Phyllotaxis Turns Over a New Leaf—A New Hypothesis
Abstract
1. Introduction
2. Hechtian Adhesion
3. Is the Hechtian Oscillator Just an Hypothesis? Direct Evidence
4. Auxin Activity Is a Proxy for the Hechtian Oscillator
5. A Molecular Pin-Ball Machine Regulates Ion Fluxes at the Plasma Membrane
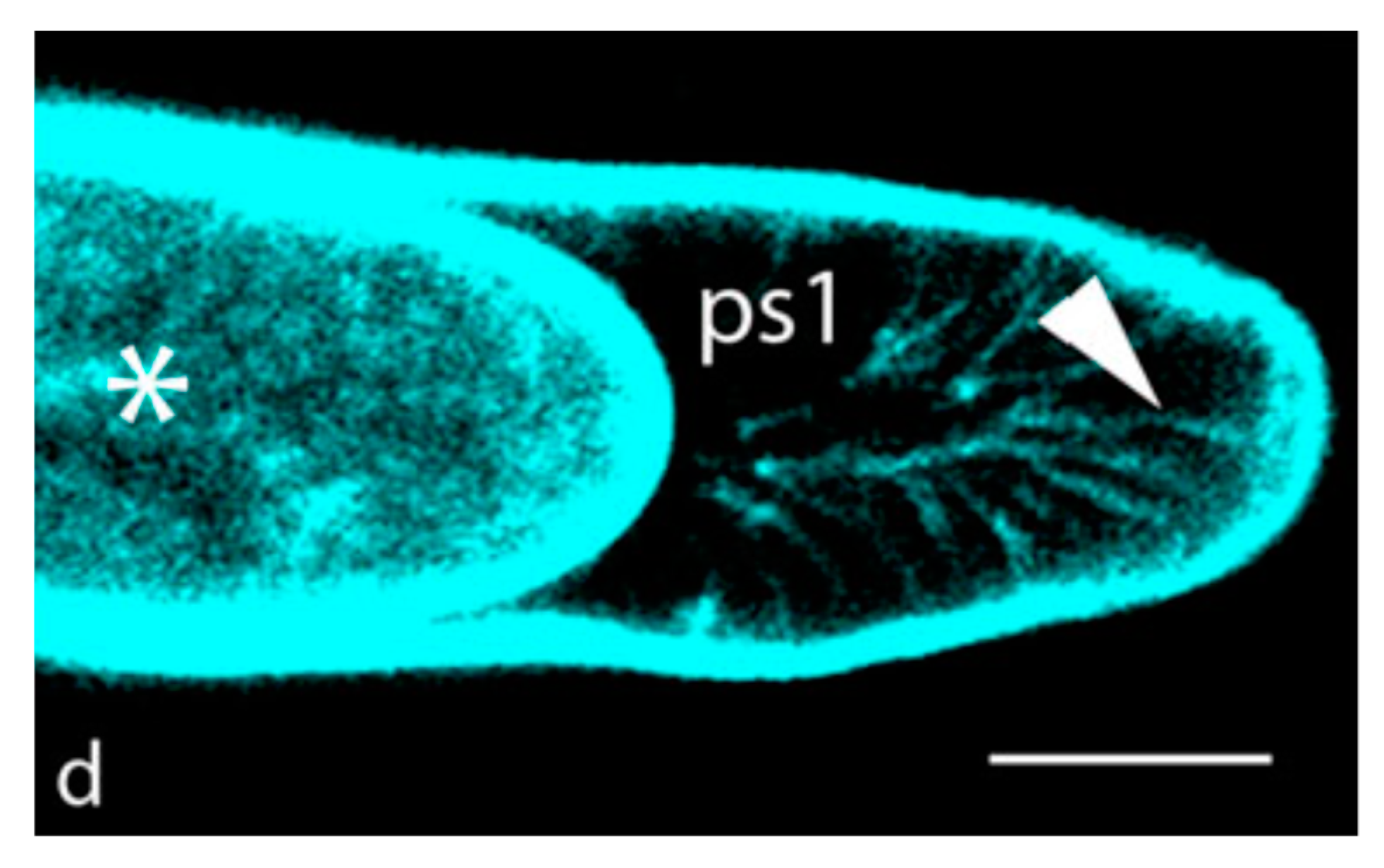
6. Transduction of the Stress Vector through the Protoderm
7. Stress, PIN Protein Redirection and Auxin Waves
8. Cell Wall Rheology
9. A Phyllotaxis Algorithm
- AGP-Ca2+ capacitor: AGPc;
- Stem apical meristem protoderm annulus radius: SAMPa;
- PP proton pump activity: PP;
- Auxin efflux activity: Aefflux (hence auxin levels: Aux);
- Ca2+ channels: Cch;
- Exocytosis is a complex variable regulated by Ca2+ influx;
- Hechtian adhesion: Had;
- Cell wall stress vector: CWsv;
10. Evolutionary Origin of Angiosperm Phyllotaxis
11. D’Arcy Thompson, Alan Turing and Peter Mitchell Revisited
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arber, A. The Natural Philosophy of Plant Form; Cambridge University Press: Cambridge, UK, 1950. [Google Scholar]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewksi, M.J. The Role of the Primary Cell Wall in Plant Morphogenesis. Int. J. Mol. Sci. 2018, 19, 2674. [Google Scholar] [CrossRef]
- Darwin, C. The Power of Movement in Plants; John Murray: London, UK, 1880. [Google Scholar]
- Pierson, E.S.; Li, Y.Q.; Zhang, H.Q.; Willemse, M.T.M.; Linskens, H.F.; Cresti, M. Pulsatory growth of pollen tubes: Investigation of a possible relationship with the periodic distribution of cell wall components. Acta Bot. Neerl. 1995, 44, 121–128. [Google Scholar] [CrossRef]
- Zoulias, N.; Duttke, S.H.C.; Garces, H.; Spencer, V.; Kim, M. The Role of Auxin in the Pattern Formation of the Asteraceae Flower Head (Capitulum). Plant Physiol. 2019, 179, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Pennybacker, M.F.; Shipman, P.D.; Newell, A.C. Phyllotaxis: Some progress, but a story far from over. Physica D 2015, 306, 48–81. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewksi, M.J. Pollen tube growth and guidance: Occam’s razor sharpened on a molecular arabinogalactan glycoprotein Rosetta Stone. New Phytol. 2018, 217, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Geitmann, A. Mechanical principles governing pollen tube growth. Funct. Plant Sci. Biotechnol. 2007, 1, 232–245. [Google Scholar]
- Adler, I.; Barabe, D.; Jean, R.V. A history of the study of Phyllotaxis. Ann. Bot. 1997, 80, 231–244. [Google Scholar] [CrossRef]
- Okabe, T. The riddle of phyllotaxis: Exquisite control of divergence angle. Acta Soc. Bot. Pol. 2016, 85, 3527. [Google Scholar] [CrossRef]
- Sachs, T. The Control of the Patterned Differentiation of Vascular Tissues. Adv. Bot. Res. 1981, 9, 151–262. [Google Scholar]
- Reinhardt, D.; Pesce, E.-R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef]
- Heisler, G.; Ohno, C.; Das, P.; Sieber, P.; Reddy, G.V.; Long, J.A.; Meyerowitz, E.M. Patterns of Auxin Transport and Gene Expression during Primordium Development Revealed by Live Imaging of the Arabidopsis Inflorescence Meristem. Curr. Biol. 2005, 15, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Heisler, M.G.; Hamant, O.; Krupinski, P.; Uyttewaal, M.; Ohno, C.; Jonsson, H.; Traas, J.; Meyerowitz, E.M. Alignment between PIN1 Polarity and Microtubule Orientation in the Shoot Apical Meristem Reveals a Tight Coupling between Morphogenesis and Auxin Transport. PLoS Biol. 2010, 8, e1000516. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Heisler, M.G. Self-organising periodicity in development: Organ positioning in plants. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Abraham-Shrauner, B.; Pickard, B.G. A model for leaf initiation Determination of phyllotaxis by waves in the generative circle. Plant Signal. Behav. 2011, 6, 1755–1768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thompson, D. On Growth and Form; Abridged ed.; Cambridge University Press: Cambridge, UK, 1961. [Google Scholar]
- Clarke, A.E.; Anderson, R.L.; Stone, B.A. Form and function of arabinogalactans and arabinogalactan-proteins. Phytochemistry 1979, 18, 521–540. [Google Scholar] [CrossRef]
- Gaspar, Y.M.; Johnson, K.L.; McKenna, J.A.; Bacic, A.; Schultz, C.J. The complex structures of arabinogalactan-proteins and the journey towards understanding. Plant Mol. Biol. 2001, 47, 161–176. [Google Scholar] [CrossRef]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef]
- Aspinall, G.O.; Malloy, J.A.; Craig, J.W.T. Extracellular polysaccharides from suspension-cultured sycamore cells. Can. J. Biochem. 1969, 47, 1063–1070. [Google Scholar] [CrossRef]
- Lamport, D.T.A. Cell wall metabolism. Ann. Rev. Plant Physiol. 1970, 21, 235–270. [Google Scholar] [CrossRef]
- Jermyn, M.A.; Yeow, Y.M. A class of lectins present in the tissues of seed plants. Aust. J. Plant Physiol. 1975, 2, 501–531. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Varnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis Cell Wall Proteoglycan Consists of Pectin and Arabinoxylan Covalently Linked to an Arabinogalactan Protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Turing, A.M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B 1952, 237, 37–72. [Google Scholar]
- Briggs, G.E. The Absorption of Salts by Plant Tissues, considered as Ionic Interchange. Ann. Bot. 1933, 46, 301–322. [Google Scholar] [CrossRef]
- Hecht, K. Studien uber den Vorgang der Plasmophysiologie der Grenzschichten lebender Pflanzenzellen. Cohns Beitr. Biol. Pflanz. 1912, 11, 137–189. [Google Scholar]
- Volgger, M.; Lang, I.; Ovecka, M.; Lichtscheidl, I. Plasmolysis and cell wall deposition in wheat root hairs under osmotic stress. Protoplasma 2010, 243, 51–62. [Google Scholar] [CrossRef]
- De Vriese, K.; Himschoot, E.; Dunser, K.; Nguyen, L.; Drozdzecki, A.; Costa, A.; Nowack, M.K.; Kleine-Vehn, J.; Audenaert, D.; Beeckman, T.; et al. Identification of Novel Inhibitors of Auxin-Induced Ca2+ Signaling via a Plant-Based Chemical Screen. Plant Physiol. 2019, 180, 480–496. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Kieliszewksi, M.J.; Showalter, A.M. Salt-stress upregulates periplasmic arabinogalactan-proteins: Using salt-stress to analyse AGP function. New Phytol. 2006, 169, 479–492. [Google Scholar] [CrossRef]
- Pfeifer, L.; Classen, B. First structural characterisation of seagrass arabinogalactan-proteins reveals habitat-driven adaptation to marine environment. Cell Wall Meet. 2019, 15, P003. [Google Scholar]
- Moller, B.; Weijers, D. Auxin Control of Embryo Patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, a001545. [Google Scholar] [CrossRef]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Palacio-Lopez, K.; Tinaz, B.; Holzinger, A.; Domozych, D.S. Arabinogalactan proteins and the extracellular matrix of Charophytes: A sticky business. Front. Plant Sci. 2019, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.; Costa, M.; Jones, B.J.; Mendes, M.A.; Pereira, L.G. Pollen grain development is compromised in Arabidopsis agp6 agp11 null mutants. J. Exp. Bot. 2009, 60, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Corral-Martinez, P.; Driouich, A.; Segui-Simarro, J.M. Dynamic Changes in Arabinogalactan-Protein, Pectin, Xyloglucan and Xylan Composition of the Cell Wall During Microspore Embryogenesis in Brassica napus. Front. Plant Sci. 2019, 10, 332. [Google Scholar] [CrossRef]
- Koji, T.; Hayashi, K.; Kinoshita, T. Auxin Activates the Plasma Membrane H+-ATPase by Phosphorylation during Hypocotyl Elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar]
- Mazhab-Jafari, M.T.; Rohou, A.; Schmidt, C.; Bueler, S.A.; Benlekbir, S.; Robinson, C.V.; Rubinstein, J.L. Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 2016, 539, 118–130. [Google Scholar] [CrossRef]
- Amdursky, N.; Lin, Y.; Aho, N.; Groenhof, G. Exploring fast proton transfer events associated with lateral proton diffusion on the surface of membranes. Proc. Natl. Acad. Sci. USA 2019, 116, 2443–2451. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Ramakrishna, P.; Duarte, P.R.; Rance, G.A.; Schubert, A.; Vordermaier, V.; Vu, L.D.; Murphy, E.; Barro, A.V.; Swarup, K.; Moirangthem, K.; et al. EXPANSIN A1-mediated radial swelling of pericycle cells positions anticlinal cell divisions during lateral root initiation. Proc. Natl. Acad. Sci. USA 2019, 116, 8597–8602. [Google Scholar] [CrossRef]
- Hamant, O.; Heisler, M.G.; Jonnson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1654. [Google Scholar] [CrossRef]
- Sampathkumar, A.; Yan, A.; Krupinski, P.; Meyerowitz, E.M. Physical Forces Regulate Plant Development and Morphogenesis. Curr. Biol. 2014, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Feraru, M.I.; Kleine-Vehn, J.; Martinie, A.; Mouille, G.; Vanneste, S.; Vernhettes, S.; Runions, J.; Friml, J. PIN Polarity Maintenance by the Cell Wall in Arabidopsis. Curr. Biol. 2011, 21, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Vernoux, T.; Besnard, F.; Traas, J. Auxin at the Shoot Apical Meristem. Cold Spring Harb. Perspect. Biol. 2010, 2, a001487. [Google Scholar] [CrossRef] [PubMed]
- Critchlow, K. The Hidden Geometry of Flowers; Floris Books: Edinburgh, UK, 2011. [Google Scholar]
- Zajaczkowski, S.; Wodzicki, T.J.; Romberger, J.A. Auxin Waves and Plant Morphogenesis. In Hormonal Regulation of Development II: The Functions of Hormones from the Level of the Cell to the Whole Plant; Scott, T.K., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 244–262. [Google Scholar]
- Komis, G.; Apostolakos, P.; Galatis, B. Altered patterns of tubulin polymerization in dividing leaf cells of Chlorophyton comosum after a hyperosmotic treatment. New Phytol. 2001, 149, 193–207. [Google Scholar] [CrossRef]
- Sato, E.M.; Hijazi, H.; Bennett, M.J.; Vissenjberg, K.; Swarup, R. New insights into root gravitropic signalling. J. Exp. Bot. 2015, 66, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Holdaway-Clarke, T.L.; Hackett, G.R.; Kunkel, J.G.; Lord, E.M.; Hepler, P.K. Uncoupling secretion and tip growth in lily pollen tubes: Evidence for the role of calcium in exocytosis. Plant J. 1999, 19, 379–386. [Google Scholar] [CrossRef]
- Heyn, A.N.J. The physiology of cell elongation. Bot. Rev. 1940, 6, 515–574. [Google Scholar] [CrossRef]
- Dyson, R.J.; Band, L.R.; Jensen, O.E. A model of crosslink kinetics in the expanding plant cell wall: Yield stress and enzyme action. J. Theor. Biol. 2012, 307, 125–136. [Google Scholar] [CrossRef]
- Pacheco-Villalobos, D.; Diaz-Moeeno, M.; van der Schuren, A.; Tamaki, T.; Kang, Y.H.; Gujas, B.; Novak, O.; Jaspert, N.; Li, Z.; Wolf, S.; et al. The Effects of High Steady State Auxin Levels on Root Cell Elongation in Brachypodium. Plant Cell 2016, 28, 1009–1024. [Google Scholar] [CrossRef]
- Tryfona, T.; Liang, H.-C.; Kotake, T.; Kaneko, S.; Marsh, J.; Ichinose, H.; Lovegrove, A.; Tsumaraya, Y.; Shewry, P.R.; Dupree, P. Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydr. Res. 2010, 345, 2656. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Peaucelle, A. Mechano-Chemical Aspects of Organ Formation in Arabidopsis thaliana: The Relationship between Auxin and Pectin. PLoS ONE 2013, 8, e57813. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Diffuse Growth of Plant Cell Walls. Plant Physiol. 2018, 176, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Altartouri, B.; Bidhendi, A.; Tani, T.; Suzuki, J.; Conrad, C.; Chebli, Y.; Liu, N.; Karunakaran, C.; Scacelli, G.; Geitmann, A. Pectin chemistry and cellulose crystallinity govern pavement cell morphogenesis in a multi-step mechanism. Plant Physiol. 2019, 181, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, V.M.; Fernandez, P.V.; Vega, A.S.; Mantese, A.I.; Federico, A.A.; Ciancia, M. Glucuronoarabinoxylans as major cell walls polymers from young shoots of the woody bamboo Phyllostachys aurea. Carbohydr. Polym. 2017, 167, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Takahashi, T.; Komeda, Y. Cloning and characterization of an LI Layer-specific gene in Arabidopsis thaliana. Plant Cell Physiol. 1999, 40, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Saare-Surminski, K.; Preil, W.; Knox, J.P.; Lieberei, R. Arabinogalactan proteins in embryogenic and non-embryogenic callus cultures of Euphorbia pulcherrima. Physiol. Plant 2000, 108, 180–187. [Google Scholar] [CrossRef]
- Potocka, I.; Godel, K.; Dobrowolska, I.; Kurczyñska, E.U. Spatio-temporal localization of selected pectic and arabinogalactan protein epitopes and the ultrastructural characteristics of explant cells that accompany the changes in the cell fate during somatic embryogenesis in Arabidopsis thaliana. Plant Physiol. Biochem. 2018, 127, 573–589. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Mitchell, P. David Keilin’s Respiratory Chain Concept and Its Chemiosmotic Consequences. Nobel Lect. Chem. 1992, 5, 295–330. [Google Scholar]
- Luckey, M. Membrane Structural Biology; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Rubery, P.H.; Sheldrake, A.R. Carrier-mediated Auxin Transport. Planta 1974, 118, 101–121. [Google Scholar] [CrossRef]
- Crompton, D.W.T. Donald Henry Northcote (1921–2004). Biogr. Mem. Fellows R. Soc. 2019, 67, 359–370. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamport, D.T.A.; Tan, L.; Held, M.; Kieliszewski, M.J. Phyllotaxis Turns Over a New Leaf—A New Hypothesis. Int. J. Mol. Sci. 2020, 21, 1145. https://doi.org/10.3390/ijms21031145
Lamport DTA, Tan L, Held M, Kieliszewski MJ. Phyllotaxis Turns Over a New Leaf—A New Hypothesis. International Journal of Molecular Sciences. 2020; 21(3):1145. https://doi.org/10.3390/ijms21031145
Chicago/Turabian StyleLamport, Derek T. A., Li Tan, Michael Held, and Marcia J. Kieliszewski. 2020. "Phyllotaxis Turns Over a New Leaf—A New Hypothesis" International Journal of Molecular Sciences 21, no. 3: 1145. https://doi.org/10.3390/ijms21031145
APA StyleLamport, D. T. A., Tan, L., Held, M., & Kieliszewski, M. J. (2020). Phyllotaxis Turns Over a New Leaf—A New Hypothesis. International Journal of Molecular Sciences, 21(3), 1145. https://doi.org/10.3390/ijms21031145




