Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure
Abstract
1. Introduction
2. Results
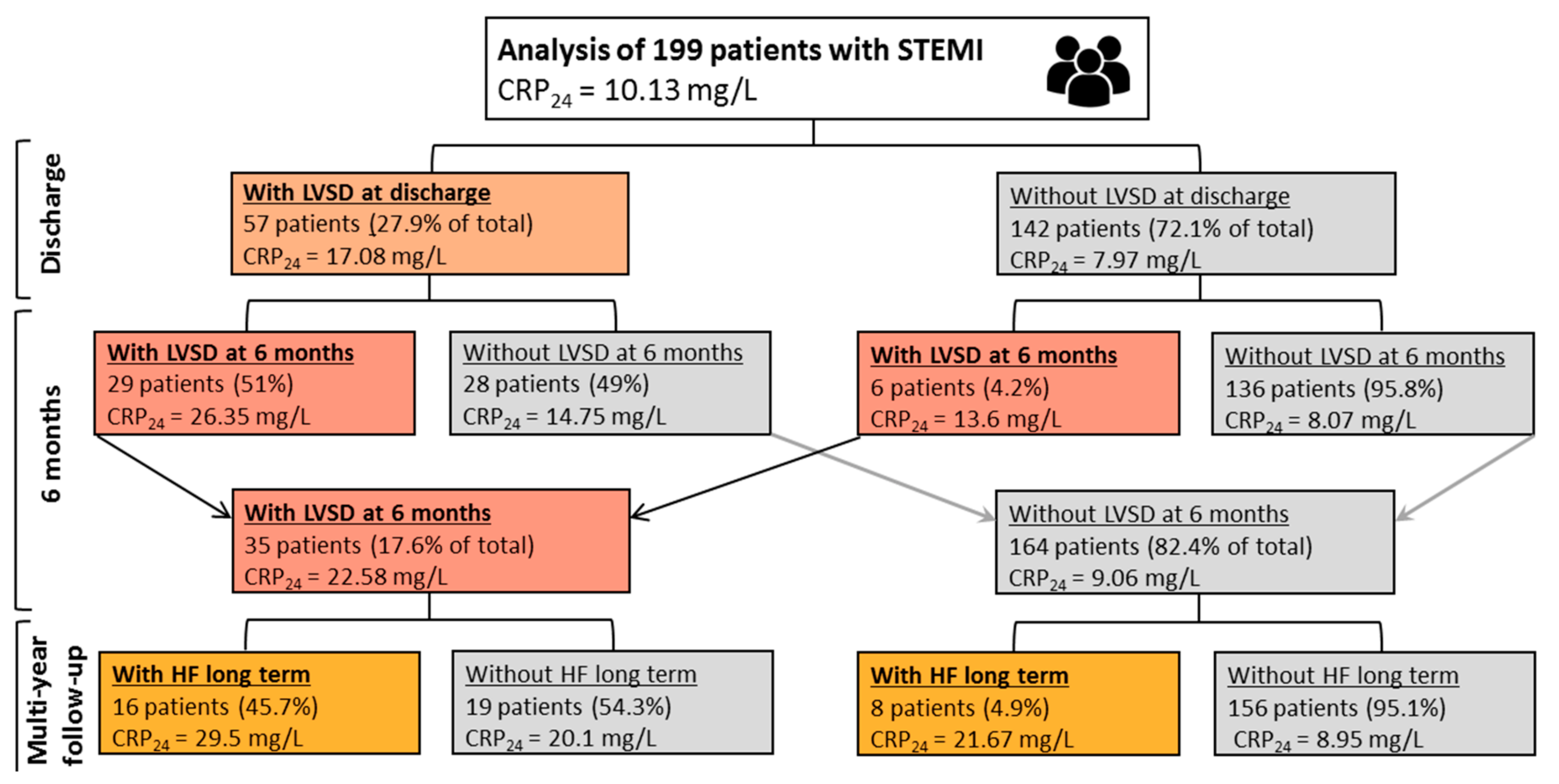
2.1. Study Endpoints
2.2. Prognostic Factors
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Therapy
4.3. Echocardiography
4.4. Blood Sampling and Biomarkers
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BNP | B-type natriuretic peptide |
| CRP | C-reactive protein |
| CRPAD | C-reactive protein concentration at hospital admission |
| CRP24 | C-reactive protein concentration 24 h after hospital admission |
| CRPDC | C-reactive protein concentration at hospital discharge |
| CRP1M | C-reactive protein concentration 1 month after hospital discharge |
| HF | heart failure |
| IL | interleukin |
| LVEF | left ventricular ejection fraction |
| LVSD | left ventricular systolic dysfunction |
| LVSDDC | left ventricular systolic dysfunction at hospital discharge |
| LVSD6M | left ventricular systolic dysfunction 6 months after hospital discharge |
| NYHA | New York Heart Association |
| PCI | percutaneous coronary intervention |
| STEMI | ST-segment elevation myocardial infarction |
| TIMI | Thrombolysis in Myocardial Infarction |
| TMPG | TIMI Myocardial Perfusion Grade |
References
- Weir, R.A.; McMurray, J.J.; Velazquez, E.J. Epidemiology of Heart Failure and Left Ventricular Systolic Dysfunction after Acute Myocardial Infarction: Prevalence, Clinical Characteristics, and Prognostic Importance. Am. J. Cardiol. 2006, 97, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Nicod, P.; Gilpin, E.; Ditrich, H.; Chappuis, F.; Ahnve, S.; Engler, R.; Henning, H.; Ross, J. Influence on Prognosis and Morbidity of left Ventricular Ejection fraction With and Without Signs of left Ventricular Failure After Acute Myocardial Infarction. Am. J. Cardiol. 1988, 81, 1165–1171. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E.; Moyé, L.A.; Basta, L.; Brown, E.J.; Cuddy, T.E.; Davis, B.R.; Geltman, E.M.; Goldman, S.; Flaker, G.C.; et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N. Engl. J. Med. 1992, 3, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Julian, D.G.; Camm, A.J.; Frangin, G.; Janse, M.J.; Munoz, A.; Schwartz, P.J.; Simon, P. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. Lancet 1997, 349, 67–74. [Google Scholar] [CrossRef]
- Daneault, B.; Genereux, P.; Kirtane, A.; Witzenbichler, B.; Guagliumi, G.; Paradis, J.M.; Fahy, M.P.; Mehran, R.; Stone, G.W. Comparison of three-year outcomes after primary percutaneous coronary intervention in patients with left ventricular ejection fraction <40% versus ≥40% (from the HORIZONS-AMI Trial). Am. J. Cardiol. 2013, 111, 12–20. [Google Scholar] [PubMed]
- Ndrepepa, G.; Mehili, J.; Martinoff, S.; Schwaiger, M.; Schömig, A.; Kastratiet, A. Evolution of left ventricular ejection fraction and its relationship to infarct size after acute myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 149–156. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Magielski, P.; Woźnicki, M.; Gierach, J.; Jabłoński, M.; Fabiszak, T.; Koziński, M.; Sukiennik, A.; Bronisz, A.; Kubica, J. Occurrence and predictors of left ventricular systolic dysfunction at hospital discharge and in long-term follow-up after acute myocardial infarction treated with primary percutaneous coronary intervention. Kardiol. Pol. 2012, 70, 157–163. [Google Scholar]
- Lewis, E.F.; Velazquez, E.J.; Solomon, S.D.; Hellkamp, A.S.; McMurray, J.J.V.; Mathias, J.; Rouleau, J.L.; Maggioni, A.P.; Swedberg, K.; Kober, L.; et al. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur. Heart J. 2008, 29, 748–756. [Google Scholar] [CrossRef]
- Sutton, N.R.; Li, S.; Thomas, L.; Wang, T.Y.; de Lemos, J.A.; Enriquez, J.R.; Shah, R.U.; Fonarow, G.C. The association of left ventricular ejection fraction with clinical outcomes after myocardial infarction: Findings from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get With the Guidelines (GWTG) Medicare-linked database. Am. Heart J. 2016, 178, 65–73. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Anzai, T. Post-Infarction inflammation and left ventricular remodeling: a double-edged sword. Circ. J. 2013, 77, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; Sonnino, C.; Van Tassell, B.W.; Biasucci, L.M.; Abbate, A. Inflammatory markers in ST-elevation acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, L.M.; Koenig, W.; Mair, J.; Mueller, C.; Plebani, M.; Lindahl, B.; Rifai, N.; Venge, P.; Hamm, C.; Giannitsis, E.; et al. How to use C-reactive protein in acute coronary care. Eur. Heart J. 2013, 34, 3687–3690. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Scirica, B.M.; Cannon, C.P.; Sabatine, M.S.; Jarolim, P.; Sloane, S.; Rifai, N.; Braunwald, E.; Morrow, D.A.; for the PROVE IT–TIMI 22 Investigators. Concentrations of C-Reactive Protein and B-Type Natriuretic Peptide 30 Days after Acute Coronary Syndromes Independently Predict Hospitalization for Heart Failure and Cardiovascular Death. Clin. Chem. 2009, 55, 265–273. [Google Scholar] [CrossRef]
- Mincu, R.I.; Jánosi, R.A.; Vinereanu, D.; Rassaf, T.; Totzeck, M. Preprocedural C-reactive protein predicts outcomes after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction a systematic meta-analysis. Sci. Rep. 2017, 7, 41530. [Google Scholar] [CrossRef]
- He, L.P.; Tang, X.Y.; Ling, W.H.; Chen, W.Q.; Chen, Y.M. Early C-reactive protein in the prediction of long-term outcomes after acute coronary syndromes: a meta-analysis of longitudinal studies. Heart 2010, 96, 339–346. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Koziński, M.; Magielski, P.; Fabiszak, T.; Kubica, A.; Sukiennik, A.; Navarese, E.P.; Odrowąż-Sypniewska, G.; Kubica, J. Value of C-reactive protein in predicting left ventricular remodelling in patients with a first ST-segment elevation myocardial infarction. Mediators Inflamm. 2012, 2012, 1–11. [Google Scholar]
- Klingenberg, R.; Aghlmandi, S.; Raber, L.; Gencer, B.; Nanchen, D.; Heg, D.; Carballo, S.; Rodondi, N.; Mach, F.; Windecker, S.; et al. Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT-proBNP and hsCRP with the GRACE score. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 129–138. [Google Scholar] [CrossRef]
- Schiele, F.; Meneveau, N.; Seronde, M.F.; Chopard, R.; Descotes-Genon, V.; Dutheil, J.; Bassand, J.P.; Reseau de Cardiologie de Franche Comte. C-reactive protein improves risk prediction in patients with acute coronary syndromes. Eur. Heart J. 2010, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Moutereau, S.; Simon, T.; Gallet, R.; Probst, V.; Ferrieres, J.; Gueret, P.; Danchin, N. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST Elevation Myocardial Infarction [FAST-MI]. Am. J. Cardiol. 2013, 111, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Morrow, D.A.; Cannon, C.P.; de Lemos, J.A.; Murphy, S.; Sabatine, M.S.; Wiviott, S.D.; Rifai, N.; McCabe, C.H.; Braunwald, E.; et al. Clinical Application of C-Reactive Protein Across the Spectrum of Acute Coronary Syndromes. Clin. Chem. 2007, 53, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Ohlmann, P.; Jaquemin, L.; Morel, O.; El Behlgiti, R.; Faure, A.; Michotey, M.O.; Beranger, N.; Roul, G.; Schneider, F.; Bareiss, P.; et al. Prognostic value of C-reactive protein and cardiac troponin I in primary percutaneous interventions for ST-elevation myocardial infarction. Am. Heart J. 2006, 152, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Ørn, S.; Manhenke, C.; Ueland, T.; Damas, J.K.; Mollnes, T.E.; Edvardsen, T.; Aukrust, P.; Dickstein, K. C-reactive protein, infarct size, microvascular obstruction, and left-ventricular remodelling following acute myocardial infarction. Eur. Heart J. 2009, 30, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Mather, A.N.; Fairbairn, T.A.; Artis, N.J.; Greenwood, J.P.; Plein, S. Relationship of cardiac biomarkers and reversible and irreversible myocardial injury following acute myocardial infarction as determined by cardiovascular magnetic resonance. Int. J. Cardiol. 2013, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Świątkiewicz, I.; Taub, P.R. The usefulness of C-reactive protein for the prediction of post-infarct left ventricular systolic dysfunction and heart failure. Kardiol. Pol. 2018, 76, 821–829. [Google Scholar] [CrossRef]
- Aggelopoulos, P.; Chrysohoou, C.; Pitsavos, C.; Papadimitriou, L.; Liontou, C.; Panagiotakos, D.; Tsiamis, E.; Stefanadis, C. Comparative value of simple inflammatory markers in the prediction of left ventricular systolic dysfunction in postacute coronary syndrome patients. Mediators Inflamm. 2009, 826297. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Koziński, M.; Magielski, P.; Gierach, J.; Fabiszak, T.; Kubica, A.; Sukiennik, A.; Navarese, E.P.; Odrowąż-Sypniewska, G.; Kubica, J. Usefulness of C-reactive protein as a marker of early post-infarct left ventricular systolic dysfunction. Inflamm. Res. 2012, 61, 725–734. [Google Scholar] [CrossRef][Green Version]
- Arruda-Olson, A.M.; Enriquez-Sarano, M.; Bursi, F.; Weston, S.A.; Jaffe, A.S.; Killian, J.M.; Roger, V.L. Left ventricular function and C-reactive protein levels in acute myocardial infarction. Am. J. Cardiol. 2010, 105, 917–921. [Google Scholar] [CrossRef]
- Suleiman, M.; Khatib, R.; Agmon, Y.; Mahamid, R.; Boulos, M.; Kapeliovich, M.; Levy, Y.; Beyar, R.; Markiewicz, W.; Hammerman, H.; et al. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction – predictive role of C-reactive protein. J. Am. Coll. Cardiol. 2006, 47, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Weston, S.A.; Killian, J.M.; Gabriel, S.E.; Jacobsen, S.J.; Roger, V.L. C-reactive protein and heart failure after myocardial infarction in the community. Am. J. Med. 2007, 120, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Lee, S.W.; Lee, C.W.; Hong, M.K.; Kim, J.J.; Park, S.W.; Park, S.J.; Park, D.W.; Kim, Y.H. Biomarkers on admission for the prediction of cardiovascular events after primary stenting in patients with ST-elevation myocardial infarction. Clin. Cardiol. 2008, 31, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Groot, H.E.; Karper, J.C.; Lipsic, E.; van Veldhuisen, D.J.; van der Horst, I.C.C.; van der Harstet, P. High-sensitivity C-reactive protein and long term reperfusion success of primary percutaneous intervention in ST-elevation myocardial infarction. Int. J. Cardiol. 2017, 248, 51–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van de Werf, F.; Ardissino, D.; Betriu, A.; Cokkinos, D.V.; Falk, E.; Fox, K.A.A.; Julian, D.; Lengyel, M.; Neumann, F.J.; Ruzyllo, W.; et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2003, 24, 28–66. [Google Scholar] [CrossRef]
- Van de Werf, F.; Bax, J.; Betriu, A.; Blomstrom-Lundqvist, C.; Crea, F.; Falk, V.; Filippatos, G.; Fox, K.; Huber, K.; Kastrati, A.; et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur. Heart J. 2008, 29, 2909–2945. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; Toldo, S.; Van Tassel, B.W.; Abbate, A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 1593–1603. [Google Scholar] [CrossRef]
- Marfella, R.; Rizzo, M.R.; Siniscalchi, M.; Paolisso, P.; Barbieri, M.; Sardu, C.; Savinelli, A.; Angelico, N.; Del Gaudio, S.; Esposito, N.; et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: Effects on myocardial salvage. Int. J. Cardiol. 2013, 168, 3954–3962. [Google Scholar] [CrossRef][Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J.; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018, 391, 319–328. [Google Scholar] [CrossRef]
- Trankle, C.R.; Canada, J.M.; Cei, L.; Abouzaki, N.; Oddi-Erdle, C.; Kadariya, D.; Christopher, S.; Viscusi, M.; Del Buono, M.; Kontos, M.C.; et al. Usefulness of Canakinumab to Improve Exercise Capacity in Patients with Long-Term Systolic Heart Failure and Elevated C-Reactive Protein. Am. J. Cardiol. 2018, 122, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J.; et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Sardu, C.; Balestrieri, M.L.; Siniscalchi, M.; Minicucci, F.; Signoriello, G.; Calabrò, P.; Mauro, C.; Pieretti, G.; Coppola, A.; et al. Effects of incretin treatment on cardiovascular outcomes in diabetic STEMI-patients with culprit obstructive and multivessel non obstructive-coronary-stenosis. Diabetol. Metab. Syndr. 2018, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Kontos, M.C.; Grizzard, J.G.; Biondi-Zoccai, G.G.L.; Van Tassell, B.W.; Robati, R.; Roach, L.M.; Arena, R.A.; Roberts, C.S.; Varma, A.; et al. Interleukin-1 Blockade With Anakinra to Prevent Adverse Cardiac Remodeling After Acute Myocardial Infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot Study). Am. J. Cardiol. 2010, 105, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of Interleukin-1 Blockade With Anakinra on Adverse Cardiac Remodeling and Heart Failure After Acute Myocardial Infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) Pilot Study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Kontos, M.C.; Abouzaki, N.A.; Melchior, R.D.; Thomas, C.; Van Tassell, B.W.; Oddi, C.; Carbone, S.; Trankle, C.R.; Roberts, C.S.; et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am. J. Cardiol. 2015, 115, 288–292. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Lipinski, M.J.; Appleton, D.; Roberts, C.S.; Kontos, M.C.; Abouzaki, N.; Melchior, R.; Mueller, G.; Garnett, J.; Canada, J.; et al. Rationale and design of the Virginia Commonwealth University–Anakinra Remodeling Trial-3 (VCU-ART3): A randomized, placebo-controlled, double-blinded, multicenter study. Clin. Cardiol. 2018, 41, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.C.; Rothman, A.M.K.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Heart J. 2015, 36, 377–384. [Google Scholar] [CrossRef]
- Gierach, J.; Gierach, M.; Świątkiewicz, I.; Woźnicki, M.; Grześk, G.; Sukiennik, A.; Koziński, M.; Kubica, J. Admission glucose and left ventricular systolic function in non-diabetic patients with acute myocardial infarction. Heart Vessels 2016, 31, 298–307. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, P.; Sacra, C.; Santamaria, M.; de Lucia, C.; Ruocco, A.; Mauro, C.; Paolisso, G.; Rizzo, M.R.; Barbieri, M.; et al. Cardiac resynchronization therapy with a defibrillator (CRTd) in failing heart patients with type 2 diabetes mellitus and treated by glucagon-like peptide 1 receptor agonists (GLP-1 RA) therapy vs. conventional hypoglycemic drugs: arrhythmic burden, hospitalizations for heart failure, and CRTd responders rate. Cardiovasc. Diabetol. 2018, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Marfella, R.; Santamaria, M.; Papini, S.; Parisi, Q.; Sacra, C.; Colaprete, D.; Paolisso, G.; Rizzo, M.R.; Barbieri, M. Stretch, Injury and Inflammation Markers Evaluation to Predict Clinical Outcomes After Implantable Cardioverter Defibrillator Therapy in Heart Failure Patients with Metabolic Syndrome. Front. Physiol. 2018, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Fedele, F.; Mancone, M.; Adamo, F.; Severino, P. Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction: The Misleading Definition of the New Guidelines. Cardiol. Rev. 2017, 25, 4–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur. J. Heart Fail. 2008, 10, 933–989. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Ryan, K.A.; Mesley, R.; Marble, S.J.; McCabe, C.H.; Van de Werf, F.; Braunwald, E.; TIMI (Thrombolysis In Myocardial Infarction) Study Group. Relationship of TIMI Myocardial Perfusion Grade to Mortality After Administration of Thrombolytic Drugs. Circulation 2000, 101, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]
- Severino, P.; Mestrini, V.; Mariani, M.V.; Birtolo, L.I.; Scarpati, R.; Mancone, M.; Fedele, F. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail. Rev. 2019. [Google Scholar] [CrossRef]



| Variable | Whole Study Group (n = 199) | Patients with LVSD 6 Months after Discharge (n = 35) | Patients without LVSD 6 Months after Discharge (n = 164) | p-Value between Patients with or without LVSD |
|---|---|---|---|---|
| Age (years) | 56.0 (50.0–64.0) | 58.0 (52.0–66.0) | 56.0 (50.0–64.0) | 0.233 |
| Gender (male:female) n (%) | 154:45 (77.4:22.6) | 26:9 (74.3:25.7) | 128:36 (78.1:21.9) | 0.633 |
| Anterior STEMI n (%) | 88 (44.2) | 31 (88.6) | 57 (34.8) | <0.001 |
| HF prior to STEMI (≤II NYHA) n (%) | 7 (3.5) | 3 (8.6) | 4 (2.4) | 0.009 |
| HF at discharge (≥II NYHA) n (%) | 20 (10.1) | 10 (28.6) | 10 (6.1) | <0.001 |
| Body mass index (kg/m²) | 26.7 (24.2–29.1) | 27.9 (26.0–30.1) | 26.2 (24.0–29.0) | 0.075 |
| Hypertension n (%) | 82 (41.2) | 19 (54.3) | 63 (38.4) | 0.083 |
| Diabetes mellitus n (%) | 35 (17.6) | 10 (27.0) | 25 (15.4) | 0.095 |
| Long-acting metoprolol at discharge n (%) | 197 (99.0) | 35 (100.0) | 162 (98.8) | 0.782 |
| Perindopril at discharge n (%) | 198 (99.5) | 35 (100.0) | 163 (99.4) | 0.393 |
| Simvastatin at discharge n (%) | 199 (100.0) | 35 (100.0) | 164 (100.0) | 1.0 |
| Spironolactone at discharge n (%) | 15 (7.5) | 9 (25.7) | 6 (3.7) | <0.001 |
| Non-potassium sparing diuretics at discharge n (%) | 12 (6.0) | 7 (20.0) | 5 (3.1) | <0.001 |
| Creatinine at admission (µmol/L) | 83.1 (72.5–97.2) | 88.4 (79.6–97.2) | 79.6 (71.6–97.2) | 0.152 |
| Glucose at admission (mmol/L) | 7.6 (6.8–9.3) | 8.61 (7.17–10.7) | 7.31 (6.67–9.19) | 0.004 |
| Leukocyte count at admission (10³/µL) | 11.2 (9.1–13.2) | 11.7 (10.6–13.4) | 10.9 (8.76–13.1) | 0.312 |
| Leukocyte count 24 h after admission (10³/µL) | 10.1 (5.4–20.1) | 12.3 (8.84–13.6) | 10.1 (8.28–11.2) | <0.001 |
| LDL cholesterol at admission (mmol/L) | 3.8 (3.2–4.4) | 3.72 (3.34–4.45) | 3.78 (3.21–4.45) | 0.452 |
| CK-MBmax (U/L) | 102.5 (57.5–159.5) | 178.0 (122.0–231.0) | 94.0 (54.0–137.0) | <0.001 |
| TnImax (ng/mL) | 44.2 (11.3–>50.0) | >50.0 (>50.0–>50.0) | 33.9 (10.1–>50.0) | <0.001 |
| CRP at admission (mg/L) | 1.74 (0.98–3.29) | 1.72 (1.12–3.06) | 1.79 (0.945–3.33) | 0.703 |
| CRP 24 h after admission (mg/L) | 10.13 (5.62–19.64) | 22.6 (12.1–33.9) | 9.06 (5.36–15.5) | <0.001 |
| CRP at discharge (mg/L) | 10.09 (4.9–17.65) | 17.9 (8.09–35.6) | 9.02 (4.47–16.2) | <0.001 |
| CRP 1 month after discharge (mg/L) | 1.7 (0.87–3.27) | 2.12 (1.01–3.17) | 1.51 (0.830–3.21) | 0.833 |
| BNP at admission (pg/mL) | 51.9 (5.7–101.4) | 74.6 (29.1–156.9) | 50.9 (25.5–89.9) | 0.001 |
| BNP at discharge (pg/mL) | 123.0 (70.4–226.9) | 336.5 (227.2–717.5) | 106.7 (62.2–169.2) | <0.001 |
| Variable | Whole Study Group (n = 199) | Patients with LVSD 6 Months after Discharge (n = 35) | Patients without LVSD 6 Months after Discharge (n = 164) | p-Value between Patients with or without LVSD |
|---|---|---|---|---|
| Angiographic indices: | ||||
| LAD/non-LAD n (%) | 92 (46.2)/107 (53.8) | 32 (91.4)/3 (8.6) | 60 (36.6)/104 (63.4) | <0.001 |
| TIMI 3 flow pre-PCI n (%) | 55 (27.6) | 3 (8.6) | 52 (31.7) | 0.035 |
| TIMI 3 flow post-PCI n (%) | 185 (93.0) | 29 (82.9) | 156 (95.1) | 0.02 |
| TMPG 3 post-PCI n (%) | 92 (46.2) | 15 (42.9) | 77 (47.0) | 0.659 |
| Multivessel coronary disease n (%) | 119 (59.8) | 22 (62.9) | 97 (59.1) | 0.638 |
| Abciximab use n (%) | 50 (25.1) | 15 (42.9) | 35 (21.3) | 0.007 |
| Intracoronary stents n (%) | 197 (99.0) | 35 (100.0) | 162 (98.8) | 0.197 |
| Echocardiographic indices at discharge: | ||||
| LVEDd (mm) | 49.0 (45.0–53.0) | 53.0 (52.0–57.0) | 48.0 (45.0–52.0) | <0.001 |
| LVESd (mm) | 34.0 (30.0–37.0) | 38.0 (36.0–45.0) | 33.0 (30.0–35.0) | <0.001 |
| LVEDVI (mL/m²) | 48.4 (44.2–60.6) | 60.4 (53.9–69.6) | 48.6 (43.0–57.9) | <0.001 |
| LVESVI (mL/m²) | 26.8 (23.1–34.9) | 37.7 (33.4–43.8) | 25.8 (22.9–30.7) | <0.001 |
| LVEF (%) | 45.0 (40.0–49.7) | 36.0 (33.5–40.0) | 46.0 (42.5–50.0) | <0.001 |
| WMSI (pts) | 1.5 (1.38–1.75) | 1.81 (1.75–1.94) | 1.44 (1.38–1.69) | <0.001 |
| S′ (cm/s) | 7.0 (6.1–8.1) | 5.9 (5.1–6.8) | 7.2 (6.4–8.4) | <0.001 |
| DT (ms) | 155.0 (145.0–185.0) | 145.0 (135.0–155.0) | 160.0 (150.0–190.0) | <0.001 |
| E/E′ (−) | 10.3 (8.4–12.6) | 11.8 (9.6–13.9) | 10.1 (8.2–12.0) | 0.005 |
| Echocardiographic indices 6 months after discharge: | ||||
| LVEDd (mm) | 50.0 (46.0–54.0) | 55.0 (50.0–56.0) | 48.5 (45.5–53.0) | <0.001 |
| LVESd (mm) | 34.0 (31.0–37.0) | 39.0 (35.0–44.0) | 33.0 (31.0–36.0) | <0.001 |
| LVEDVI (mL/m²) | 57.4 (48.8–68.6) | 76.9 (68.1–83.1) | 54.5 (43.2–65.0) | <0.001 |
| LVESVI (mL/m²) | 29.3 (24.8–39.0) | 48.2 (40.9–56.4) | 27.7 (24.1–33.6) | <0.001 |
| LVEF (%) | 46.0 (42.3–52.0) | 36.0 (34.0–38.6) | 47.7 (44.2–52.5) | <0.001 |
| WMSI (pts) | 1.44 (1.31–1.63) | 1.88 (1.69–1.94) | 1.38 (1.31–1.5) | <0.001 |
| S′ (cm/s) | 7.0 (6.1–8.1) | 5.6 (4.8–6.9) | 7.1 (6.3–8.2) | <0.001 |
| DT (ms) | 170.0 (155.0–195.0) | 150.0 (135.0–190.0) | 175.0 (155.0–200.0) | <0.001 |
| E/E′ (−) | 9.5 (8.0–11.7) | 13.1 (9.7–16.3) | 9.1 (7.9–10.8) | <0.001 |
| Variable | With HF Hospitalization in Long-Term Follow-Up (n = 16) | Without HF Hospitalization in Long-Term Follow-Up (n = 19) | p-Value |
|---|---|---|---|
| Age (years) | 57.0 (53.0–64.0) | 61.0 (50.0–67.0) | 0.935 |
| Gender (male:female) n (%) | 11:5 (68.8:31.3) | 15:4 (78.9:21.1) | 0.492 |
| Anterior location of STEMI n (%) | 15 (93.8) | 16 (84.2) | 0.365 |
| HF prior to STEMI (I/II NYHA) n (%) | 1 (6.3) | 2 (10.5) | 0.082 |
| HF at discharge for STEMI (≥II NYHA) n (%) | 5 (31.3) | 5 (26.3) | 0.418 |
| Body mass index (kg/m²) | 29.4 (27.3–30.7) | 27.4 (24.5–29.4) | 0.088 |
| Hypertension n (%) | 8 (50) | 11 (57.9) | 0.640 |
| Diabetes mellitus n (%) | 5 (31.3) | 5 (26.3) | 0.748 |
| Creatinine at admission (µmol/L) | 88.4 (79.6–98.1) | 88.4 (70.7–91.3) | 0.656 |
| Glucose at admission (mmol/L) | 7.75 (6.75–10.8) | 9.00 (7.94–10.7) | 0.125 |
| Leukocyte count on admission (10³/µL) | 12.6 (10.9–13.6) | 11.7 (9.50–12.5) | 0.441 |
| Leukocyte count 24 h after admission (10³/µL) | 12.4 (9.38–14.0) | 12.3 (8.74–13.4) | 0.461 |
| LDL cholesterol at admission (mmol/L) | 3.63 (3.26–5.01) | 3.72 (3.44–4.22) | 0.781 |
| CK-MBmax (U/L) | 203.5 (157.5–240.0) | 148.0 (119.0–206.0) | 0.172 |
| TnImax (ng/mL) | >50.0 (>50.0–>50.0) | >50.0 (>50.0–>50.0) | 0.350 |
| CRP at admission (mg/L) | 2.59 (1.42–4.24) | 1.6 (0.82–1.77) | 0.014 |
| CRP 24 h after admission (mg/L) | 29.5 (17.8–43.8) | 20.11 (6.21–26.7) | 0.056 |
| CRP at discharge (mg/L) | 21.9 (12.36–43.7) | 15.4 (7.95–24.66) | 0.161 |
| CRP 1 month after discharge (mg/L) | 2.57 (1.69–3.48) | 1.54 (0.65–2.72) | 0.052 |
| BNP at admission (pg/mL) | 108.9 (23.2–269.7) | 61.9 (31.0–132.4) | 0.502 |
| BNP at discharge (pg/mL) | 384.8 (198.8–756.0) | 336.5 (233.0–717.5) | 0.781 |
| LAD/non-LAD n (%) | 15 (93.8) | 17 (89.8) | 0.649 |
| TIMI 3 flow pre-PCI n (%) | 2 (12.5) | 1 (5.3) | 0.733 |
| TIMI 3 flow post-PCI n (%) | 14 (87.5) | 15 (78.9) | 0.978 |
| TMPG 3 post-PCI n (%) | 7 (43.8) | 8 (42.1) | 0.615 |
| Multivessel coronary disease n (%) | 11 (68.8) | 11 (57.9) | 0.507 |
| Abciximab use n (%) | 7 (43.8) | 8 (42.1) | 0.922 |
| LVEF at discharge for STEMI (%) | 36.0 (32.8–39.7) | 36.8 (35.0–40.4) | 0.301 |
| WMSI at discharge for STEMI (pts) | 1.88 (1.81–1.97) | 1.81 (1.75–1.88) | 0.095 |
| LVEF 6 months after discharge for STEMI (%) | 35.8 (31.3–37.0) | 36.9 (35.0–39.2) | 0.056 |
| WMSI 6 months after discharge for STEMI (pts) | 1.88 (1.84–1.97) | 1.75 (1.63–1.88) | 0.029 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Univariate analysis: | |||
| WMSI at discharge (for a 1-point increase) | 8255.0 | 387.65–175,791.3 | <0.001 |
| LAD vs. non-LAD | 17.10 | 4.99–58.62 | <0.00001 |
| Anterior vs. nonarterior wall STEMI | 14.54 | 4.86–43.55 | <0.000003 |
| Abciximab use vs. no use | 2.80 | 1.29–6.07 | <0.01 |
| BNP at discharge (for a 100 pg/mL increase) | 1.81 | 1.44–2.28 | <0.000001 |
| CRP at 24 h after admission (for a 10 mg/L increase) | 1.62 | 1.29–2.03 | <0.00006 |
| BNP at admission (for a 100 pg/mL increase) | 1.48 | 1.06–2.05 | <0.03 |
| CRP at discharge (for a 10 mg/L increase) | 1.45 | 1.17–1.80 | <0.0008 |
| WBC 24 h after admission (for a 10³/µL increase) | 1.27 | 1.10–1.46 | <0.001 |
| LVESd at discharge (for a 1 mm increase) | 1.22 | 1.12–1.33 | <0.00001 |
| LVEDd at discharge (for a 1 mm increase) | 1.16 | 1.08–1.26 | <0.0002 |
| LVESVI at discharge (for a 1 mL/m² increase) | 1.15 | 1.09–1.20 | <0.0000003 |
| E/E’ at discharge (for a 1-point increase) | 1.13 | 1.03–1.24 | <0.01 |
| CK-MBmax (for a 10 U/L increase) | 1.10 | 1.05–1.15 | <0.00006 |
| Body mass index (for a 10 kg/m² increase) | 1.09 | 0.99–1.20 | 0.08 |
| LVEDVI at discharge (for a 1 mL/m² increase) | 1.07 | 1.04–1.10 | <0.00003 |
| TnImax (for a 1 ng/mL increase) | 1.06 | 1.03–1.10 | <0.0003 |
| WBC at admission (for a 10³/µL increase) | 1.06 | 0.94–1.20 | 0.313 |
| DT at discharge (for a 1 ms increase) | 0.97 | 0.96–0.99 | <0.002 |
| CRP at admission (for a 10 mg/L increase) | 0.96 | 0.09–5.19 | 0.698 |
| LVEF at discharge (for a 1% increase) | 0.70 | 0.62–0.79 | <0.0000002 |
| TIMI flow pre-PCI (for a 1-point increase) | 0.63 | 0.44–0.88 | <0.008 |
| S’ at discharge (for a 1 cm/s increase) | 0.45 | 0.32–0.64 | <0.00002 |
| Multivariable analysis (MA): | |||
| BNP at discharge (for a 100 pg/mL increase) | 1.44 | 1.11–1.84 | <0.0002 |
| LVEF at discharge (for a 1% increase) | 0.73 | 0.65–0.83 | <0.00002 |
| MA with LVEF at discharge excluded: | |||
| LAD vs. non-LAD | 7.36 | 1.95–27.7 | <0.004 |
| BNP at discharge (for a 100 pg/mL increase) | 1.59 | 1.26–2.01 | <0.0002 |
| CRP at 24 h after admission (for a 10 mg/L increase) | 1.47 | 1.10–1.97 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świątkiewicz, I.; Magielski, P.; Kubica, J.; Zadourian, A.; DeMaria, A.N.; Taub, P.R. Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure. Int. J. Mol. Sci. 2020, 21, 807. https://doi.org/10.3390/ijms21030807
Świątkiewicz I, Magielski P, Kubica J, Zadourian A, DeMaria AN, Taub PR. Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure. International Journal of Molecular Sciences. 2020; 21(3):807. https://doi.org/10.3390/ijms21030807
Chicago/Turabian StyleŚwiątkiewicz, Iwona, Przemysław Magielski, Jacek Kubica, Adena Zadourian, Anthony N. DeMaria, and Pam R. Taub. 2020. "Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure" International Journal of Molecular Sciences 21, no. 3: 807. https://doi.org/10.3390/ijms21030807
APA StyleŚwiątkiewicz, I., Magielski, P., Kubica, J., Zadourian, A., DeMaria, A. N., & Taub, P. R. (2020). Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure. International Journal of Molecular Sciences, 21(3), 807. https://doi.org/10.3390/ijms21030807





