Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria
Abstract
:1. Introduction
2. Results
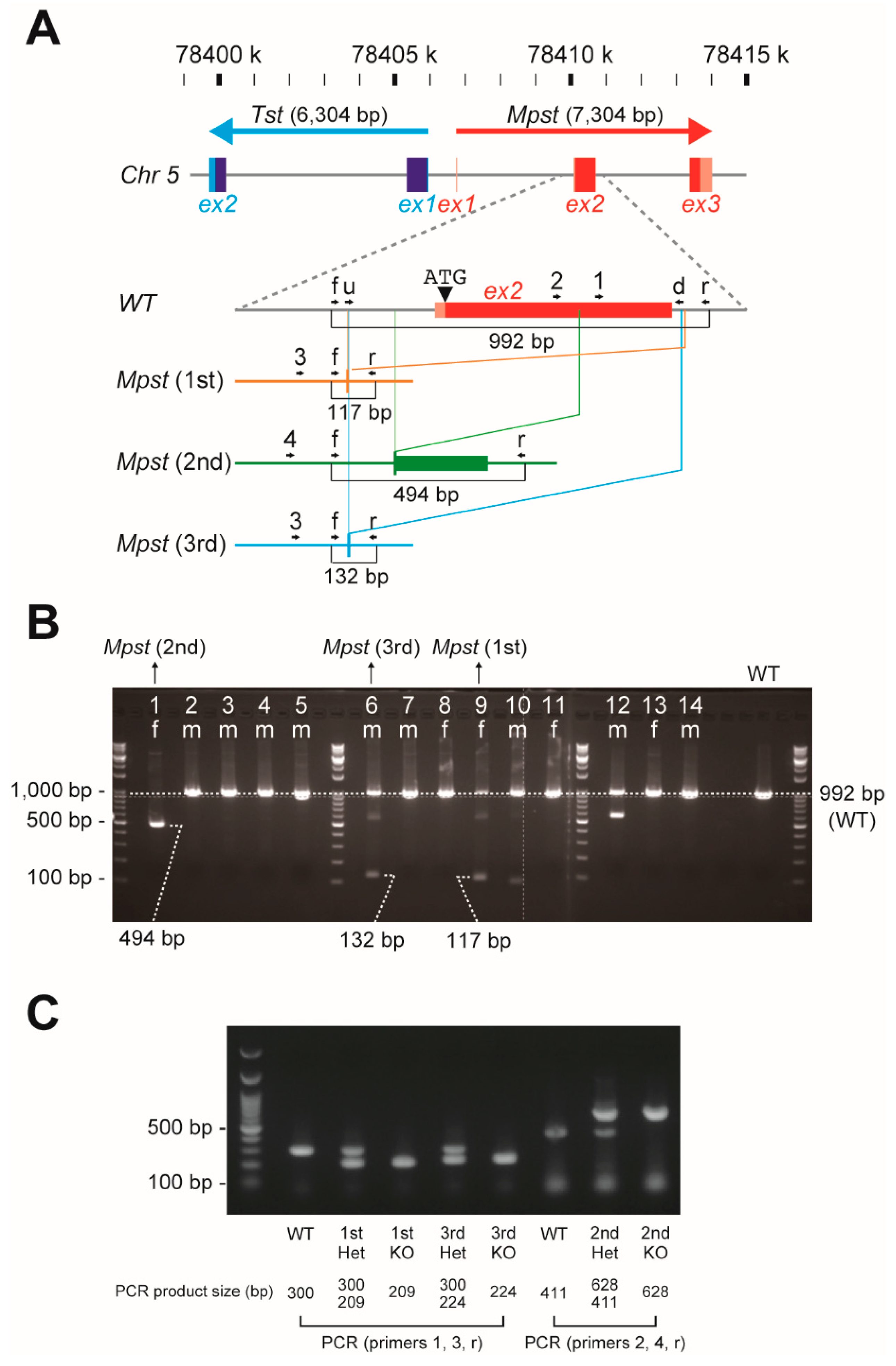
2.1. Establishment of Three Independent Mpst Mutant Lines
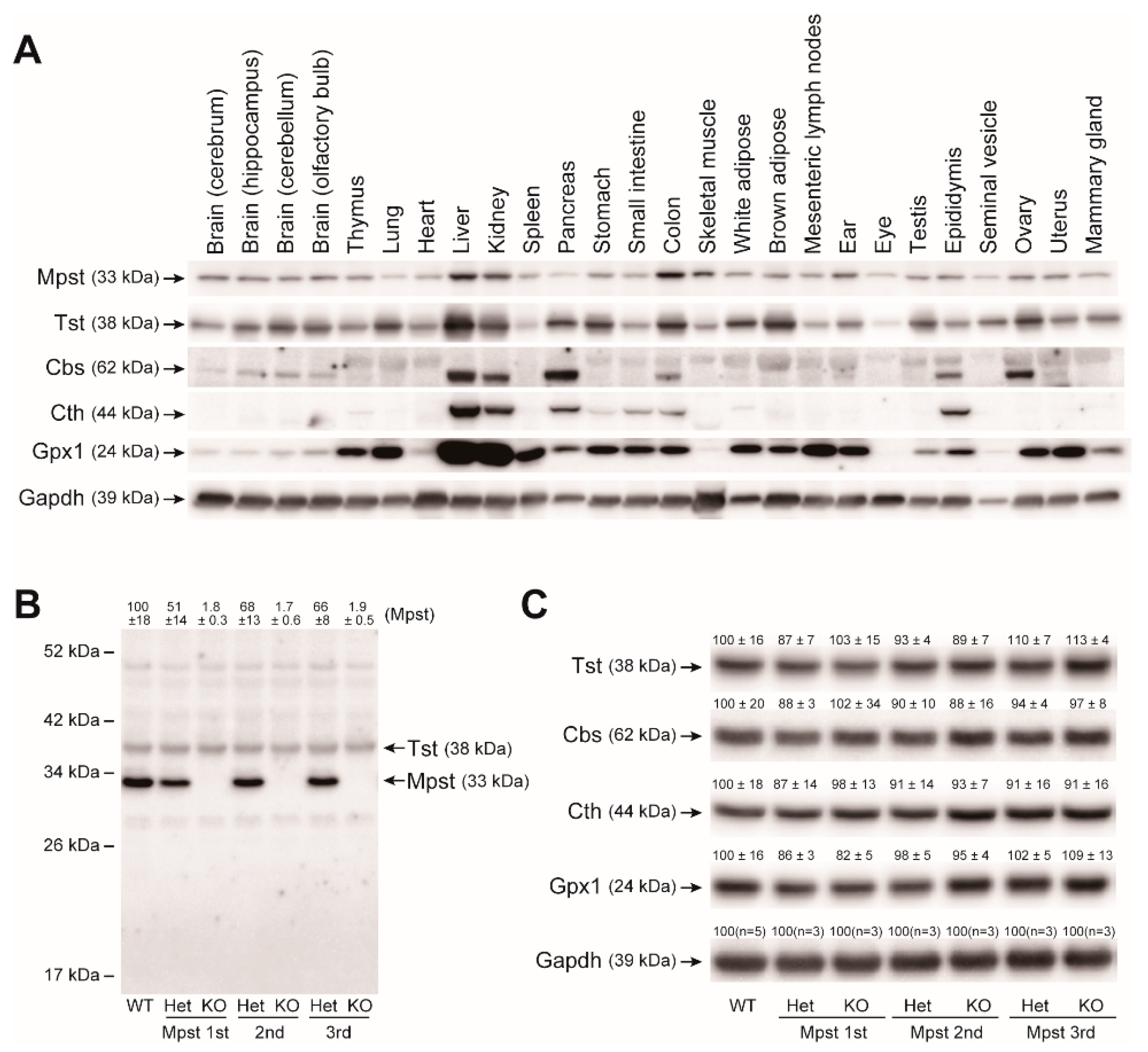
2.2. Mpst, Tst, Cbs, and Cth Expression in Mpst Mutant Livers
2.3. Increased Urinary Excretion of 3-Mercaptolactate in Mpst-KO Mice
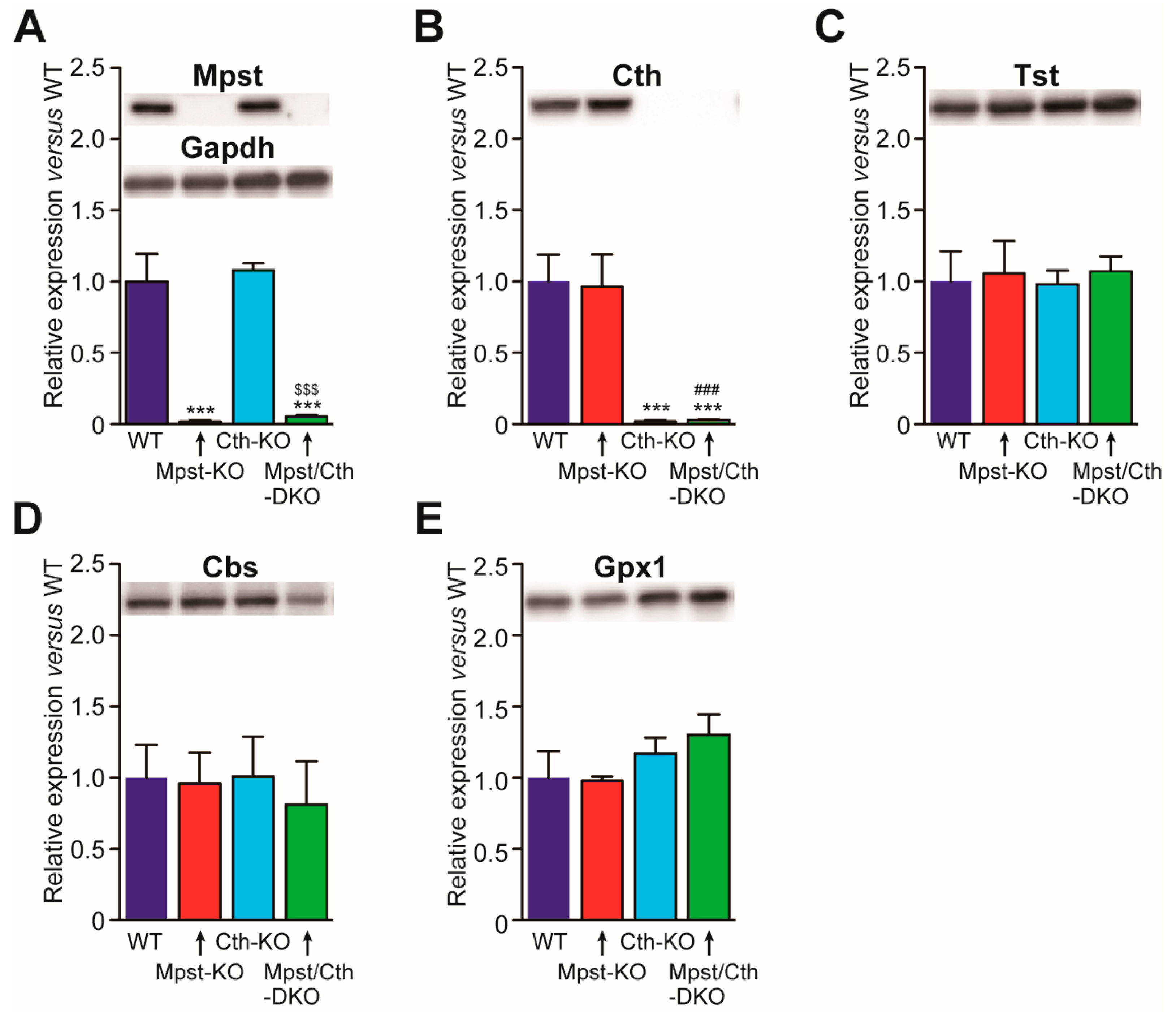
2.4. Generation of Mice Lacking Both Mpst and Cth
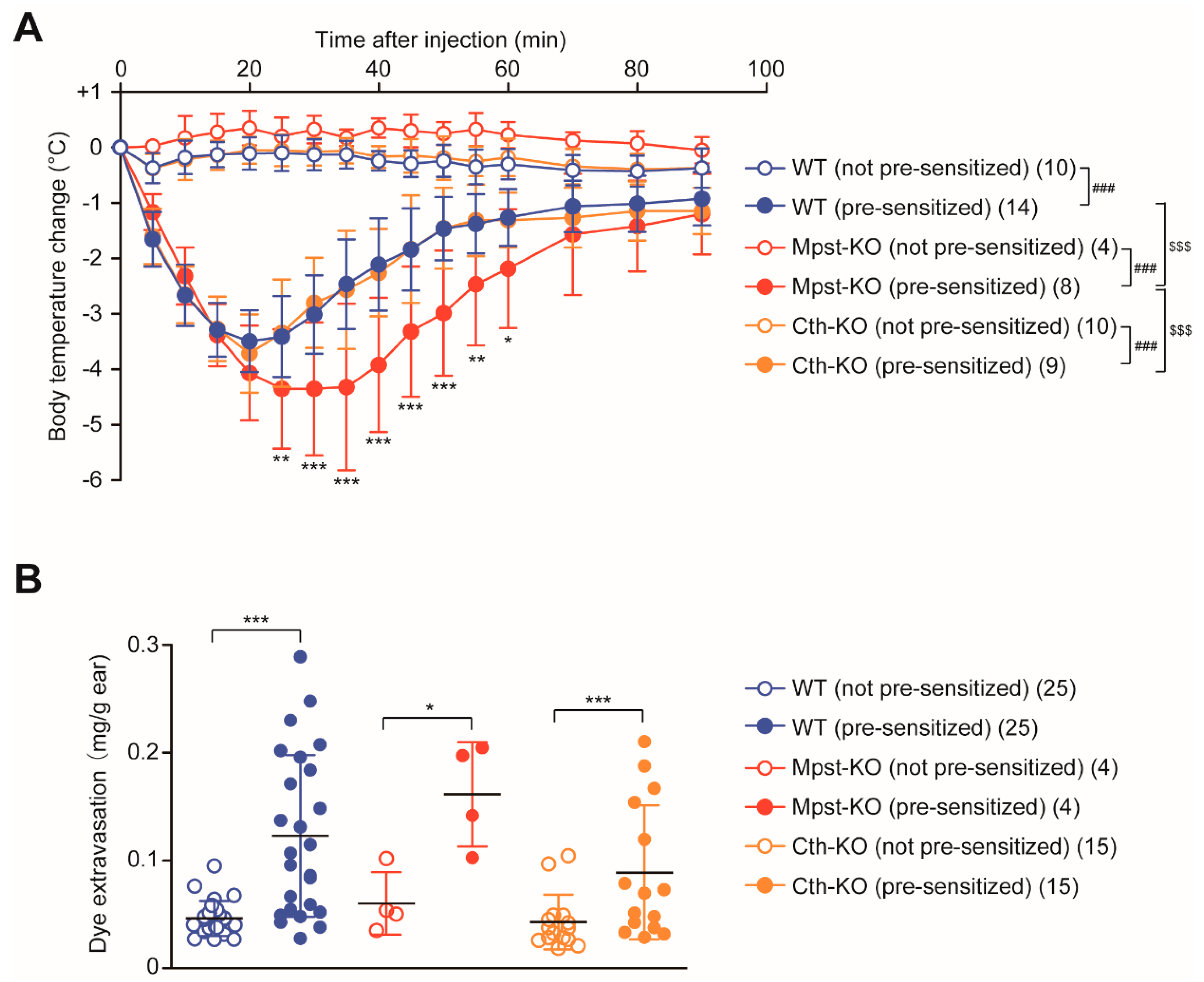
2.5. Enhanced PSA Response in Mpst-KO Mice
3. Discussion
4. Materials and Methods
4.1. Generation of Mpst (1st–3rd)-KO Mice
4.2. Preparation of Mpst and Tst Recombinant Proteins
4.3. Rabbit Polyclonal Mpst Antibody Production
4.4. Western Blot Analyses
4.5. Mpst and Tst Activity Assays
4.6. Measurements of Amino Acid/Thiol Compound Levels and Biochemical Parameters
4.7. PSA and PCA Assays
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BUN | Blood urea nitrogen |
| Cars2 | Cysteinyl-tRNA synthetase 2 |
| Cbs | Cystathionine β-synthase |
| CPK | Creatine phosphokinase |
| CRE | Creatinine |
| Cth | Cystathionine γ-lyas |
| DKO | Double knockout |
| DNP | 2,4-dinitrophenyl |
| Gpx1 | Glutathione peroxidase 1 |
| GSH | Glutathione |
| GSSG | Glutathione (oxidized form) |
| Hcy | Homocysteine |
| H2S | Hydrogen sulfide |
| LDH | Lactate dehydrogenase |
| MCDU | Mercaptolactate-cysteine disulfiduria |
| 3-ML | 3-Mercaptolactate |
| 3-MP | 3-Mercaptopyruvate |
| Mpst | Mercaptopyruvate sulfurtransferase |
| PCA | Passive cutaneous anaphylaxis |
| PSA | Passive systemic anaphylaxis |
| RSS | Reactive sulfur species |
| TBARS | Thiobarbituric acid reactive substances |
| Tst | Thiosulfate sulfurtransferase |
| UA | Uric acid |
| WT | Wild-type |
References
- Ampola, M.G.; Efron, M.L.; Bixby, E.M.; Meshorer, E. Mental deficiency and a new aminoaciduria. Am. J. Dis. Child 1969, 117, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Crawhall, J.C.; Parker, R.; Sneddon, W.; Young, E.P.; Ampola, M.G.; Efron, M.L.; Bixby, E.M. Beta mercaptolactate-cysteine disulfide: Analog of cystine in the urine of a mentally retarded patient. Science 1968, 160, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Crawhall, J.C.; Parker, R.; Sneddon, W.; Young, E.P. Beta-mercaptolactate-cysteine disulfide in the urine of a mentally retarded patient. Am. J. Dis. Child 1969, 117, 71–82. [Google Scholar] [PubMed]
- Crawhall, J.C.; Bir, K.; Purkiss, P.; Stanbury, J.B. Sulfur amino acids as precursors of beta-mercaptolactate-cysteine disulfide in human subjects. Biochem. Med. 1971, 5, 109–115. [Google Scholar] [CrossRef]
- Hannestad, U.; Martensson, J.; Sjodahl, R.; Sorbo, B. 3-mercaptolactate cysteine disulfiduria: Biochemical studies on affected and unaffected members of a family. Biochem. Med. 1981, 26, 106–114. [Google Scholar] [CrossRef]
- Niederwiesler, A.; Giliberti, P.; Baerlocher, K. Beta-mercaptolactate cysteine disulfiduria in two normal sisters. Isolation and characterization of beta-mercaptolactate cysteine disulfide. Clin. Chim. Acta 1973, 43, 405–416. [Google Scholar] [CrossRef]
- Nagahara, N.; Nagano, M.; Ito, T.; Shimamura, K.; Akimoto, T.; Suzuki, H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: A model for human mercaptolactate-cysteine disulfiduria. Sci. Rep. 2013, 3, 1986. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N. Catalytic site cysteines of thiol enzyme: Sulfurtransferases. J. Amino Acids 2011, 2011, 709404. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Okazaki, T.; Nishino, T. Cytosolic mercaptopyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J. Biol. Chem. 1995, 270, 16230–16235. [Google Scholar]
- Nagahara, N.; Tanaka, M.; Tanaka, Y.; Ito, T. Novel Characterization of antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice: Overexpression of the evolutionarily-related enzyme rhodanese. Antioxidants 2019, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Ubuka, T.; Ohta, J.; Akagi, R.; Hosaki, Y.; Ishimoto, Y.; Kiguchi, S.; Ikeda, T.; Ishino, K. Metabolism ofL-cysteine via transamination pathway (3-mercaptopyruvate pathway). Amino Acids 1992, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Minami, M. Mercaptopyruvate sulfurtransferase as a defense against cyanide toxication: Molecular properties and mode of detoxification. Histol. Histopathol. 1999, 14, 1277–1286. [Google Scholar] [PubMed]
- Wrobel, M.; Jurkowska, H.; Sliwa, L.; Srebro, Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.A.; Kelly, S.M.; Mottram, J.C.; Coombs, G.H. 3-Mercaptopyruvate sulfurtransferase of Leishmania contains an unusual C-terminal extension and is involved in thioredoxin and antioxidant metabolism. J. Biol. Chem. 2003, 278, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef]
- Kimura, Y.; Toyofuku, Y.; Koike, S.; Shibuya, N.; Nagahara, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015, 5, 14774. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine gamma-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Unoki, T.; Shinkai, Y.; Ishii, I.; Ida, T.; Akaike, T.; Yamamoto, M.; Kumagai, Y. Environmental electrophile-mediated toxicity in mice lacking Nrf2, CSE, or both. Environ. Health Perspect. 2019, 127, 67002. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, Y.; Kamata, S.; Mitsuoka, S.; Okada, N.; Yoshida, S.; Yamamoto, J.; Ohkubo, R.; Abiko, Y.; Yamada, H.; Akahoshi, N.; et al. Hemizygosity of transsulfuration genes confers increased vulnerability against acetaminophen-induced hepatotoxicity in mice. Toxicol. Appl. Pharmacol. 2015, 282, 195–206. [Google Scholar] [CrossRef]
- Yamada, H.; Akahoshi, N.; Kamata, S.; Hagiya, Y.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Takano, N.; Mori, M.; Ishizaki, Y.; et al. Methionine excess in diet induces acute lethal hepatitis in mice lacking cystathionine gamma-lyase, an animal model of cystathioninuria. Free Radic. Biol. Med. 2012, 52, 1716–1726. [Google Scholar] [CrossRef]
- Nakano, S.; Ishii, I.; Shinmura, K.; Tamaki, K.; Hishiki, T.; Akahoshi, N.; Ida, T.; Nakanishi, T.; Kamata, S.; Kumagai, Y.; et al. Hyperhomocysteinemia abrogates fasting-induced cardioprotection against ischemia/reperfusion by limiting bioavailability of hydrogen sulfide anions. J. Mol. Med. 2015, 93, 879–889. [Google Scholar] [CrossRef]
- Han, S.J.; Noh, M.R.; Jung, J.M.; Ishii, I.; Yoo, J.; Kim, J.I.; Park, K.M. Hydrogen sulfide-producing cystathionine gamma-lyase is critical in the progression of kidney fibrosis. Free Radic. Biol. Med. 2017, 112, 423–432. [Google Scholar] [CrossRef]
- Akahoshi, N.; Handa, H.; Takemoto, R.; Kamata, S.; Yoshida, M.; Onaka, T.; Ishii, I. Preeclampsia-like features and partial lactation failure in mice lacking cystathionine gamma-lyase-an animal model of cystathioninuria. Int. J. Mol. Sci. 2019, 20, 3507. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, M. H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 2015, 230, 165–180. [Google Scholar]
- Flannigan, K.L.; Wallace, J.L. Hydrogen sulfide-based anti-inflammatory and chemopreventive therapies: An experimental approach. Curr. Pharm. Des. 2015, 21, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.; Ardusso, L.R.; Bilo, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Lacourciere, G.M.; Ishii, K.; Stadtman, T.C. Characterization of potential selenium-binding proteins in the selenophosphate synthetase system. Proc. Natl. Acad. Sci. USA 2005, 102, 1012–1016. [Google Scholar] [CrossRef] [Green Version]
- Balbino, B.; Sibilano, R.; Starkl, P.; Marichal, T.; Gaudenzio, N.; Karasuyama, H.; Bruhns, P.; Tsai, M.; Reber, L.L.; Galli, S.J. Pathways of immediate hypothermia and leukocyte infiltration in an adjuvant-free mouse model of anaphylaxis. J. Allergy Clin. Immunol. 2017, 139, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Makabe-Kobayashi, Y.; Hori, Y.; Adachi, T.; Ishigaki-Suzuki, S.; Kikuchi, Y.; Kagaya, Y.; Shirato, K.; Nagy, A.; Ujike, A.; Takai, T.; et al. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J. Allergy Clin. Immunol. 2002, 110, 298–303. [Google Scholar] [CrossRef]
- Soriano, R.N.; Braga, S.P.; Breder, J.S.C.; Batalhao, M.E.; Oliveira-Pelegrin, G.R.; Ferreira, L.F.R.; Rocha, M.J.A.; Carnio, E.C.; Branco, L.G.S. Endogenous peripheral hydrogen sulfide is propyretic: Its permissive role in brown adipose tissue thermogenesis in rats. Exp. Physiol. 2018, 103, 397–407. [Google Scholar] [CrossRef]
- Harvey, R.A.; Ferrier, D.A. Lippincott’s Illustrated Reviews: Biochemistry, 5th ed.; Woulters Kluwer–Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 261–276. [Google Scholar]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice deficient in cystathionine beta-synthase: Animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Akahoshi, N.; Kobayashi, C.; Ishizaki, Y.; Izumi, T.; Himi, T.; Suematsu, M.; Ishii, I. Genetic background conversion ameliorates semi-lethality and permits behavioral analyses in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. Hum. Mol. Genet. 2008, 17, 1994–2005. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Yamashita, Y.; Takemoto, T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 2016, 418, 1–9. [Google Scholar] [CrossRef]
- Namekata, K.; Enokido, Y.; Ishii, I.; Nagai, Y.; Harada, T.; Kimura, H. Abnormal lipid metabolism in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. J. Biol. Chem. 2004, 279, 52961–52969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarabak, R.; Westley, J. 3-Mercaptopyruvate sulfurtransferase: Rapid equilibrium-ordered mechanism with cyanide as the acceptor substrate. Biochemistry 1980, 19, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rao, P.; Bhattacharya, R. Dose and time-dependent effects of cyanide on thiosulfate sulfurtransferase, 3-mercaptopyruvate sulfurtransferase, and cystathionine lambda-lyase activities. J. Biochem. Mol. Toxicol. 2013, 27, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kotanska, M.; Knutelska, J.; Bednarski, M.; Zygmunt, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Gorny, M.; Sokolowska-Jezewicz, M. The effect of NaCl on the level of reduced sulfur compounds in rat liver. Implications for blood pressure increase. Postepy Hig. Med. Dosw. 2017, 71, 564–576. [Google Scholar] [CrossRef]
- Yamamoto, J.; Kamata, S.; Miura, A.; Nagata, T.; Kainuma, R.; Ishii, I. Differential adaptive responses to 1- or 2-day fasting in various mouse tissues revealed by quantitative PCR analysis. FEBS Open Bio 2015, 5, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Isokawa, M.; Funatsu, T.; Tsunoda, M. Fast and simultaneous analysis of biothiols by high-performance liquid chromatography with fluorescence detection under hydrophilic interaction chromatography conditions. Analyst 2013, 138, 3802–3808. [Google Scholar] [CrossRef] [Green Version]
- Isokawa, M.; Shimosawa, T.; Funatsu, T.; Tsunoda, M. Determination and characterization of total thiols in mouse serum samples using hydrophilic interaction liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1019, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Taketomi, Y.; Ueno, N.; Kojima, T.; Sato, H.; Murase, R.; Yamamoto, K.; Tanaka, S.; Sakanaka, M.; Nakamura, M.; Nishito, Y.; et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 2013, 14, 554–563. [Google Scholar] [CrossRef]

| Parental Genotypes | Genotypes of Offsprings (≥3-week-old) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | × | Female | Total Litter No. | Average Litter Size | Mpst-WT | Mpst-Het | Mpst-KO | Sex Ratio Male:Female | ||||||||||
| Mpst | Cth | Mpst | Cth | Cth-WT | Cth-Het | Cth-KO | Cth-WT | Cth-Het | Cth-KO | Cth-WT | Cth-Het | Cth-KO | ||||||
| Mpst single mutant | ||||||||||||||||||
| Het (1st) | WT | 27 | 5.1 ± 2.6 | 59 | 78 | 52:85 | ||||||||||||
| Het (1st) | Het (1st) | 81 | 5.7 ± 2.2 | 114 | 234 | 112 | 244:216 | |||||||||||
| KO (1st) | KO (1st) | 5 | 5.2 ± 1.8 | 26 | 16:10 | |||||||||||||
| Het (2nd) | WT | 14 | 5.5 ± 2.0 | 37 | 40 | 41:36 | ||||||||||||
| Het (2nd) | Het (2nd) | 17 | 5.0 ± 2.3 | 28 | 36 | 21 | 35:50 | |||||||||||
| KO (2nd) | KO (2nd) | 10 | 4.0 ± 2.0 | 40 | 21:19 | |||||||||||||
| Het (3rd) | WT | 9 | 3.6 ± 2.4 | 16 | 16 | 16:16 | ||||||||||||
| Het (3rd) | Het (3rd) | 30 | 5.1 ± 2.0 | 30 | 73 | 49 | 83:69 | |||||||||||
| KO (3rd) | KO (3rd) | 15 | 5.4 ± 2.4 | 81 | 39:42 | |||||||||||||
| Mpst (1st)/Cth double mutant | ||||||||||||||||||
| Het | KO | Het | KO | 11 | 4.6 ± 1.6 | 12 | 25 | 14 | 27:24 | |||||||||
| KO | Het | KO | Het | 29 | 5.8 ± 2.4 | 50 | 87 | 32 | 88:81 | |||||||||
| KO | KO | KO | KO | 8 | 3.6 ± 1.9 | 29 | 18:11 | |||||||||||
| Mpst | Mpst (1st)/Cth | |||||
|---|---|---|---|---|---|---|
| Mice | WT (a) | 1st KO (b) | 2nd KO | 3rd KO | Cth-KO (c) | -DKO (d) |
| Serum (µM) | (n = 10) | (n = 7) | (n = 7) | (n = 7) | (n = 5) | (n = 10) |
| Ala | 479 ± 128 | 377 ± 56 | 439 ± 79 | 430 ± 88 | 574 ± 239 | 407 ± 88 |
| Arg | 120 ± 19 c | 104 ± 23 c | 120 ± 14 | 113 ± 24 | 168 ± 37 abd | 146 ± 26 c |
| Asn/Asp | 6.28 ± 2.66 c | 9.33 ± 6.68 c | 17.4 ± 9.4 | 6.01 ± 4.41 | 28.1 ± 17.8 abd | 14.3 ± 6.4 c |
| Gln | 807 ± 135 | 752 ± 133 | 762 ± 74 | 801 ± 35 | 935 ± 172 d | 654 ± 147 c |
| Glu | 27.4 ± 21.4 c | 26.1 ± 17.9 c | 36.7 ± 15.7 | 19.9 ± 8.1 | 54.5 ± 17.9 ab | 39.2 ± 10.7 |
| Gly | 328 ± 75 | 291 ± 38 | 352 ± 28 | 306 ± 64 | 368 ± 109 | 345 ± 49 |
| His | 42.0 ± 24.5 cd | 44.2 ± 23.3 cd | 85.1 ± 19.1 | 39.3 ± 20 | 131 ± 58 ab | 90.4 ± 10.4 ab |
| Ile | 109 ± 18 | 106 ± 18 | 113 ± 21 | 105 ± 14 | 123 ± 20 | 106 ± 20 |
| Leu | 159 ± 21 | 155 ± 22 | 170 ± 26 | 169 ± 15 | 196 ± 11 d | 156 ± 32 c |
| Lys | 217 ± 37 | 226 ± 42 | 211 ± 48 | 255 ± 33 | 298 ± 24 | 184 ± 44 |
| Met | 75.3 ± 19.4 | 69.9 ± 16.7 c | 86.9 ± 8.0 | 67.6 ± 11.0 | 104 ± 25 b | 96.6 ± 22 |
| Phe | 95.6 ± 26.9 | 79.5 ± 13.9 | 86.0 ± 7.0 | 89.8 ± 6.2 | 106 ± 23 | 83.3 ± 13.4 |
| Pro | 131 ± 68 | 91.2± 26.7 | 120 ± 30 | 94.5 ± 28.4 | 177 ± 89 | 135 ± 36 |
| Ser | 145 ± 58 | 120 ± 18 | 143 ± 18 | 125 ± 29 | 175 ± 62 | 141 ± 30 |
| Thr | 175 ± 42 | 151 ± 35 | 180 ± 21 | 163 ± 45 | 199 ± 35 | 194 ± 43 |
| Trp | 71.2 ± 17.1 | 73.9 ± 11.1 | 61.5 ± 16.5 | 62.9 ± 12.0 | 69.9 ± 9.6 | 82.9 ± 22.3 |
| Tyr | 94.8 ± 32.0 | 87.6 ± 25.0 | 110 ± 18 | 101 ± 26 | 99.2 ± 58.9 | 80.3 ± 30.4 |
| Val | 242 ± 58 | 225 ± 40 c | 253 ± 29 | 226 ± 27 | 304 ± 43 b | 251 ± 41 |
| Cystathionine | 44.1 ± 19.7 cd | 39.5 ± 12.8 cd | 56.2 ± 12.6 | 37.6 ± 8.1 | 124 ± 15 ab | 133 ± 32 ab |
| Citrulline | 79.2 ± 19.3 cd | 63.0 ± 15.2 cd | 68.6 ± 14.1 | 67.2 ± 12.7 | 169 ± 24 ab | 135 ± 34 ab |
| Ornithine | 42.1 ± 10.9 c | 35.1 ± 10.2 c | 35.7 ± 6.6 | 43.0 ± 16.2 | 66.4 ± 19.1 ab | 49.5 ± 13.8 |
| Taurine | 675 ± 349 | 491 ± 136 | 642 ± 181 | 514 ± 88 | 601 ± 240 | 664 ± 235 |
| Total Cys | 319 ± 40 d | 322 ± 52 d | 341 ± 78 | 315 ± 32 | 274 ± 11 | 261 ± 51 ab |
| Total Hcy | 5.78 ± 1.22 cd | 5.90 ± 2.05 cd | 5.58 ± 0.77 | 6.23 ± 1.11 | 83.6 ± 18.6 abd | 121 ± 15 abc |
| Total GSH | 252 ± 71 cd | 230 ± 141 d | 221 ± 42 | 224 ± 37 | 124 ± 59 a | 107 ± 42 ab |
| Total Cys-Gly | 3.23 ± 0.39 b | 4.43 ± 0.59 ac | 4.79 ± 0.55 | 3.93 ± 0.39 | 3.15 ± 0.93 b | 3.87 ± 0.86 |
| Total γ Glu-Cys | 10.4 ± 2.8 | 13.2 ± 4.0 cd | 12.1 ± 2.4 | 8.37 ± 1.94 | 8.17 ± 0.45 b | 8.32 ± 2.16 b |
| Urine (mmol/mol creatinine) | ||||||
| (n = 10) | (n = 9) | (n = 7) | (n = 7) | (n = 6) | (n = 9) | |
| 3-ML | 10.4 ± 2.0 bd | 61.3 ± 9.9 acd | 57.4 ± 29.4 | 75.9 ± 20.7 | 16.9 ± 3.2 bd | 91.2 ± 21.0 abc |
| Total Cys | 156 ± 28 cd | 187 ± 24 cd | 222 ± 50 | 211 ± 84 | 624 ± 272 ab | 601 ± 115 ab |
| Total Hcy | 7.32 ± 2.66 cd | 6.24 ± 2.81 cd | 6.15 ± 1.11 | 5.44 ± 1.24 | 169 ± 51 ab | 195 ± 69 ab |
| Total GSH | 2.00 ± 1.28 | 1.40 ± 0.81 | 2.31 ± 1.09 | 1.74 ± 0.44 | 2.67 ± 1.84 | 2.15 ± 1.09 |
| Total Cys-Gly | 26.6 ± 10.0 cd | 25.4 ± 7.1 cd | 41.3 ± 9.6 | 32.4 ± 12.0 | 85.8 ± 37.7 ab | 82.4 ± 33.9 ab |
| Total γ Glu-Cys | 10.2 ± 4.9 cd | 9.45 ± 3.70 cd | 21.9 ± 7.0 | 14.3 ± 3.5 | 24.3 ± 13.0 ab | 26.8 ± 7.7 ab |
| Liver | ||||||
| Total GSH (nmol/mg protein; n = 5 each) | ||||||
| 10.6 ± 1.5 cd | 10.9 ± 1.7 cd | N.T. | N.T. | 6.51 ± 0.75 ab | 6.25 ± 1.25 ab | |
| GSSG/tGSH (%; n = 5 each) | ||||||
| 4.04 ± 0.48 cd | 3.82 ± 0.48 cd | N.T. | N.T. | 5.42 ± 0.62 ab | 5.69 ± 0.89 ab | |
| Mpst | Mpst (1st)/Cth | |||||
|---|---|---|---|---|---|---|
| Mice | WT (a) | 1st KO (b) | 2nd KO | 3rd KO | Cth-KO (c) | -DKO (d) |
| Serum | (8) | (7) | (7) | (7) | (5) | (10) |
| Albumin (g/dL) | 2.10 ± 0.12 | 2.21 ± 0.18 d | 2.14 ± 0.10 | 2.19 ± 0.20 | 2.00 ± 0.19 | 1.97 ± 0.16 b |
| ALT (IU/L) | 17.5 ± 10.8 | 12.1 ± 2.2 d | 16.3 ± 5.0 | 16.0 ± 5.3 | 22.0 ± 8.1 | 29.2 ± 12.6 b |
| AST (IU/L) | 45.3 ± 9.7 | 39.6 ± 12.3 d | 42.6 ± 16.6 | 42.1 ± 14.0 | 51.0 ± 20.4 | 75.2 ± 40.4 b |
| BUN (mg/dL) | 25.2 ± 3.3 | 25.4 ± 3.4 | 25.8 ± 1.9 | 26.8 ± 5.3 | 26.5 ± 2.5 | 26.5 ± 3.1 |
| CPK (IU/L) | 394 ± 110 | 306 ± 96 d | 448 ± 222 | 271 ± 62 | 351 ± 84 | 470 ± 154 b |
| CRE (mg/dL) | < 0.2 | < 0.2 | < 0.2 | < 0.2 | < 0.2 | < 0.2 |
| LDH (IU/L) | 173 ± 48 d | 157 ± 16 d | 282 ± 93 | 184 ± 28 | 225 ± 54 | 308 ± 80 ab |
| T-bilirubin (mg/dL) | 0.29 ± 0.11 | 0.30 ± 0.12 | 0.37 ± 0.11 | 0.27 ± 0.08 | 0.36 ± 0.11 | 0.35 ± 0.07 |
| T-protein (g/dL) | 4.59 ± 0.22 d | 4.71 ± 0.44 d | 4.39 ± 0.23 | 4.63 ± 0.49 | 4.26 ± 0.27 | 4.13 ± 0.23 ab |
| UA (mg/dL) | 2.81 ± 0.79 cd | 1.97 ± 1.12 | 1.74 ± 0.60 | 2.17 ± 0.79 | 1.60 ± 0.39 a | 1.63 ± 0.40 a |
| (6) | (5) | (6) | (8) | |||
| TBARS (nmol/mL) | 1.68 ± 0.22 | 1.68 ± 0.23 | N.T. | N.T. | 1.87 ± 0.41 | 1.50 ± 0.17 |
| Liver | ||||||
| TBARS | (6) | (5) | (6) | (8) | ||
| (nmol/µg protein) | 522 ± 29 d | 546 ± 77 | N.T. | N.T. | 659 ± 78 | 702 ± 169 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akahoshi, N.; Minakawa, T.; Miyashita, M.; Sugiyama, U.; Saito, C.; Takemoto, R.; Honda, A.; Kamichatani, W.; Kamata, S.; Anan, Y.; et al. Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria. Int. J. Mol. Sci. 2020, 21, 818. https://doi.org/10.3390/ijms21030818
Akahoshi N, Minakawa T, Miyashita M, Sugiyama U, Saito C, Takemoto R, Honda A, Kamichatani W, Kamata S, Anan Y, et al. Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria. International Journal of Molecular Sciences. 2020; 21(3):818. https://doi.org/10.3390/ijms21030818
Chicago/Turabian StyleAkahoshi, Noriyuki, Tatsuro Minakawa, Masashi Miyashita, Uran Sugiyama, Chihiro Saito, Rintaro Takemoto, Akihiro Honda, Waka Kamichatani, Shotaro Kamata, Yasumi Anan, and et al. 2020. "Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria" International Journal of Molecular Sciences 21, no. 3: 818. https://doi.org/10.3390/ijms21030818
APA StyleAkahoshi, N., Minakawa, T., Miyashita, M., Sugiyama, U., Saito, C., Takemoto, R., Honda, A., Kamichatani, W., Kamata, S., Anan, Y., & Ishii, I. (2020). Increased Urinary 3-Mercaptolactate Excretion and Enhanced Passive Systemic Anaphylaxis in Mice Lacking Mercaptopyruvate Sulfurtransferase, a Model of Mercaptolactate-Cysteine Disulfiduria. International Journal of Molecular Sciences, 21(3), 818. https://doi.org/10.3390/ijms21030818






