no. 3: 1063. https://doi.org/10.3390/ijms21031063
APA StyleVilcaes, A. A., Garbarino-Pico, E., Torres Demichelis, V., & Daniotti, J. L. (2020). Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages. International Journal of Molecular Sciences, 21(3), 1063. https://doi.org/10.3390/ijms21031063




