Testosterone Promotes the Proliferation of Chicken Embryonic Myoblasts Via Androgen Receptor Mediated PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
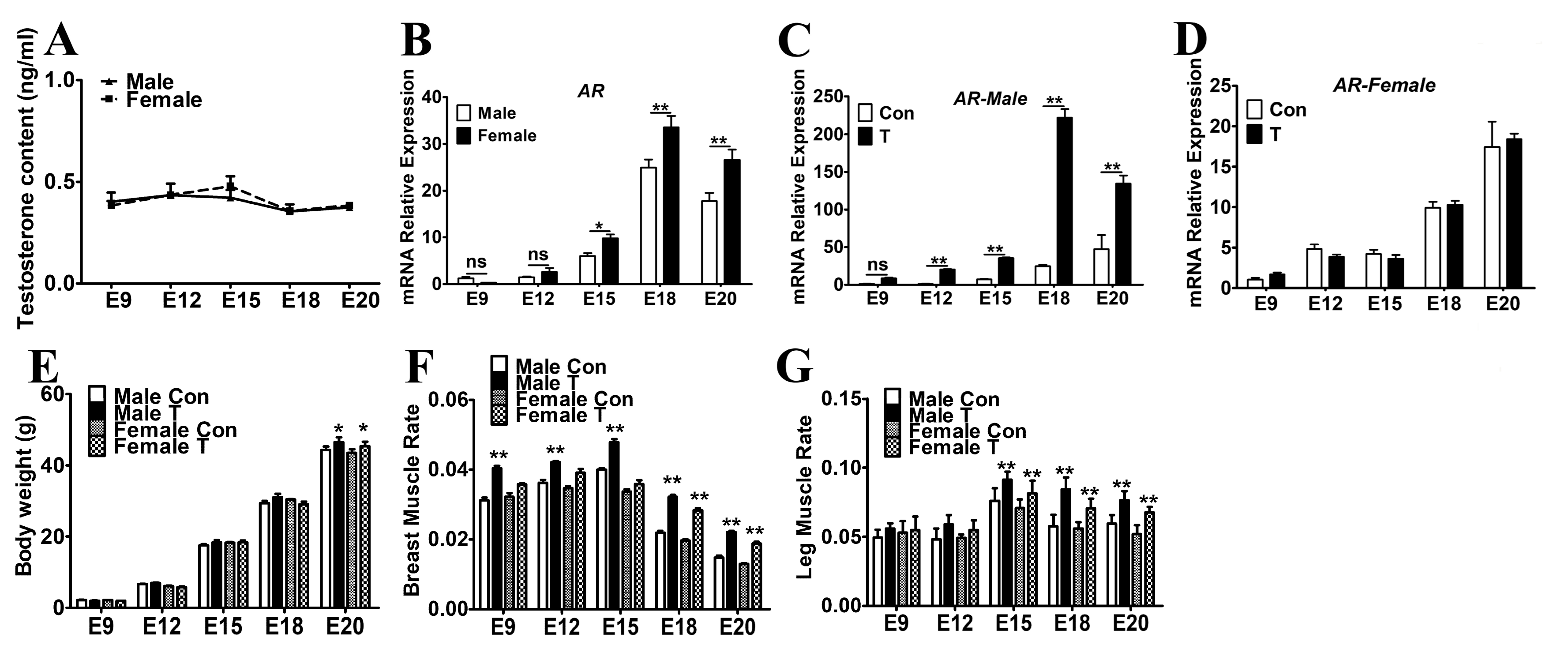
2.1. Exogenous T Increased the Skeletal Muscle Mass in Chicken Embryos
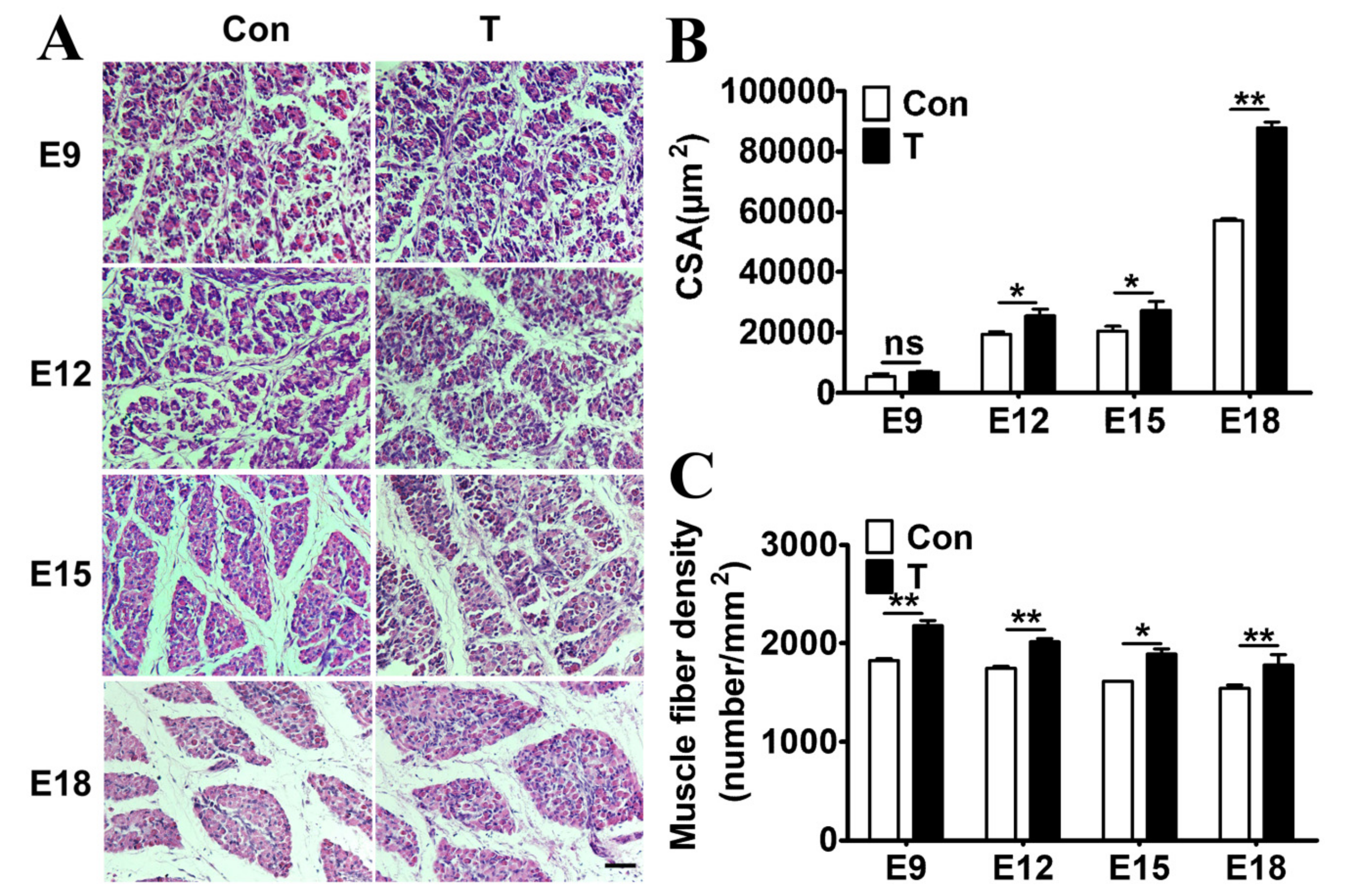
2.2. Exogenous T Augmented the Skeletal Muscle Fiber Proliferation
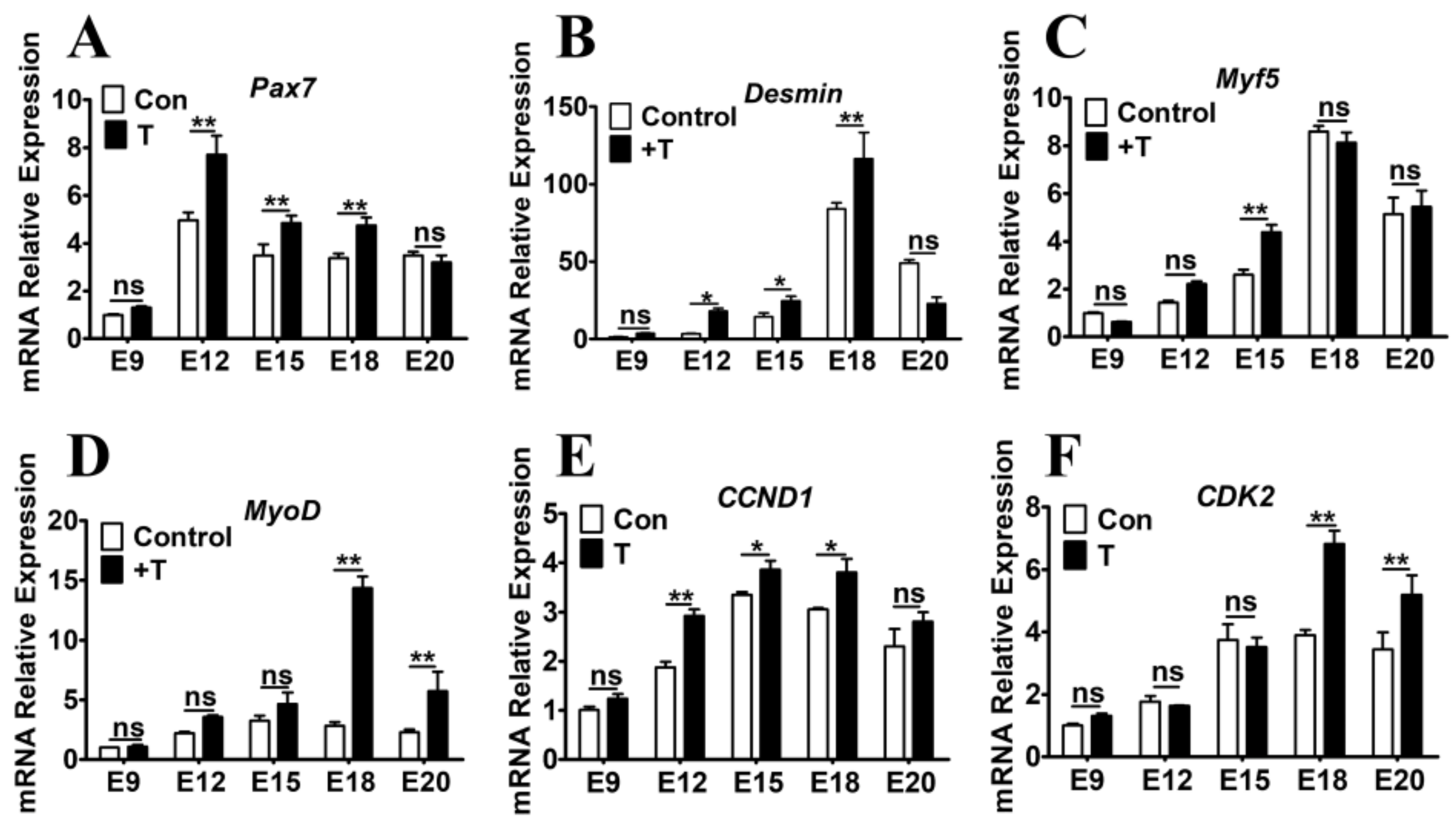
2.3. Exogenous T Upregulated the Expression of MRFs and Cell Cycle Related Genes
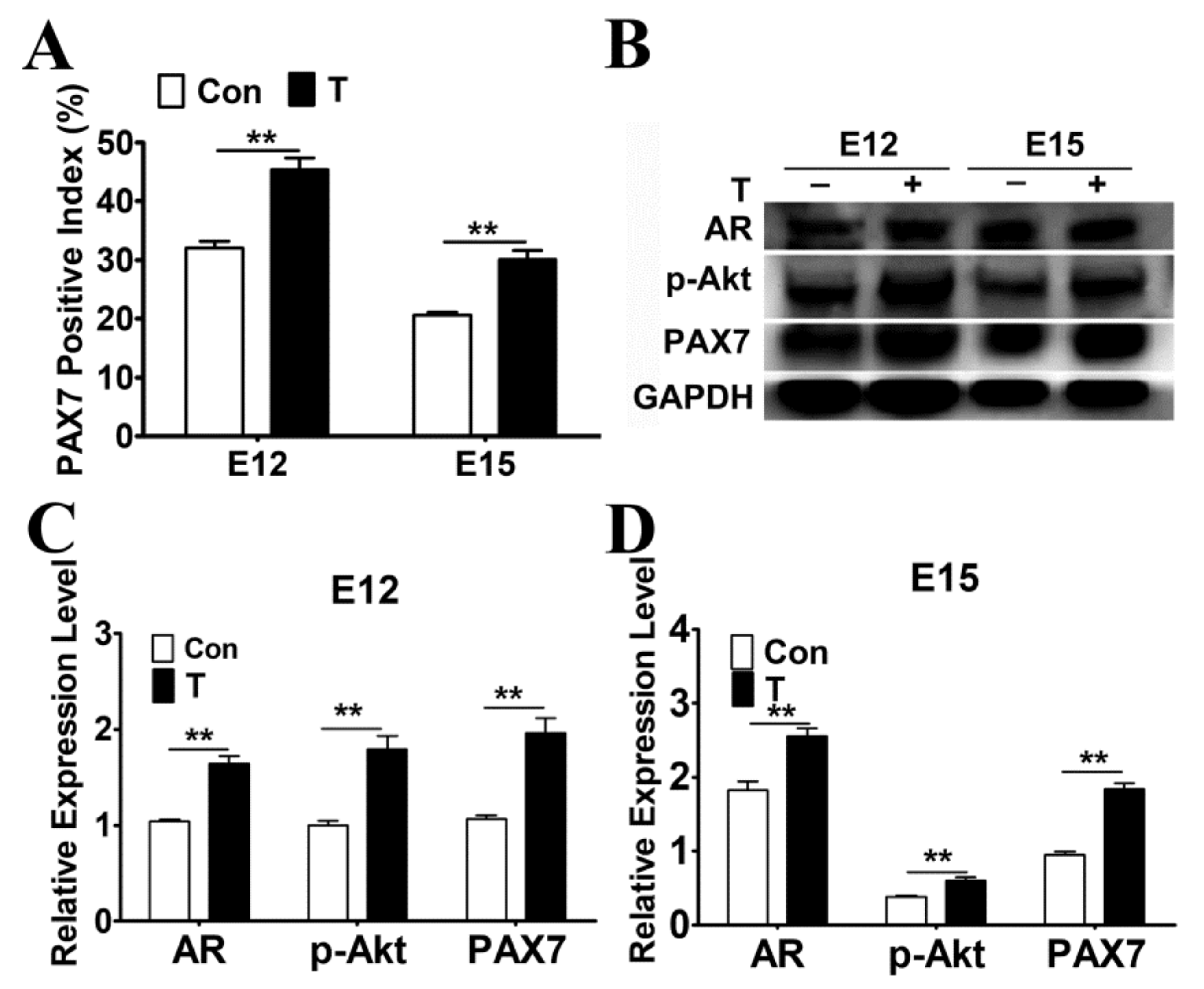
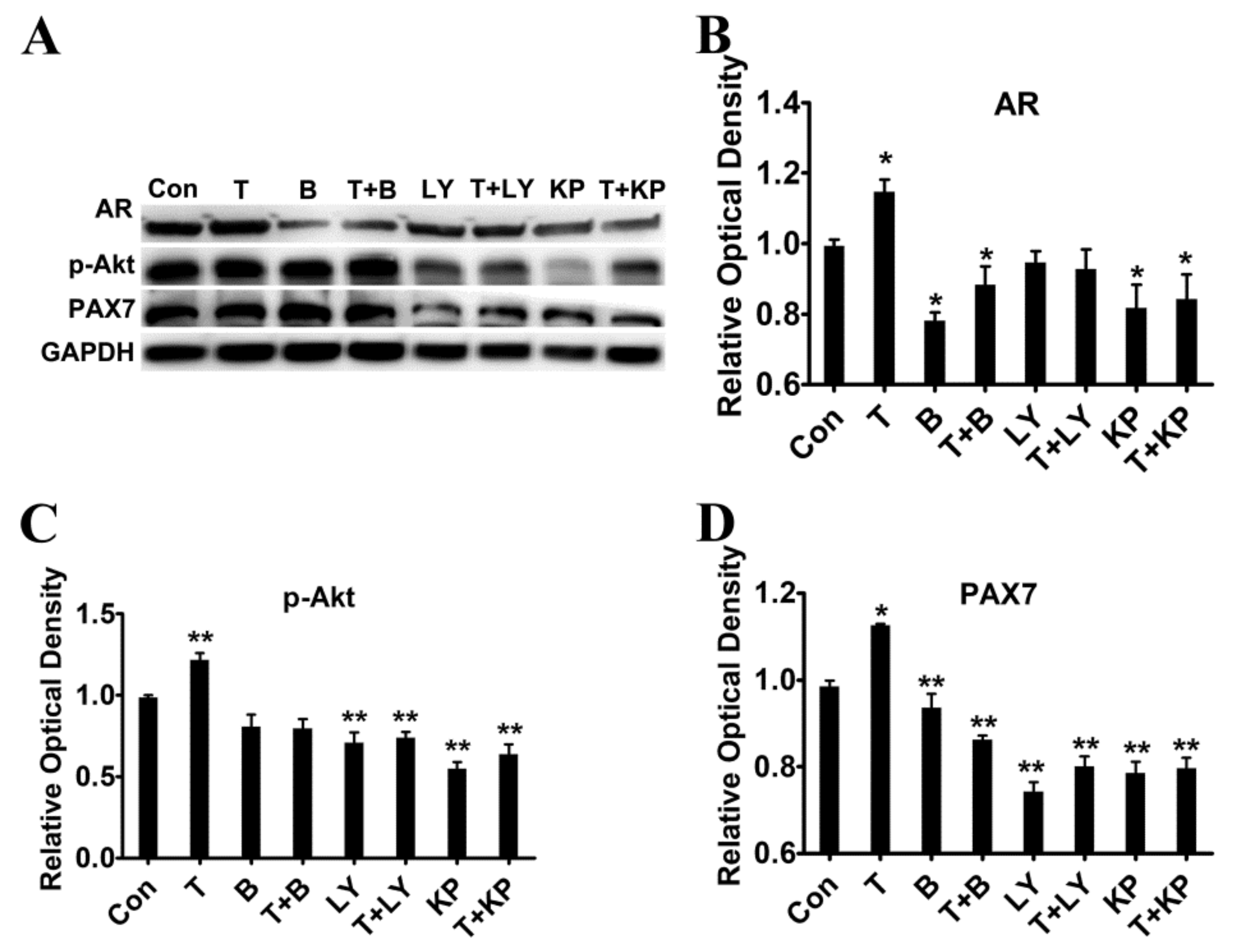
2.4. Exogenous T Promoted the Protein Expressions of AR, p-Akt and PAX7
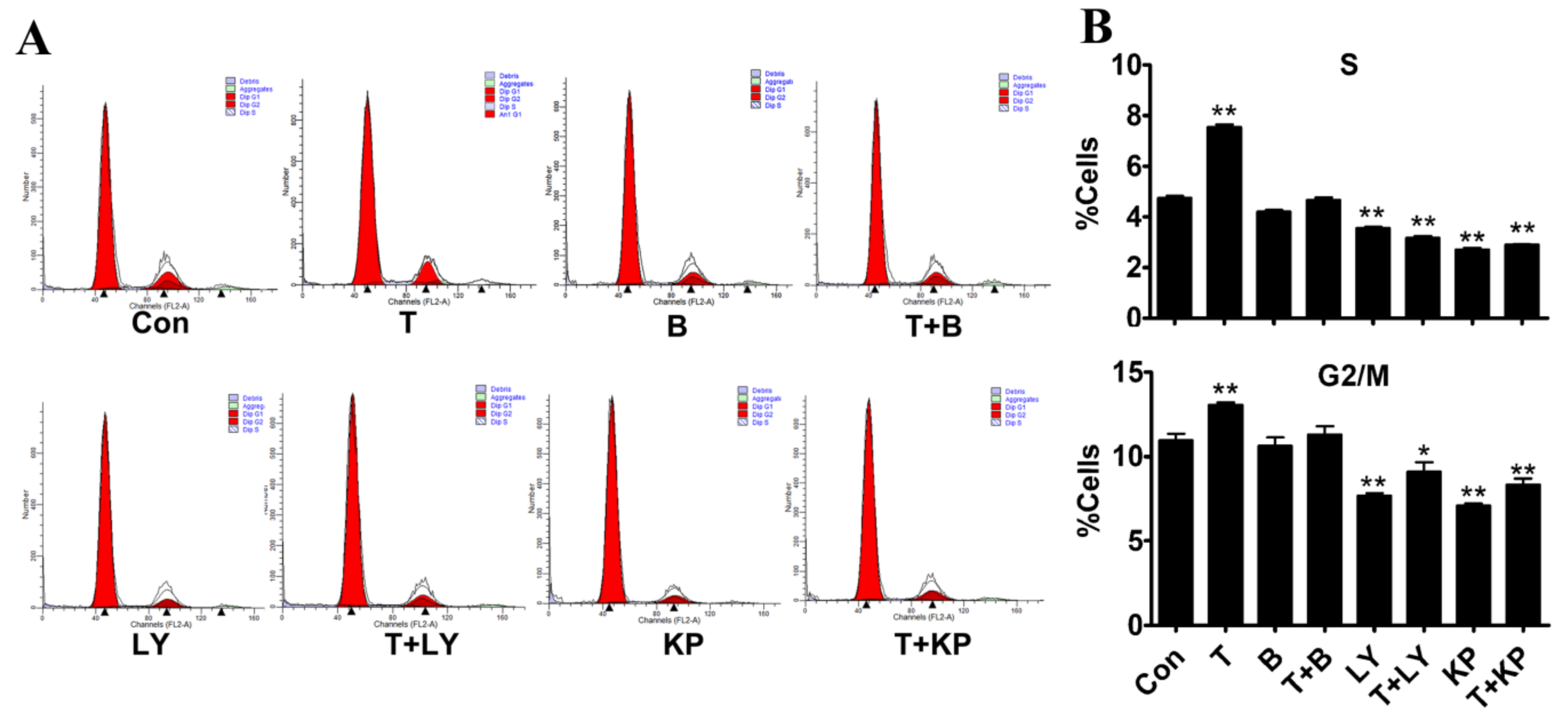
2.5. Effects of T and Inhibitors on the Cell Cycle of Myoblasts
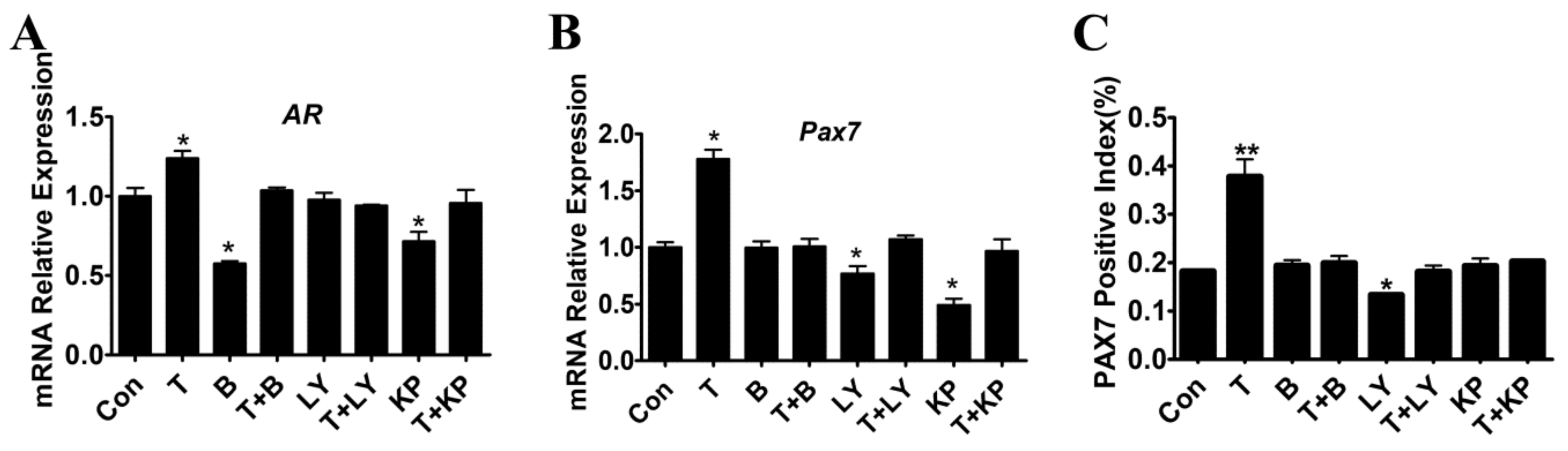
2.6. Effects of T and Inhibitors on the Expression of PAX7 in Myoblasts
2.7. Effects of T on the Activation of PI3K/Akt Signalling Pathway
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Tissue Samples
4.2. T Radioimmunoassay
4.3. Hematoxylin-Eosin (HE) Staining
4.4. Cell Culture and Treatments
4.5. RNA Isolation and Qualitative RT-PCR
4.6. Immunofluorescence Staining
4.7. Flow Cytometry Analysis (FACS)
4.8. Western Blotting Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex. Differ. 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabl, H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem Physiol. A Physiol. 1996, 114, 271–276. [Google Scholar] [CrossRef]
- Sandell, M.I.; Tobler, M.; Hasselquist, D. Yolk androgens and the development of avian immunity: An experiment in jackdaws (Corvus monedula). J. Exp. Biol. 2009, 212, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, K.L.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef]
- Dalbo, V.J.; Roberts, M.D.; Mobley, C.B.; Ballmann, C.; Kephart, W.C.; Fox, C.D.; Santucci, V.A.; Conover, C.F.; Beggs, L.A.; Balaez, A.; et al. Testosterone and trenbolone enanthate increase mature myostatin protein expression despite increasing skeletal muscle hypertrophy and satellite cell number in rodent muscle. Andrologia 2017, 49, e12622. [Google Scholar] [CrossRef]
- Gharahdaghi, N.; Rudrappa, S.; Brook, M.S.; Idris, I.; Crossland, H.; Hamrock, C.; Abdul Aziz, M.H.; Kadi, F.; Tarum, J.; Greenhaff, P.L.; et al. Testosterone therapy induces molecular programming augmenting physiological adaptations to resistance exercise in older men. J. Cachexia Sarcopenia Muscle 2019, 10, 1276–1294. [Google Scholar] [CrossRef] [Green Version]
- Sattler, F.; Bhasin, S.; He, J.; Chou, C.P.; Castaneda-Sceppa, C.; Yarasheski, K.; Binder, E.; Schroeder, E.T.; Kawakubo, M.; Zhang, A. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: The HORMA trial. Afr. Stud. Rev. 2011, 66, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Carlo, S.; Frances, T.; Sara, R.; Daniel, L.; Gianluca, T.; Nicolae Lucian, S.; Anqi, Z.; Ravi, J.; Shalender, B. Testosterone improves the regeneration of old and young mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Kovacheva, E.L.; Hikim, A.P.; Shen, R.; Sinha, I.; Sinha-Hikim, I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010, 151, 628–638. [Google Scholar] [CrossRef]
- Carlo, S.; Nicolae Lucian, S.; Hyeran, J.; Daniel, L.; Gianluca, T.; Tyler, G.; Siu, W.; Anqi, Z.; Wen, G.; Ravi, J. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: Differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology 2013, 154, 4594–4606. [Google Scholar] [CrossRef] [Green Version]
- Wannenes, F.; Caprio, M.; Gatta, L.; Fabbri, A.; Bonini, S.; Moretti, C. Androgen receptor expression during C2C12 skeletal muscle cell line differentiation. Mol. Cell Endocrinol. 2008, 292, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nick, S.; Solomon, A.M.; Sinanan, A.C.M.; Bouloux, P.M.G.; Fergal, G.; Lewis, M.P. Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med. Sci. Sports Exerc. 2012, 44, 610–615. [Google Scholar] [CrossRef]
- Carla, B.A.; Gonzalo, J.; Francisco, A.; Enrique, J.; Manuel, E. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med. Sci. Sports Exerc 2013, 45, 1712–1720. [Google Scholar] [CrossRef]
- Deane, C.S.; Hughes, D.C.; Sculthorpe, N.; Lewis, M.P.; Stewart, C.E.; Sharples, A.P. Impaired hypertrophy in myoblasts is improved with testosterone administration. J. Steroid. Biochem. Mol. Biol. 2013, 138, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlo, S.; Shalender, B.; Frances, T.; Barton, E.R.; Michelle, G.; Anqi, Z.; Janet, S.; Vandenburgh, H.H.; Travison, T.G.; Ravi, J. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology 2011, 152, 193–206. [Google Scholar] [CrossRef]
- White, J.P.; Song, G.; Puppa, M.J.; Shuichi, S.; Welle, S.L.; Carson, J.A. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol. Cell Endocrinol. 2013, 365, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Yin, L.; Lin, X.; Lu, J.; Wang, X. Effects of Cyclic Mechanical Stretch on the Proliferation of L6 Myoblasts and Its Mechanisms: PI3K/Akt and MAPK Signal Pathways Regulated by IGF-1 Receptor. Int. J. Mol. Sci. 2018, 19, 1649. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Wang, H.; Xu, Y.; Yu, D.; Li, D.; Liu, X.; Du, W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol. Int. 2015, 39, 910–922. [Google Scholar] [CrossRef]
- Wang, C.L.; Kung, H.N.; Wu, C.H.; Huang, C.J. Dietary wild bitter gourd displays selective androgen receptor modulator like activity and improves the muscle decline of orchidectomized mice. Food Funct. 2019, 10, 125–139. [Google Scholar] [CrossRef]
- Shen, Q.; Bi, H.; Yu, F.; Fan, L.; Zhu, M.; Jia, X.; Kang, J. Nontargeted metabolomic analysis of skeletal muscle in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Mol. Reprod. Dev. 2019, 86, 370–378. [Google Scholar] [CrossRef]
- Kadi, F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br. J. Pharm. 2008, 154, 522–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, M.; Khan, J.; Kumar, R.; Surolia, A.; Gupta, S. Testosterone supplementation improves insulin responsiveness in HFD fed male T2DM mice and potentiates insulin signaling in the skeletal muscle and C2C12 myocyte cell line. PLoS ONE 2019, 14, e0224162. [Google Scholar] [CrossRef] [PubMed]
- Pronsato, L.; Milanesi, L.; Vasconsuelo, A.; La Colla, A. Testosterone modulates FoxO3a and p53-related genes to protect C2C12 skeletal muscle cells against apoptosis. Steroids 2017, 124, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, M.L.; Steiner, J.L.; Gordon, B.S. Androgen-mediated regulation of skeletal muscle protein balance. Mol. Cell Endocrinol. 2017, 447, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharples, A.P.; Nasser, A.S.; Lewis, M.P.; Stewart, C.E. Reduction of myoblast differentiation following multiple population doublings in mouse C2 C12 cells: A model to investigate ageing? J. Cell Biochem. 2011, 112, 3773–3785. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′–3′) | Ref. Sequence Number | Amplicon Length (bp) |
|---|---|---|---|
| β-actin | F: ATG AAG CCC AGA GCA AAA GA | NM_205518 | 223 |
| R: GGG GTG TTG AAG GTC TCA AA | |||
| AR | F: AAG AAG CTG GGC AGT CTG AA | NC_006091.5 | 214 |
| R: AGC AGG TTG GAG AAG GAG TC | |||
| Pax7 | F: AGT TCG ATT AGC CGT GTG CT | NM_205065 | 185 |
| R: CTC TTC AAA GGC AGG TCT GG | |||
| Myf5 | F: CCC ATC CGA GCT CTT CTA TG | NM_001030363 | 223 |
| R: CAT GGT GGT GGA TTT CCT CT | |||
| MyoD | F: ATG TCC CAT ACT GCC TCC AG | NM_204214 | 235 |
| R: GTC TTG GAG CTT GGC TGA AC | |||
| Desmin | F: CTG CTT TCA GAG CTG ACG TG | XM_004942838 | 155 |
| R: CTG GAT GTG CTG CTC CTG TA | |||
| CDK6 | F: TTG TTT GAT GTG TGC ACC GT | NM_001007892 | 165 |
| R: AGT CCC CGA AAC AGC TGA AG | |||
| Cyclin D1 | F:GTG CGT GCA GAA GGA AAT CT | NM_205381 | 225 |
| R:GCG GTC AGA GGA ATC GTT TC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wang, Q.; Shi, K.; Lu, Y.; Yu, D.; Shi, X.; Du, W.; Yu, M. Testosterone Promotes the Proliferation of Chicken Embryonic Myoblasts Via Androgen Receptor Mediated PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1152. https://doi.org/10.3390/ijms21031152
Li D, Wang Q, Shi K, Lu Y, Yu D, Shi X, Du W, Yu M. Testosterone Promotes the Proliferation of Chicken Embryonic Myoblasts Via Androgen Receptor Mediated PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2020; 21(3):1152. https://doi.org/10.3390/ijms21031152
Chicago/Turabian StyleLi, Dongfeng, Qin Wang, Kai Shi, Yinglin Lu, Debing Yu, Xiaoli Shi, Wenxing Du, and Minli Yu. 2020. "Testosterone Promotes the Proliferation of Chicken Embryonic Myoblasts Via Androgen Receptor Mediated PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 21, no. 3: 1152. https://doi.org/10.3390/ijms21031152
APA StyleLi, D., Wang, Q., Shi, K., Lu, Y., Yu, D., Shi, X., Du, W., & Yu, M. (2020). Testosterone Promotes the Proliferation of Chicken Embryonic Myoblasts Via Androgen Receptor Mediated PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences, 21(3), 1152. https://doi.org/10.3390/ijms21031152






