Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Results
2.1. 5-HT-ir Descending Fibres in the E17.5 SOD1G93A SC Versus the E17.5 WT SC
2.2. 5-HT Content in the Lumbar SC
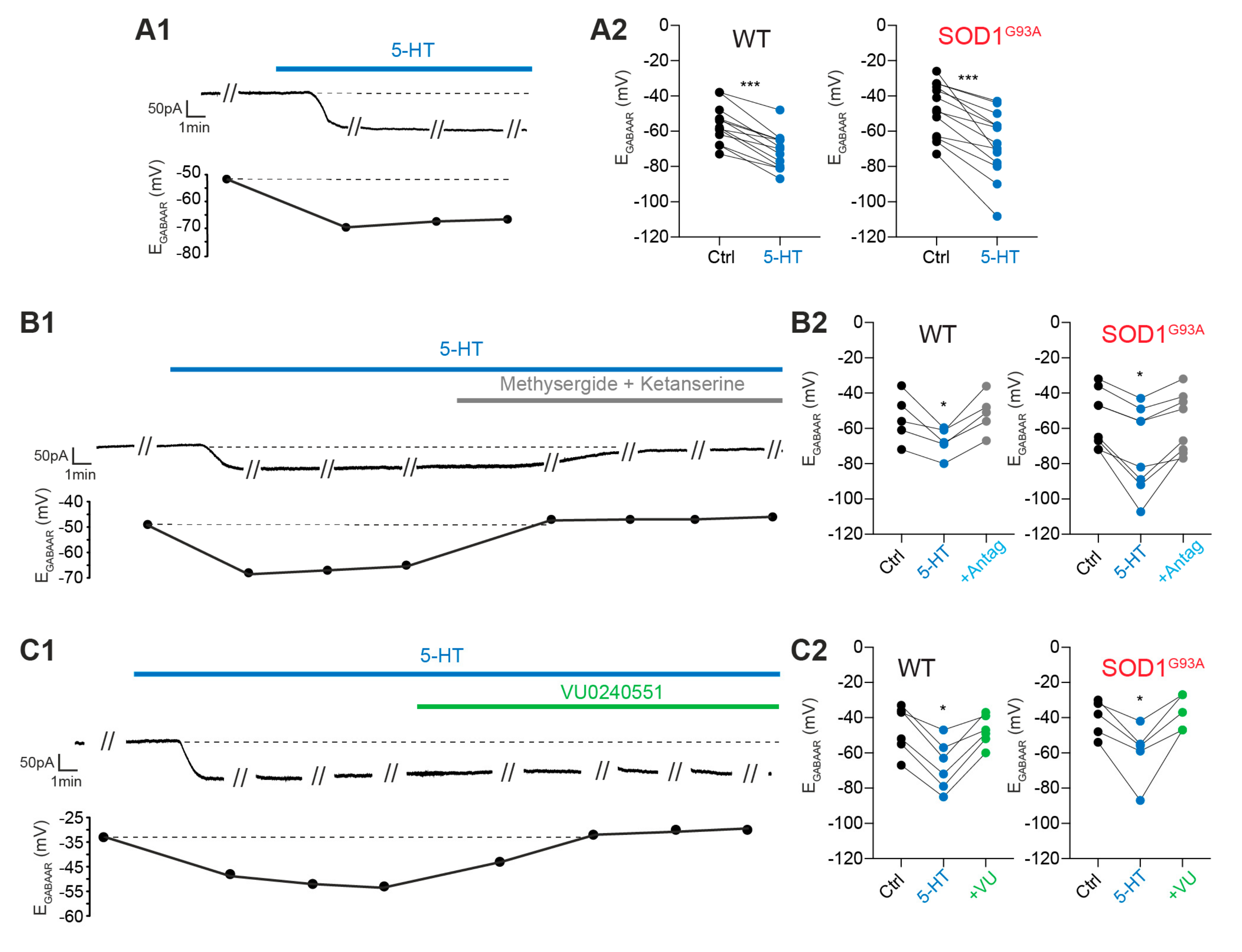
2.3. Modulation of the EGABAAR by 5-HT
2.3.1. Exogenous 5-HT Hyperpolarizes the EGABAAR
2.3.2. 5-HT2R Is Involved in EGABAAR Hyperpolarization
2.3.3. KCC2 Is Involved in EGABAAR Hyperpolarization
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations and Mouse Model
4.2. Dissection and Isolation of the ex vivo Embryonic Spinal Cord
4.3. Immunohistochemistry
4.4. Confocal Microscopy
4.5. Electrophysiological Procedures and Data Analysis
4.6. Tissue Collection and Postmortem High-performance Liquid Chromatography (HPLC) Measurements
4.7. Pharmacology
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| 5-HT | 5-hydroxytryptamine |
| 5-HT-ir | 5-HT-immunoreactive |
| 5-HTR | 5-HT receptor |
| CNS | central nervous system |
| ECl | chloride ion equilibrium |
| EGABAAR | reversal potential of GABAAR |
| HPLC | high-performance liquid chromatography |
| KCC2 | K+-Cl− cotransporter type 2 |
| NKCC1 | Na+-K+-2Cl− cotransporter isoform1 |
| MN | motoneuron |
| SC | spinal cord |
| SOD1 | superoxide dismutase 1 |
| TTX | tetrodotoxin |
References
- Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Modelling amyotrophic lateral sclerosis: Progress and possibilities. Dis. Model. Mech. 2017, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, L.; Ghilardi, A.; Rottoli, E.; De Maglie, M.; Prosperi, L.; Perego, C.; Baruscotti, M.; Bucchi, A.; Del Giacco, L.; Francolini, M. INaP selective inhibition reverts precocious inter- and motorneurons hyperexcitability in the Sod1-G93R zebrafish ALS model. Sci. Rep. 2016, 6, 24515. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Cazenave, W.; Cattaert, D.; Branchereau, P. Embryonic alteration of motoneuronal morphology induces hyperexcitability in the mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 54, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Koschnitzky, J.E.; Quinlan, K.A.; Lukas, T.J.; Kajtaz, E.; Kocevar, E.J.; Mayers, W.F.; Siddique, T.; Heckman, C.J. Effect of fluoxetine on disease progression in a mouse model of ALS. J. Neurophysiol. 2014, 111, 2164–2176. [Google Scholar] [CrossRef]
- Rubenstein, J.L. Development of serotonergic neurons and their projections. Biol. Psychiatry 1998, 44, 145–150. [Google Scholar] [CrossRef]
- Vermeiren, Y.; Janssens, J.; Van Dam, D.; De Deyn, P.P. Serotonergic Dysfunction in Amyotrophic Lateral Sclerosis and Parkinson’s Disease: Similar Mechanisms, Dissimilar Outcomes. Front. Neurosci. 2018, 12, 185. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 1964, 62, 1–55. [Google Scholar]
- Lidov, H.G.; Molliver, M.E. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res. Bull. 1982, 9, 559–604. [Google Scholar] [CrossRef]
- Ye, W.; Shimamura, K.; Rubenstein, J.L.; Hynes, M.A.; Rosenthal, A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 1998, 93, 755–766. [Google Scholar] [CrossRef]
- Rajaofetra, N.; Sandillon, F.; Geffard, M.; Privat, A. Pre- and post-natal ontogeny of serotonergic projections to the rat spinal cord. J. Neurosci. Res. 1989, 22, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Ballion, B.; Branchereau, P.; Chapron, J.; Viala, D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res. Dev. Brain Res. 2002, 137, 81–88. [Google Scholar] [CrossRef]
- Wallace, J.A.; Lauder, J.M. Development of the serotonergic system in the rat embryo: An immunocytochemical study. Brain Res. Bull. 1983, 10, 459–479. [Google Scholar] [CrossRef]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. 2003, 4, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Branchereau, P.; Chapron, J.; Meyrand, P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J. Neurosci. 2002, 22, 2598–2606. [Google Scholar] [CrossRef]
- Allain, A.E.; Meyrand, P.; Branchereau, P. Ontogenic changes of the spinal GABAergic cell population are controlled by the serotonin (5-HT) system: Implication of 5-HT1 receptor family. J. Neurosci. 2005, 25, 8714–8724. [Google Scholar] [CrossRef]
- Delpy, A.; Allain, A.E.; Meyrand, P.; Branchereau, P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J. Physiol. 2008, 586, 1059–1075. [Google Scholar] [CrossRef]
- Russell, J.M. Sodium-potassium-chloride cotransport. Physiol. Rev. 2000, 80, 211–276. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Fogarty, M.J. Driven to decay: Excitability and synaptic abnormalities in amyotrophic lateral sclerosis. Brain Res. Bull. 2018, 140, 318–333. [Google Scholar] [CrossRef]
- Branchereau, P.; Martin, E.; Allain, A.E.; Cazenave, W.; Supiot, L.; Hodeib, F.; Laupenie, A.; Dalvi, U.; Zhu, H.; Cattaert, D. Relaxation of synaptic inhibitory events as a compensatory mechanism in fetal SOD spinal motor networks. Elife 2019, 8. [Google Scholar] [CrossRef]
- Bos, R.; Sadlaoud, K.; Boulenguez, P.; Buttigieg, D.; Liabeuf, S.; Brocard, C.; Haase, G.; Bras, H.; Vinay, L. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc. Natl. Acad. Sci. USA 2013, 110, 348–353. [Google Scholar] [CrossRef]
- Brustein, E.; Drapeau, P. Serotoninergic modulation of chloride homeostasis during maturation of the locomotor network in zebrafish. J. Neurosci. 2005, 25, 10607–10616. [Google Scholar] [CrossRef] [PubMed]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Oueghlani, Z.; Juvin, L.; Lambert, F.M.; Cardoit, L.; Courtand, G.; Masmejean, F.; Cazalets, J.R.; Barriere, G. Serotonergic modulation of sacral dorsal root stimulation-induced locomotor output in newborn rat. Neuropharmacology 2019. [Google Scholar] [CrossRef] [PubMed]
- Slawinska, U.; Miazga, K.; Jordan, L.M. The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiol. Exp. 2014, 74, 172–187. [Google Scholar]
- Slawinska, U.; Miazga, K.; Jordan, L.M. 5-HT(2) and 5-HT(7) receptor agonists facilitate plantar stepping in chronic spinal rats through actions on different populations of spinal neurons. Front. Neural Circuits 2014, 8, 95. [Google Scholar] [CrossRef]
- Murray, K.C.; Nakae, A.; Stephens, M.J.; Rank, M.; D’Amico, J.; Harvey, P.J.; Li, X.; Harris, R.L.; Ballou, E.W.; Anelli, R.; et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 2010, 16, 694–700. [Google Scholar] [CrossRef]
- Murray, K.C.; Stephens, M.J.; Ballou, E.W.; Heckman, C.J.; Bennett, D.J. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J. Neurophysiol. 2011, 105, 731–748. [Google Scholar] [CrossRef]
- Lucas-Osma, A.M.; Li, Y.; Murray, K.; Lin, S.; Black, S.; Stephens, M.J.; Ahn, A.H.; Heckman, C.J.; Fenrich, K.K.; Fouad, K.; et al. 5-HT1D receptors inhibit the monosynaptic stretch reflex by modulating C-fiber activity. J. Neurophysiol. 2019, 121, 1591–1608. [Google Scholar] [CrossRef]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharm. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Zifa, E.; Fillion, G. 5-Hydroxytryptamine receptors. Pharm. Rev. 1992, 44, 401–458. [Google Scholar] [PubMed]
- Navailles, S.; De Deurwaerdere, P. Presynaptic control of serotonin on striatal dopamine function. Psychopharmacol. (Berl.) 2011, 213, 213–242. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R. Serotonergic mechanisms in amyotrophic lateral sclerosis. Int. J. Neurosci. 2006, 116, 775–826. [Google Scholar] [CrossRef]
- Milan, L.; Barriere, G.; De Deurwaerdere, P.; Cazalets, J.R.; Bertrand, S.S. Monoaminergic control of spinal locomotor networks in SOD1G93A newborn mice. Front. Neural Circuits 2014, 8, 77. [Google Scholar] [CrossRef]
- Dentel, C.; Palamiuc, L.; Henriques, A.; Lannes, B.; Spreux-Varoquaux, O.; Gutknecht, L.; Rene, F.; Echaniz-Laguna, A.; Gonzalez de Aguilar, J.L.; Lesch, K.P.; et al. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: A link to spasticity. Brain 2013, 136, 483–493. [Google Scholar] [CrossRef]
- El Oussini, H.; Scekic-Zahirovic, J.; Vercruysse, P.; Marques, C.; Dirrig-Grosch, S.; Dieterle, S.; Picchiarelli, G.; Sinniger, J.; Rouaux, C.; Dupuis, L. Degeneration of serotonin neurons triggers spasticity in amyotrophic lateral sclerosis. Ann. Neurol. 2017. [Google Scholar] [CrossRef]
- Tramu, G.; Pillez, A.; Leonardelli, J. Serotonin axons of the ependyma and circumventricular organs in the forebrain of the guinea pig. An immunohistochemical study. Cell Tissue Res. 1983, 228, 297–311. [Google Scholar] [CrossRef]
- Bormann, J.; Hamill, O.P.; Sakmann, B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 1987, 385, 243–286. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.X.; Ziskind-Conhaim, L. Development of glycine- and GABA-gated currents in rat spinal motoneurons. J. Neurophysiol. 1995, 74, 113–121. [Google Scholar] [CrossRef]
- Fitoussi, A.; Dellu-Hagedorn, F.; De Deurwaerdere, P. Monoamines tissue content analysis reveals restricted and site-specific correlations in brain regions involved in cognition. Neuroscience 2013, 255, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Dellu-Hagedorn, F.; Fitoussi, A.; De Deurwaerdere, P. Correlative analysis of dopaminergic and serotonergic metabolism across the brain to study monoaminergic function and interaction. J. Neurosci. Methods 2017, 280, 54–63. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, E.; Cazenave, W.; Allain, A.-E.; Cattaert, D.; Branchereau, P. Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 1107. https://doi.org/10.3390/ijms21031107
Martin E, Cazenave W, Allain A-E, Cattaert D, Branchereau P. Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences. 2020; 21(3):1107. https://doi.org/10.3390/ijms21031107
Chicago/Turabian StyleMartin, Elodie, William Cazenave, Anne-Emilie Allain, Daniel Cattaert, and Pascal Branchereau. 2020. "Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis" International Journal of Molecular Sciences 21, no. 3: 1107. https://doi.org/10.3390/ijms21031107
APA StyleMartin, E., Cazenave, W., Allain, A.-E., Cattaert, D., & Branchereau, P. (2020). Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences, 21(3), 1107. https://doi.org/10.3390/ijms21031107




