Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats
Abstract
:1. Introduction
2. Results
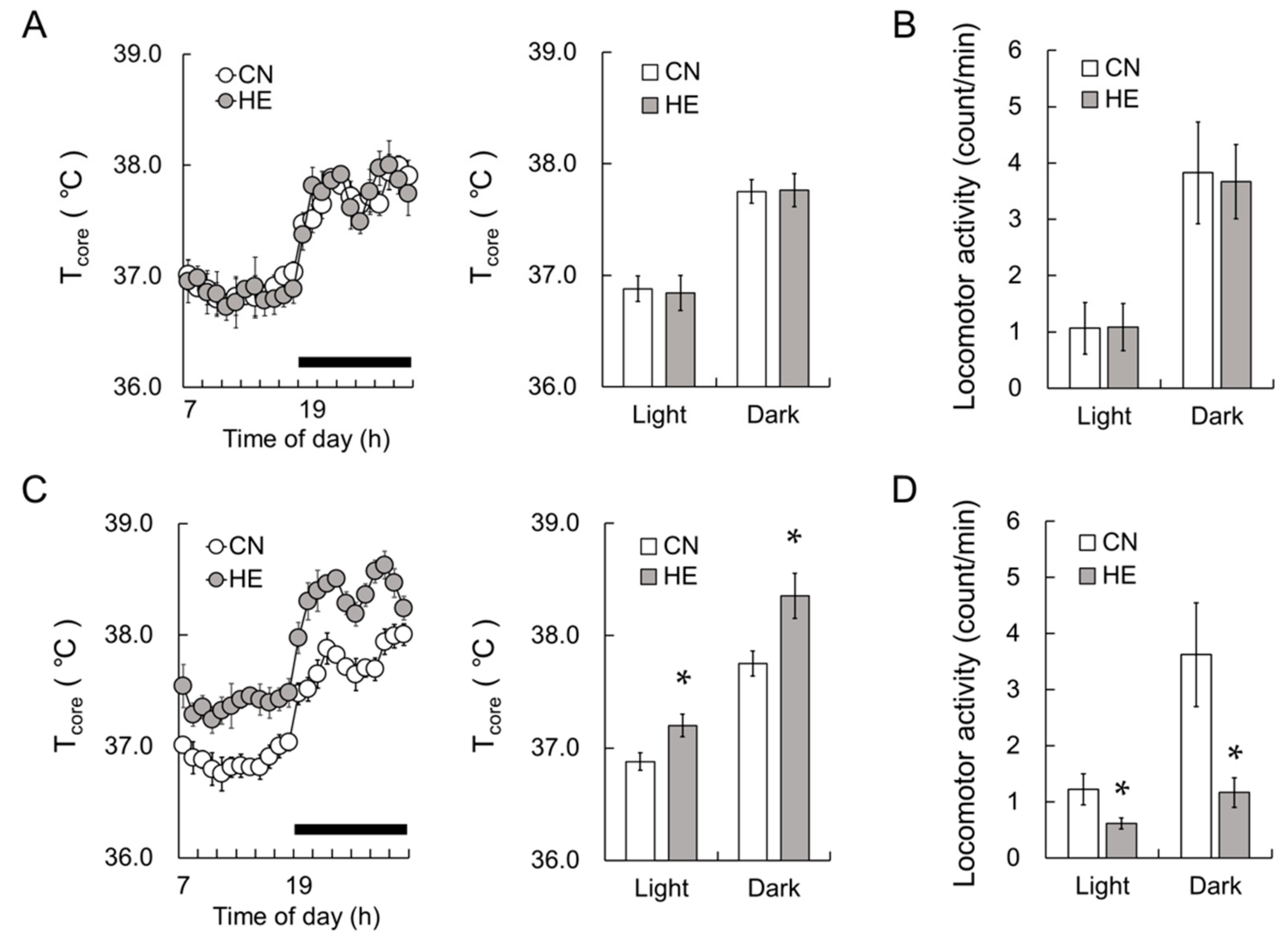
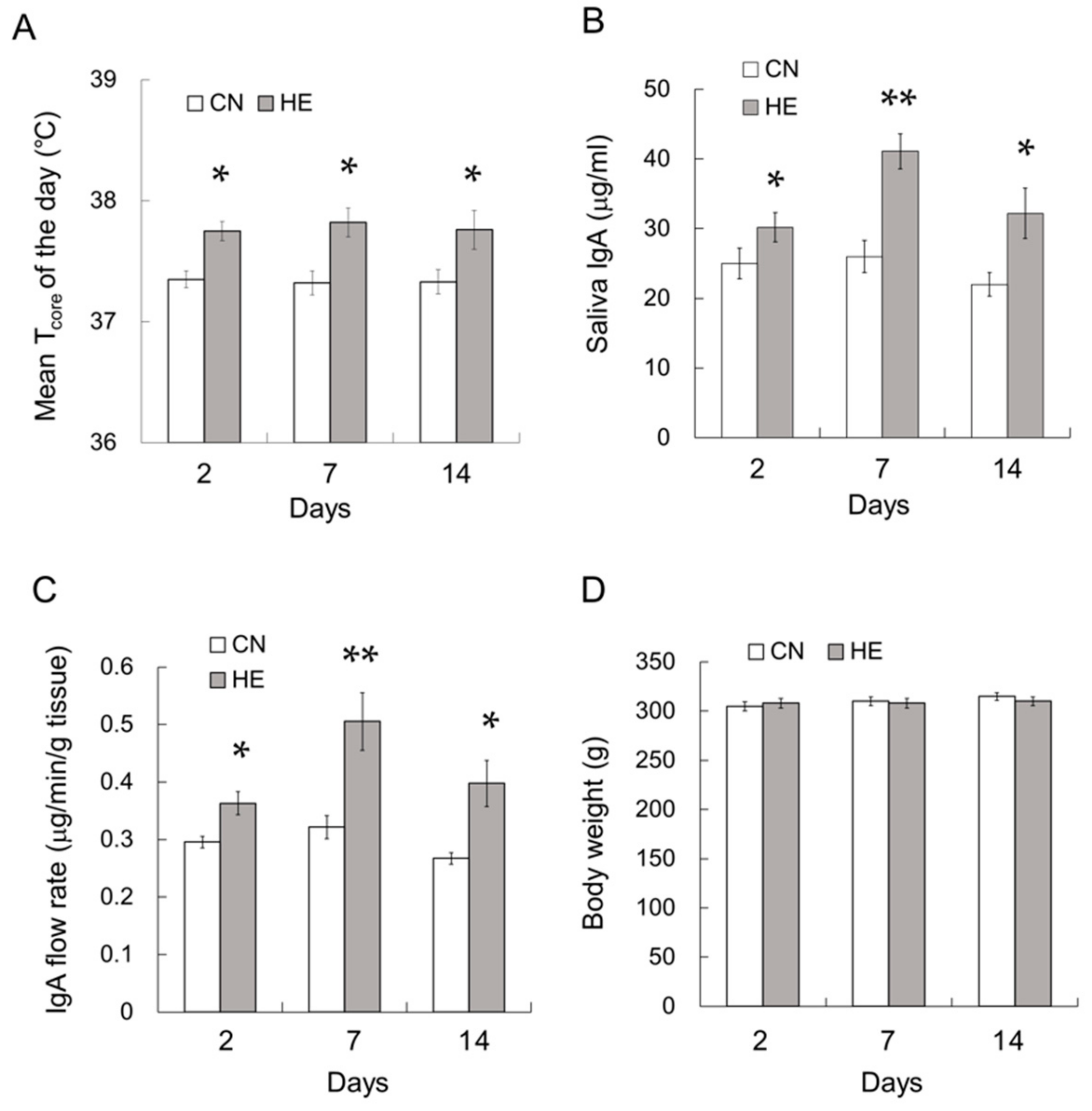
2.1. Tcore and Locomotor Activity
2.2. Body, SMG, and Adrenal Gland (AG) Weight
2.3. Blood Cell Counts
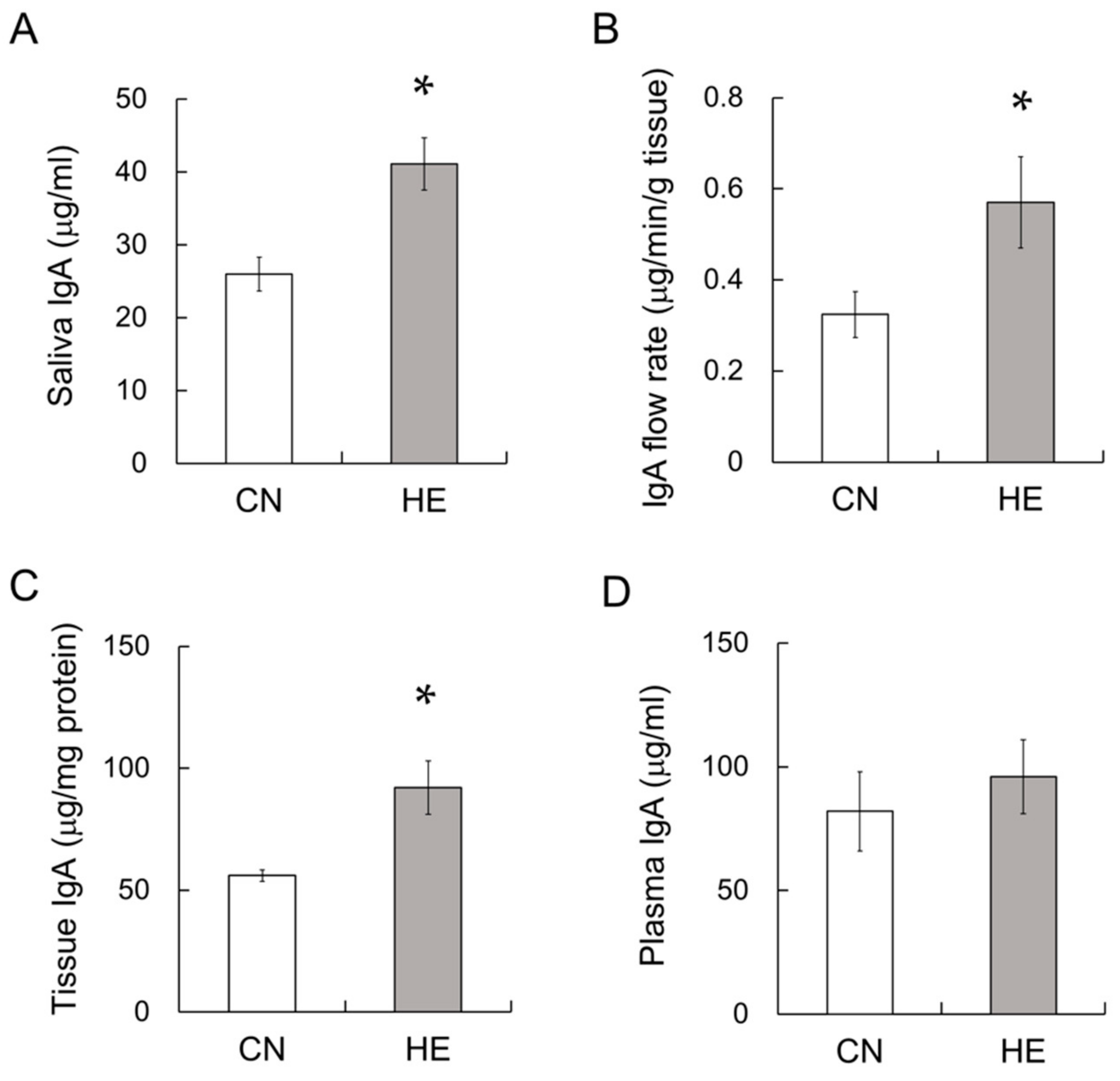
2.4. IgA Concentration in the Saliva, SMGs and Plasma
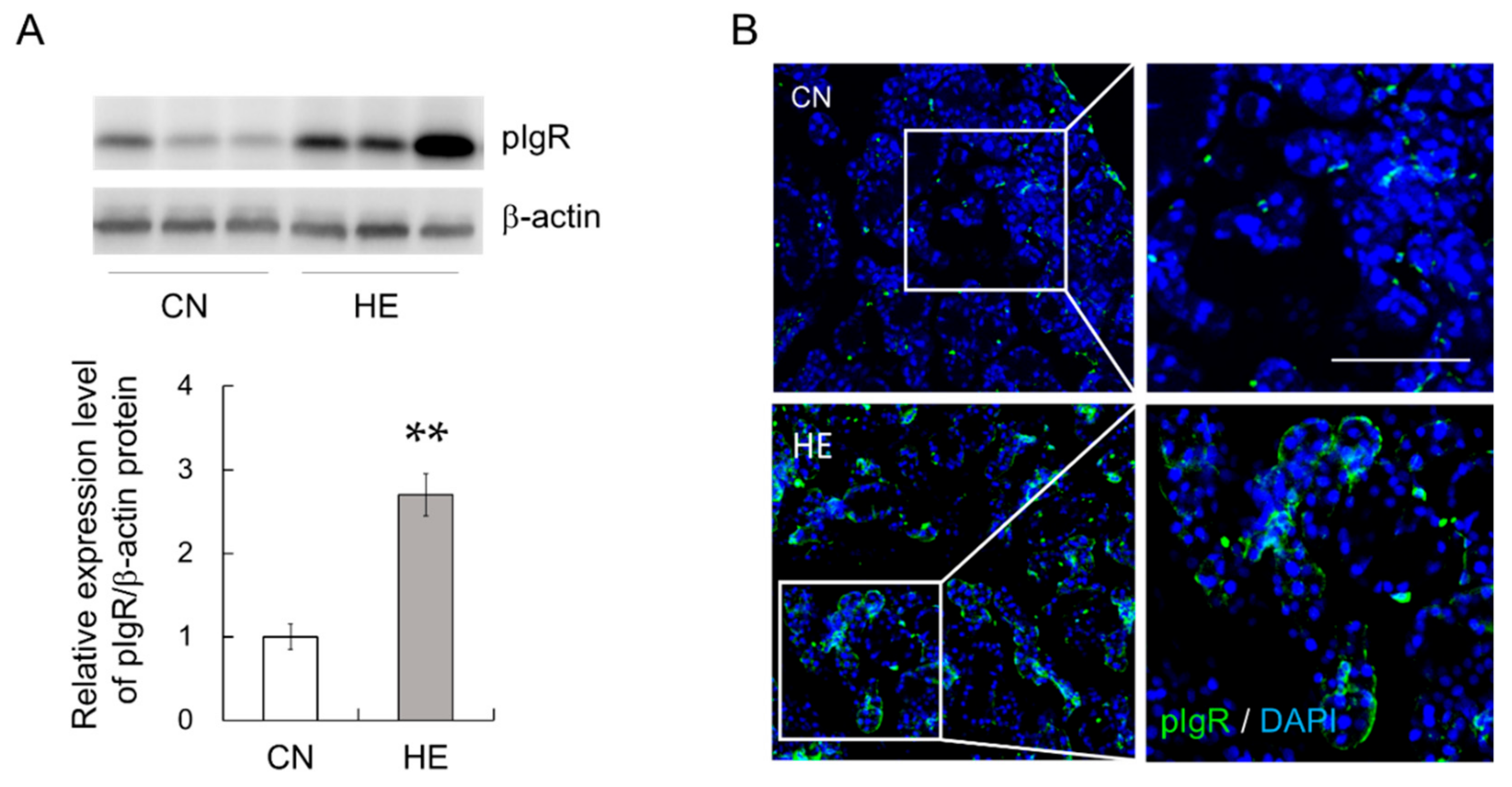
2.5. pIgR Expression in the SMGs
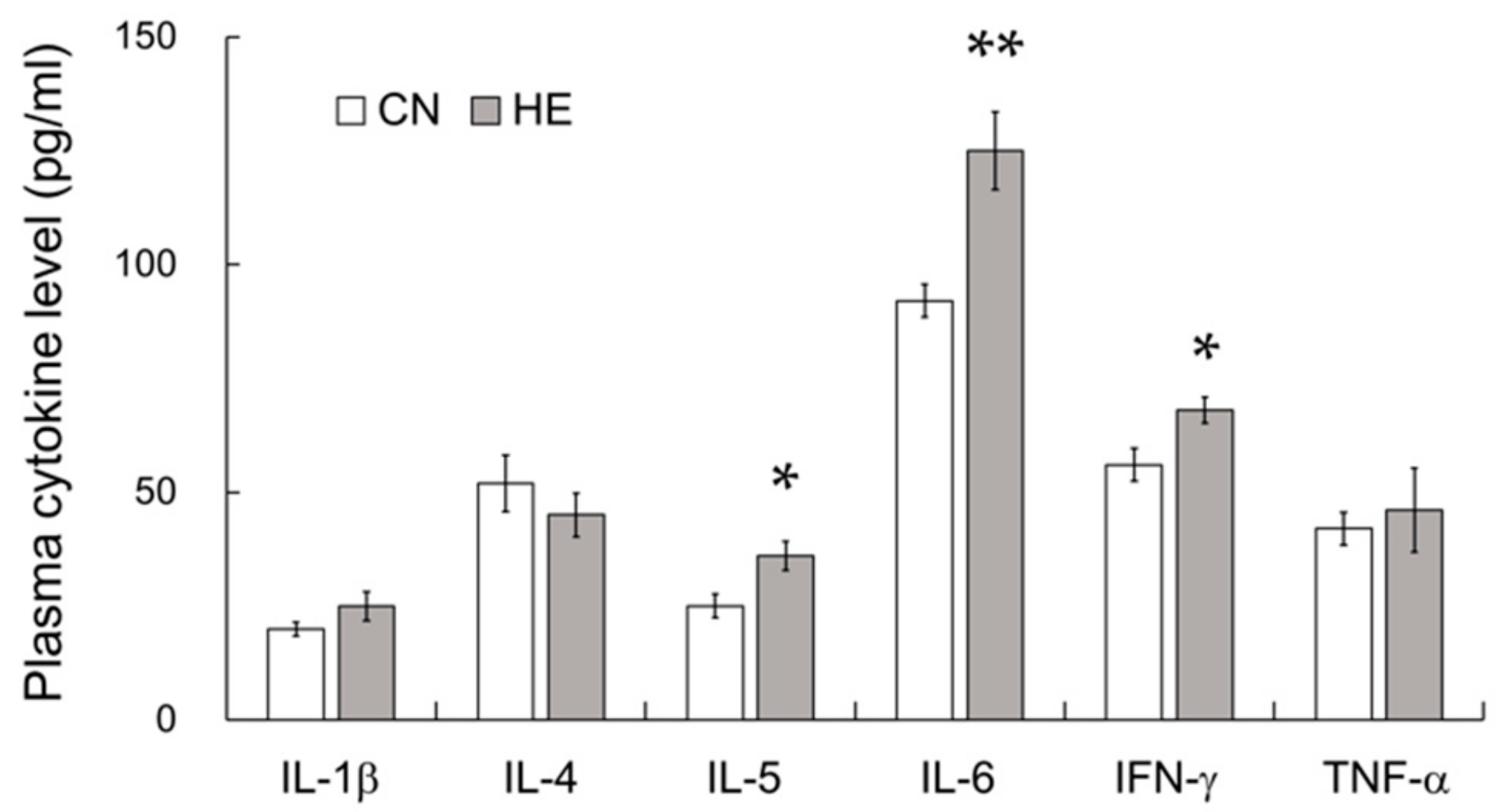
2.6. Plasma Cytokine Levels
2.7. Plasma GC and GC Receptor (GR) Levels in the SMGs
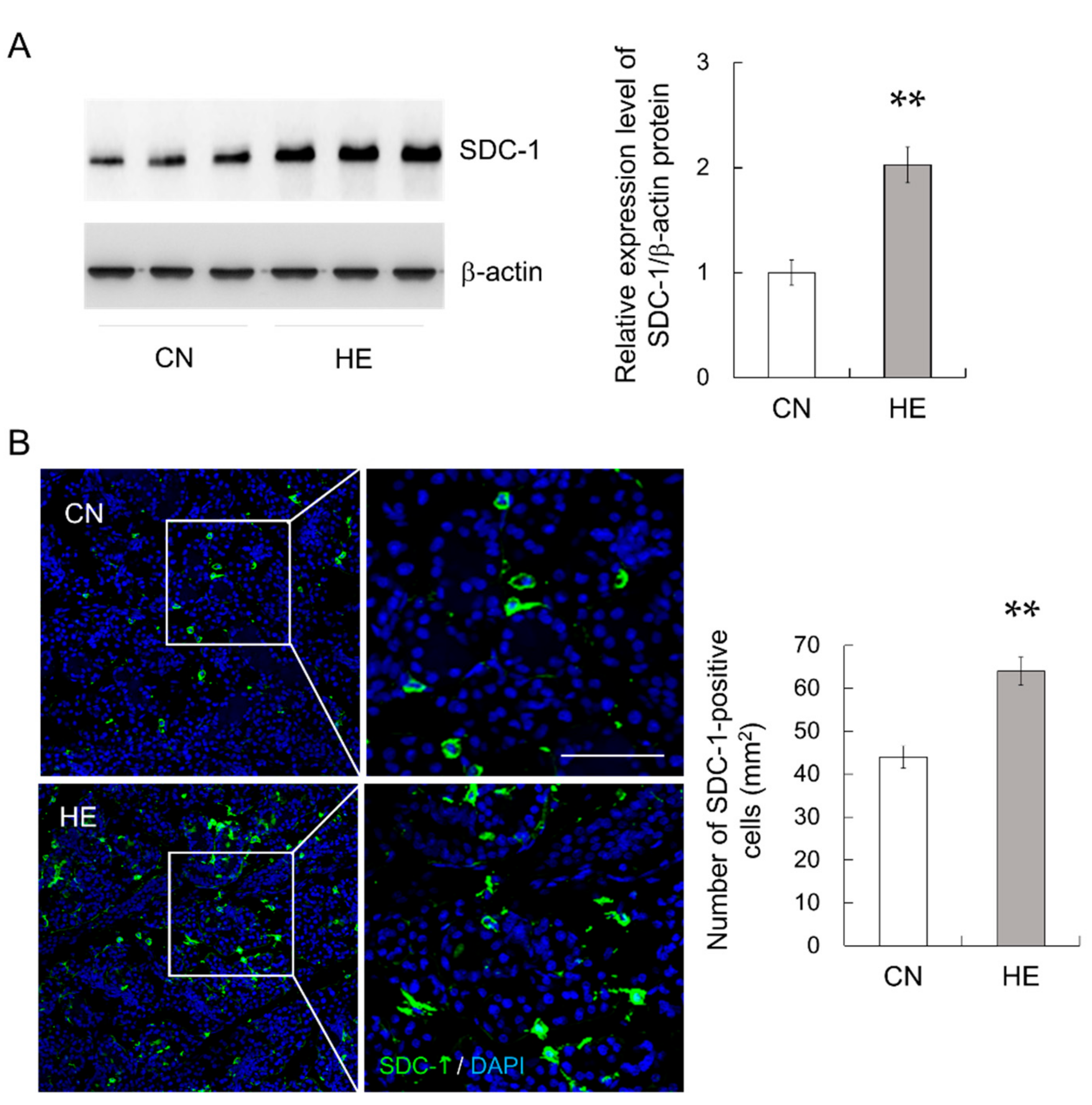
2.8. Syndecan-1 (SDC-1) Expression in the SMGs
2.9. Time Course Analysis of the Effect of Heat Exposure on IgA Secretion
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Schedule
4.3. Saliva Collection
4.4. Blood and Tissue Collection
4.5. Blood Cell Counts
4.6. Western Blot Analysis
4.7. ELISA
4.8. Immunohistochemistry
4.9. Cytokine Measurements
4.10. Time Course Effects of Heat Exposure on IgA Secretion
4.11. Data Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Adrenal gland |
| CN | Control |
| ELISA | Enzyme-linked immunosorbent assay |
| GC | Glucocorticoid |
| GR | Glucocorticoid receptor |
| HE | Heat-exposed |
| HGB | Hemoglobin |
| HTC | Hematocrit |
| IFNγ | Interferon-γ |
| IgA | Immunoglobulin A |
| IL | Interleukin |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| pIgR | Polymeric immunoglobulin receptor |
| PLT | Platelet |
| RBC | Red blood cell |
| SC | Secretory component |
| SMG | Submandibular gland |
| TNFα | Tumor necrosis factor-α |
| WBC | White blood cell |
References
- Williams, R.C.; Gibbons, R.J. Inhibition of bacterial adherence by secretory immunoglobulin A: A mechanism of antigen disposal. Science 1972, 177, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann. N. Y. Acad. Sci. 2007, 1098, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, F.E.; Kaetzel, C.S. Regulation of the polymeric immunoglobulin receptor and IgA transport: New advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011, 4, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Turula, H.; Wobus, C.E. The role of the polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Rose, P.T.; Gregory, R.L.; Gfell, L.E.; Hughes, C.V. IgA antibodies to Streptococcus mutans in caries-resistant and -susceptible children. Pediatr. Dent. 1994, 16, 272–275. [Google Scholar]
- Neville, V.; Gleeson, M.; Folland, J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sports Exerc. 2008, 40, 1228–1236. [Google Scholar] [CrossRef] [Green Version]
- Sollid, L.M.; Kvale, D.; Brandtzaeg, P.; Markussen, G.; Thorsby, E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J. Immunol. 1987, 138, 4303–4306. [Google Scholar]
- Kvale, D.; Lovhaug, D.; Sollid, L.M.; Brandtzaeg, P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J. Immunol. 1988, 140, 3086–3089. [Google Scholar]
- Hayashi, M.; Takenouchi, N.; Asano, M.; Kato, M.; Tsurumachi, T.; Saito, T.; Moro, I. The polymeric immunoglobulin receptor (secretory component) in a human intestinal epithelial cell line is up-regulated by interleukin-1. Immunology 1997, 92, 220–225. [Google Scholar] [CrossRef]
- Asano, M.; Komiyama, K. Polymeric immunoglobulin receptor. J. Oral Sci. 2011, 53, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincheval-Arnold, A.; Belair, L.; Cencic, A.; Djiane, J. Up-regulation of polymeric immunoglobulin receptor mRNA in mammary epithelial cells by IFN-gamma. Mol. Cell. Endocrinol. 2002, 194, 95–105. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Godínez-Victoria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Reyna-Garfias, H.; Arciniega-Martínez, I.M.; Kormanovski-Kovsova, A.; Campos-Rodriguez, R. Moderate exercise enhances expression of SIgA in mouse ileum. Int. J. Sports Med. 2012, 33, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary exercise increases IgA concentration and polymeric Ig receptor expression in the rat submandibular gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496. [Google Scholar] [CrossRef] [Green Version]
- Wijburg, O.L.; Uren, T.K.; Simpfendorfer, K.; Johansen, F.E.; Brandtzaeg, P.; Strugnell, R.A. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 2006, 203, 21–26. [Google Scholar] [CrossRef]
- Davids, B.J.; Palm, J.E.; Housley, M.P.; Smith, J.R.; Andersen, Y.S.; Martin, M.G.; Hendrickson, B.A.; Johansen, F.E.; Svard, S.G.; Gillin, F.D.; et al. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J. Immunol. 2006, 177, 6281–6290. [Google Scholar] [CrossRef] [Green Version]
- Hainsworth, F.R. Saliva spreading, activity, and body temperature regulation in the rat. Am. J. Physiol. 1967, 212, 1288–1292. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.C.; Cristiano, R.; Vizin, L.; Carrettiero, D.C. Current understanding on the neurophysiology of behavioral thermoregulation. Temperature 2015, 2, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Wyndham, C.H. Effect of acclimatization on the sweat rate-rectal temperature relationship. J. Appl. Physiol. 1967, 22, 27–30. [Google Scholar] [CrossRef]
- Horowitz, M.; Kaspler, P.; Simon, E.; Gerstberger, R. Heat acclimation and hypohydration: involvement of central angiotensin II receptors in thermoregulation. Am. J. Physiol. 1999, 277, R47–R55. [Google Scholar] [CrossRef]
- Sugimoto, N.; Shido, O.; Matsuzaki, K.; Ohno-Shosaku, T.; Hitomi, Y.; Tanaka, M.; Sawaki, T.; Fujita, Y.; Kawanami, T.; Masaki, Y.; et al. Cellular heat acclimation regulates cell growth, cell morphology, mitogen-activated protein kinase activation, and expression of aquaporins in mouse fibroblast cells. Cell. Physiol. Biochem. 2012, 30, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Shido, O.; Matsuzaki, K. Involvement of neurogenesis in the hypothalamic area in establishing long-term heat acclimation in rats. Temperature 2015, 3, 362–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shido, O.; Matsuzaki, K.; Katakura, M. Neurogenesis in the Thermoregulatory System. Handb. Clin. Neurol. 2018, 156, 457–463. [Google Scholar]
- Horowitz, M.; Meiri, U. Thermoregulatory activity in the rat: effects of hypohydration, hypovolemia and hypertonicity and their interaction with short-term heat acclimation. Comp. Biochem. Physiol. A Comp. Physiol. 1985, 82, 577–582. [Google Scholar] [CrossRef]
- Oron, Y.; Falach, O.; Marmary, I.; Horowitz, M. Long-term heat adaptation results in an enhanced efficiency of muscarinically-induced water secretion in rat submaxillary glands. Comp. Biochem. Physiol. A Comp. Physiol. 1989, 94, 673–676. [Google Scholar] [CrossRef]
- Furuyama, F.; Murakami, M.; Oiwa, T.; Nishino, H. Differences in thermal salivation between the FOK rat (a model of genotypic heat adaptation) and three other rat strains. Physiol. Behav. 1998, 63, 787–793. [Google Scholar] [CrossRef]
- Sugimoto, N.; Matsuzaki, K.; Ishibashi, H.; Tanaka, M.; Sawaki, T.; Fujita, Y.; Kawanami, T.; Masaki, Y.; Okazaki, T.; Sekine, J.; et al. Upregulation of aquaporin expression in the salivary glands of heat-acclimated rats. Sci. Rep. 2013, 3, 1763. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Fujioka, T.; Hashimoto, M.; Nakamura, S. Stress and brain angiotensin II receptors. Crit. Rev. Neurobiol. 1998, 12, 305–317. [Google Scholar] [CrossRef]
- Mikami, Y.; Iwase, T.; Komiyama, Y.; Matsumoto, N.; Oki, H.; Komiyama, K. Secretory leukocyte protease inhibitor inhibits expression of polymeric immunoglobulin receptor via the NF-κB signaling pathway. Mol. Immunol. 2015, 67, 568–574. [Google Scholar] [CrossRef]
- O’Connell, F.P.; Pinkus, J.L.; Pinkus, G.S. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am. J. Clin. Pathol. 2004, 121, 254–263. [Google Scholar] [CrossRef]
- Welc, S.S.; Phillips, N.A.; Oca-Cossio, J.; Wallet, S.M.; Chen, D.L.; Clanton, T.L. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am. J. Physiol. Cell. Physiol. 2012, 303, C455–C466. [Google Scholar] [CrossRef]
- Obi, S.; Nakajima, T.; Hasegawa, T.; Kikuchi, H.; Oguri, G.; Takahashi, M.; Nakamura, F.; Yamasoba, T.; Sakuma, M.; Toyoda, S.; et al. Heat induces interleukin-6 in skeletal muscle cells via TRPV1/PKC/CREB pathways. J. App. Phys. 2017, 122, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, G.; Ring, C.; Carroll, D.; Evans, P.; Clow, A.; Hucklebridge, F. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic and cold pressor. Psychophysiology 1998, 35, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Zeier, H.; Brauchli, P.; Joller-Jemelka, H.I. Effects of work demands on immunoglobulin A and cortisol in air traffic controllers. Biol. Psychol. 1996, 42, 413–423. [Google Scholar] [CrossRef]
- Yamamoto, S.; Motomura, A.; Akahoshi, A.; Takahashi, K.; Minami, H. Immunoglobulin secretions in the mesenteric lymph node in stressed rats. J. Nutr. Sci. Vitaminol. 2009, 55, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Laurent, H.K.; Stroud, L.R.; Brush, B.; D’Angelo, C.; Granger, D.A. Secretory IgA reactivity to social threat in youth: relations with HPA, ANS, and behavior. Psychoneuroendocrinology 2015, 59, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Campos-Rodríguez, R.; Godínez-Victoria, M.; Abarca-Rojano, E.; Pacheco-Yépez, J.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Stress modulates intestinal secretory immunoglobulin A. Front. Integr. Neurosci. 2013, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, H.; Lu, A.; Zhong, Y.; Hou, X.; Wang, N.; Jia, D.; Zan, J.; Zhao, H.; Xu, J.; et al. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation. Int. J. Hyperthermia 2012, 28, 756–765. [Google Scholar] [CrossRef]
- Furukawa, M.; Kawamoto, T.; Noshiro, M.; Honda, K.K.; Sakai, M.; Fujimoto, K.; Honma, S.; Honma, K.; Hamada, T.; Kato, Y. Clock gene expression in the submandibular glands. J. Dent. Res. 2005, 84, 1193–1197. [Google Scholar] [CrossRef]
- Wada, M.; Orihara, K.; Kamagata, M.; Hama, K.; Sasaki, H.; Haraguchi, A.; Miyakawa, H.; Nakao, A.; Shibata, S. Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice. Sci. Rep. 2017, 7, 8802. [Google Scholar] [CrossRef]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejerdy, P.; Fabian, G. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Sőti, C.; Nguyen, M.T.; Csermely, P.; Fejérdy, P. Expected functions of salivary HSP70 in the oral cavity. In Heat Shock Proteins: New Research, 1st ed.; Morel, E., Vincent, C., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2008; pp. 321–340. [Google Scholar]
- Fábián, T.K.; Beck, A.; Fejérdy, P.; Hermann, P.; Fábián, G. Molecular mechanisms of taste recognition: considerations about the role of saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.K.; Anand, E.; Bleck, C.K.E.; Anes, E.; Griffiths, G. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLOS ONE 2010, 5, e10136. [Google Scholar] [CrossRef] [PubMed]
- Vanmuylder, N.; Evrard, L.; Daelemans, P.; Dourov, N. Chaperones in the Parotid Gland: Localization of Heat Shock Proteins in Human Adult Salivary Glands. Cells Tissues Organs 2000, 167, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Katakura, M.; Maruyama, M.; Enhkjargal, B.; Matsuzaki, K.; Hashimoto, M.; Shido, O. Changes of noradrenaline-induced contractility and gene expression in aorta of rats acclimated to heat in two different modes. Eur. J. Appl. Physiol. 2008, 104, 29–40. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Katakura, M.; Hara, T.; Li, G.; Hashimoto, M.; Shido, O. Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflüg. Arch. 2009, 458, 661–673. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Katakura, M.; Inoue, T.; Hara, T.; Hashimoto, M.; Shido, O. Aging attenuates acquired heat tolerance and hypothalamic neurogenesis in rats. J. Comp. Neurol. 2015, 523, 1190–1201. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Katakura, M.; Sugimoto, N.; Hara, T.; Hashimoto, M.; Shido, O. β-amyloid infusion into lateral ventricle alters behavioral thermoregulation and attenuates acquired heat tolerance in rats. Temperature 2015, 2, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Katakura, M.; Sugimoto, N.; Hara, T.; Hashimoto, M.; Shido, O. Neural progenitor cell proliferation in the hypothalamus is involved in acquired heat tolerance in long-term heat-acclimated rats. PLOS ONE 2017, 12, e0178787. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Sugimoto, N.; Katakura, M.; Sumiyoshi, E.; Hara, T.; Hashimoto, M.; Shido, O. Daily voluntary exercise enhances pilocarpine-induced saliva secretion and aquaporin 1 expression in rat submandibular glands. FEBS Open Bio. 2018, 8, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Ishii, H.; Nakagawa, Y. Stress response to surgical procedures in the submandibular region and its influence on saliva secretion in mice. Arch. Oral Biol. 2001, 46, 387–390. [Google Scholar] [CrossRef]
- Islam, R.; Matsuzaki, K.; Sumiyoshi, E.; Hossain, M.E.; Hashimoto, M.; Katakura, M.; Sugimoto, N.; Shido, O. Theobromine improves working memory by activating the CaMKII/CREB/BDNF pathway in rats. Nutrients 2019, 11, E888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.E.; Matsuzaki, K.; Katakura, M.; Sugimoto, N.; Mamun, A.A.; Islam, R.; Hashimoto, M.; Shido, O. Direct exposure to mild heat promotes proliferation and neuronal differentiation of neural stem/progenitor cells in vitro. PLOS ONE 2017, 12, e0190356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, A.A.; Romanovsky, A.A. Energy trade-offs in Host Defense: Immunology Meets Physiology. Trends Endocrinol. Metab. 2019, 30, 875–878. [Google Scholar] [CrossRef]

| CN | HE | P Value | |
|---|---|---|---|
| BW (g) | 310.7 ± 3.2 | 305.5 ± 3.9 | 0.083 |
| SMG/BW (mg/g) | 0.98 ± 0.01 | 1.00 ± 0.02 | 0.505 |
| AG/BW (mg/g) | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.442 |
| CN | HE | P Value | |
|---|---|---|---|
| WBC (× 102/μL) | 66.7 ± 3.2 | 68.5 ± 3.9 | 0.130 |
| RBC (× 105/μL) | 86.0 ± 3.6 | 87.5 ± 4.6 | 0.579 |
| PLT (× 104/μL) | 68.4 ± 2.4 | 65.7 ± 4.8 | 0.234 |
| HGB (g/dL) | 15.1 ± 1.1 | 15.5 ± 0.8 | 0.505 |
| HTC (%) | 48.0 ± 2.8 | 50.2 ± 3.3 | 0.161 |
| MVC (fl) | 54.9 ± 1.2 | 55.4 ± 1.5 | 0.195 |
| MCH (pg) | 17.5 ± 1.0 | 18.0 ± 1.2 | 0.234 |
| MCHC (g/dL) | 30.2 ± 2.0 | 31.8 ± 2.1 | 0.195 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, K.; Sugimoto, N.; Islam, R.; Hossain, M.E.; Sumiyoshi, E.; Katakura, M.; Shido, O. Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats. Int. J. Mol. Sci. 2020, 21, 815. https://doi.org/10.3390/ijms21030815
Matsuzaki K, Sugimoto N, Islam R, Hossain ME, Sumiyoshi E, Katakura M, Shido O. Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats. International Journal of Molecular Sciences. 2020; 21(3):815. https://doi.org/10.3390/ijms21030815
Chicago/Turabian StyleMatsuzaki, Kentaro, Naotoshi Sugimoto, Rafiad Islam, Md Emon Hossain, Eri Sumiyoshi, Masanori Katakura, and Osamu Shido. 2020. "Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats" International Journal of Molecular Sciences 21, no. 3: 815. https://doi.org/10.3390/ijms21030815





