Reciprocal Regulatory Interaction between TRPV1 and Kinin B1 Receptor in a Rat Neuropathic Pain Model
Abstract
1. Introduction
2. Results
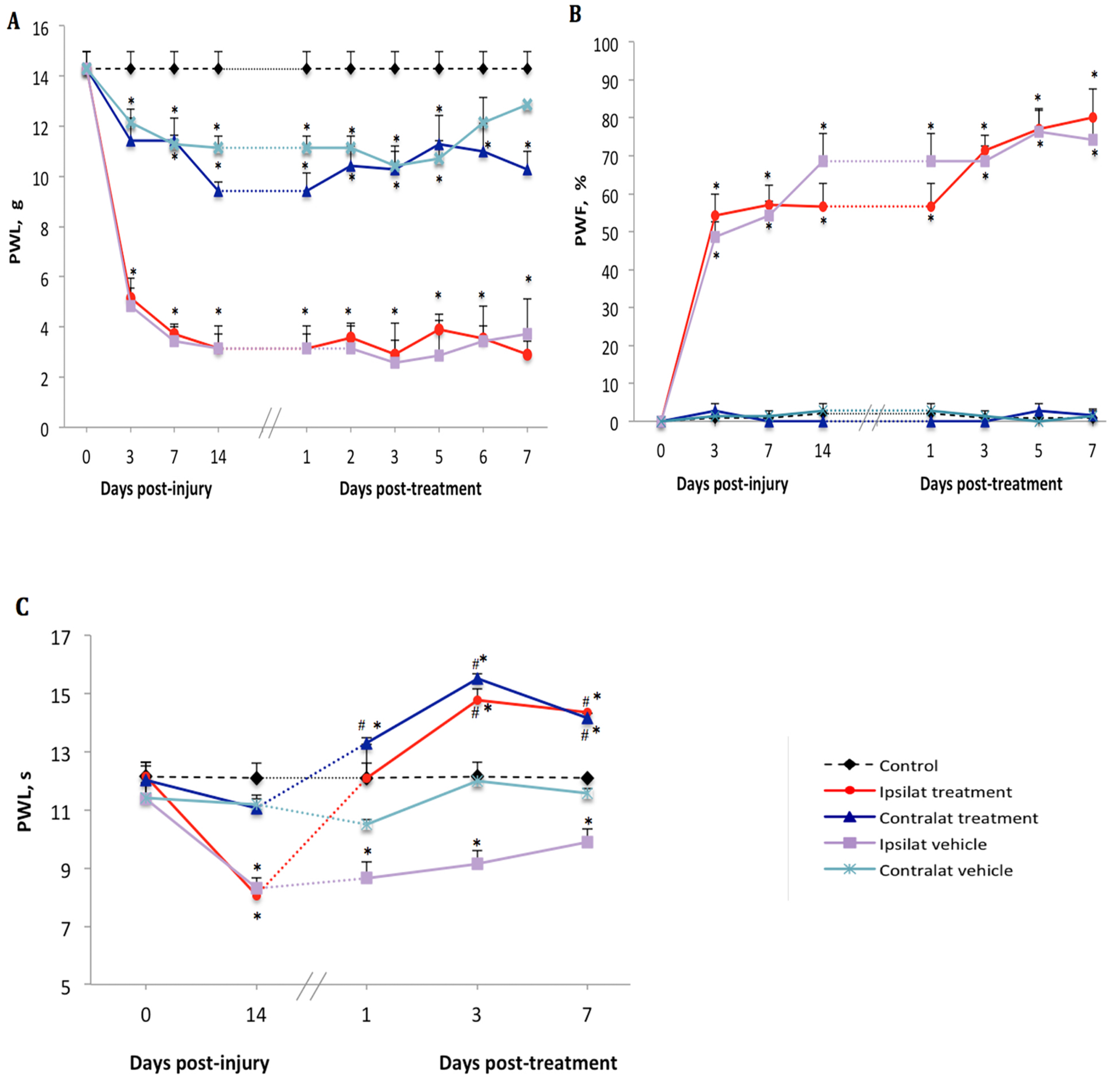
2.1. Changes of Nociceptive Behavior after PSNL
2.2. Effect of the B1R Antagonist on PSNL-Induced Nociceptive Behavior
2.3. Effect of the TRPV1 Antagonist on PSNL-Induced Nociceptive Behavior
2.4. Effect of B1R and TRPV1 Antagonists on Spinal Cord mRNA Levels
2.5. Effect of B1R and TRPV1 Antagonists on mRNA Levels in DRG
2.6. B1R Protein Expression in the Ipsilateral Dorsal Horn (iDH)
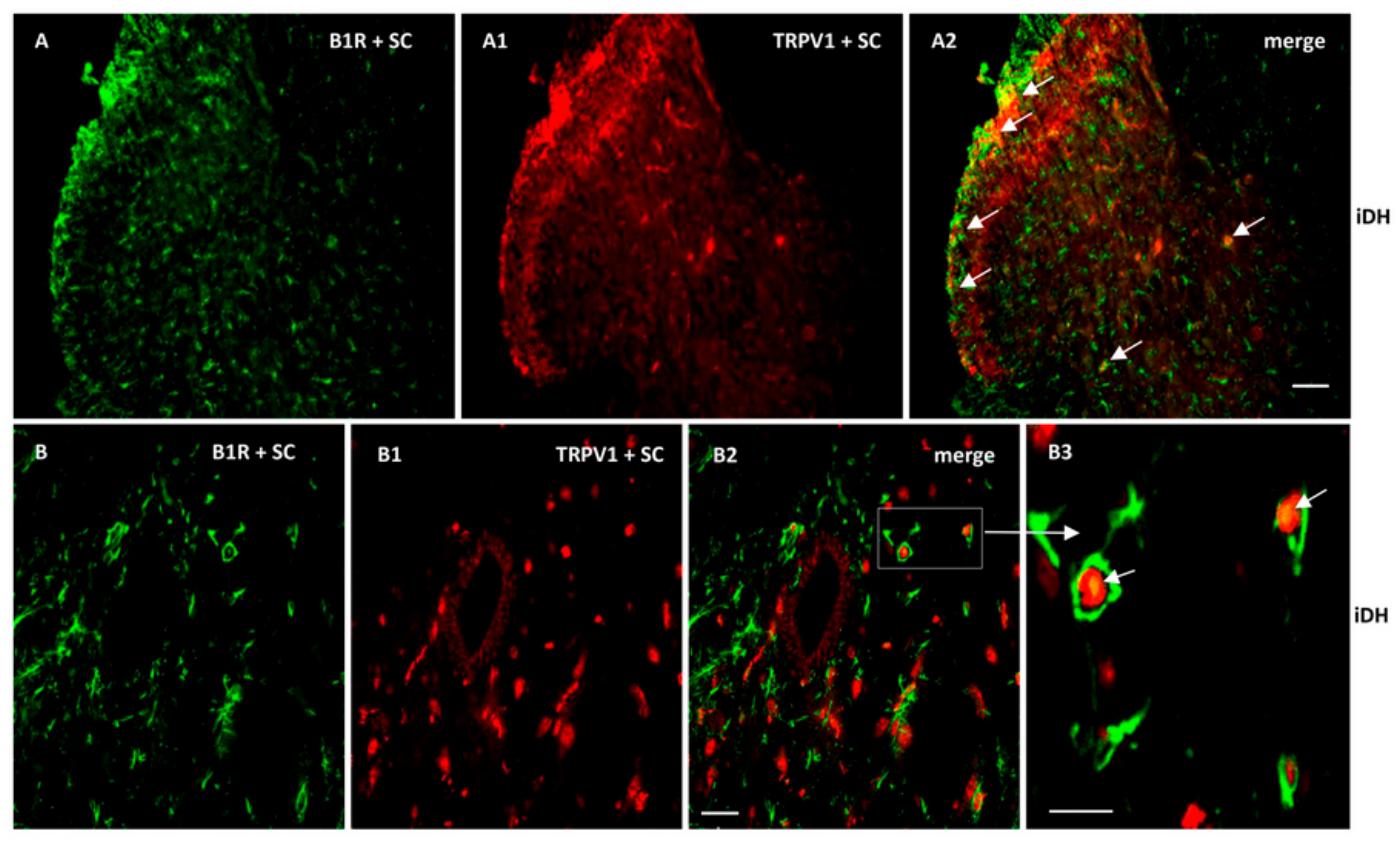
2.7. Localization of B1R and TRPV1 in the Ipsilateral Dorsal Horn (iDH) and DRG (iDRG)
3. Discussion
3.1. B1R in Neuropathic Pain
3.2. TRPV1 in Neuropathic Pain
4. Materials and Methods
4.1. Animal Care Procedure
4.2. Partial Sciatic Nerve Ligation (PSNL)
4.3. Group Identification and Distribution of Animals
4.4. Behavioral Assessment
4.5. Tactile Allodynia Test
4.6. Cold Allodynia Test
4.7. Thermal Hyperalgesia Test
4.8. Pharmacological Treatments
4.9. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.10. Immunohistochemical and Immunofluorescence Procedures
4.11. Densitometric Analysis
4.12. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Dissecting out mechanisms responsible for peripheral neuropathic pain: Implications for diagnosis and therapy. Life Sci. 2004, 74, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Couture, R.; Harrisson, M.; Vianna, R.M.; Cloutier, F. Kinin receptors in pain and inflammation. Eur. J. Pharmacol. 2001, 429, 161–176. [Google Scholar] [CrossRef]
- Calixto, J.B.; Medeiros, R.; Fernandes, E.S.; Ferreira, J.; Cabrini, D.A.; Campos, M.M. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br. J. Pharmacol. 2004, 143, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Petcu, M.; Dias, J.P.; Ongali, B.; Thibault, G.; Neugebauer, W.; Couture, R. Role of kinin B1 and B2 receptors in a rat model of neuropathic pain. Int. Immunopharmacol. 2008, 8, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Chahmi, E.; Dias, J.P.; Couture, R. Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J. Neuroinflamm. 2010, 7, 36. [Google Scholar] [CrossRef]
- Levy, D.; Zochodne, D.W. Increased mRNA expression of the B1 and B2 bradykinin receptors and antinociceptive effects of their antagonists in an animal model of neuropathic pain. Pain 2000, 86, 265–271. [Google Scholar] [CrossRef]
- Yamaguchi-Sase, S.; Hayashi, I.; Okamoto, H.; Nara, Y.; Matsuzaki, S.; Hoka, S.; Majima, M. Amelioration of hyperalgesia by kinin receptor antagonists or kininogen deficiency in chronic constriction nerve injury in rats. Inflamm. Res. 2003, 52, 164–169. [Google Scholar] [CrossRef]
- Gougat, J.; Ferrari, B.; Sarran, L.; Planchenault, C.; Poncelet, M.; Maruani, J.; Alonso, R.; Cudennec, A.; Croci, T.; Guagnini, F.; et al. SSR240612 [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-[[(6-methoxy-2-naphthyl)sulfonyl]amino] propanoyl)amino]-3-(4-[[2R,6S)-2,6-dimethylpiperidinyl]methyl]phenyl)-N-isopropyl -N-methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin B1 receptor: Biochemical and pharmacological characterization. J. Pharmacol. Exp. Ther. 2004, 309, 661–669. [Google Scholar] [CrossRef]
- Quintao, N.L.; Passos, G.F.; Medeiros, R.; Paszcuk, A.F.; Motta, F.L.; Pesquero, J.B.; Campos, M.M.; Calixto, J.B. Neuropathic pain-like behavior after brachial plexus avulsion in mice: The relevance of kinin B1 and B2 receptors. J. Neurosci. 2008, 28, 2856–2863. [Google Scholar] [CrossRef]
- Gabra, B.H.; Berthiaume, N.; Sirois, P.; Nantel, F.; Battistini, B. The kinin system mediates hyperalgesia through the inducible bradykinin B1 receptor subtype: Evidence in various experimental animal models of type 1 and type 2 diabetic neuropathy. Biol. Chem. 2006, 387, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.P.; Talbot, S.; Senecal, J.; Carayon, P.; Couture, R. Kinin B1 receptor enhances the oxidative stress in a rat model of insulin resistance: Outcome in hypertension, allodynia and metabolic complications. PLoS ONE 2010, 5, e12622. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Motta, E.M.; Dutra, R.C.; Manjavachi, M.N.; Bento, A.F.; Malinsky, F.R.; Pesquero, J.B.; Calixto, J.B. Anti-nociceptive effect of kinin B(1) and B(2) receptor antagonists on peripheral neuropathy induced by paclitaxel in mice. Br. J. Pharmacol. 2011, 164, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C.; Bento, A.F.; Leite, D.F.; Manjavachi, M.N.; Marcon, R.; Bicca, M.A.; Pesquero, J.B.; Calixto, J.B. The role of kinin B1 and B2 receptors in the persistent pain induced by experimental autoimmune encephalomyelitis (EAE) in mice: Evidence for the involvement of astrocytes. Neurobiol. Dis. 2013, 54, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Couture, R. Emerging role of microglial kinin B1 receptor in diabetic pain neuropathy. Exp. Neurol. 2012, 234, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Leeb-Lundberg, L.M.; Marceau, F.; Muller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Quirion, R. Inflammatory mediators modulating the transient receptor potential vanilloid 1 receptor: Therapeutic targets to treat inflammatory and neuropathic pain. Expert Opin. Ther. Targets 2007, 11, 307–320. [Google Scholar] [CrossRef]
- Chuang, H.H.; Prescott, E.D.; Kong, H.; Shields, S.; Jordt, S.E.; Basbaum, A.I.; Chao, M.V.; Julius, D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001, 411, 957–962. [Google Scholar] [CrossRef]
- Sugiura, T.; Tominaga, M.; Katsuya, H.; Mizumura, K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J. Neurophysiol. 2002, 88, 544–548. [Google Scholar] [CrossRef]
- Ma, Q.P. The expression of bradykinin B(1) receptors on primary sensory neurones that give rise to small caliber sciatic nerve fibres in rats. Neuroscience 2001, 107, 665–673. [Google Scholar] [CrossRef]
- Wotherspoon, G.; Winter, J. Bradykinin B1 receptor is constitutively expressed in the rat sensory nervous system. Neurosci. Lett. 2000, 294, 175–178. [Google Scholar] [CrossRef]
- DomBourian, M.G.; Turner, N.A.; Gerovac, T.A.; Vemuganti, R.; Miranpuri, G.S.; Tureyen, K.; Satriotomo, I.; Miletic, V.; Resnick, D.K. B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine 2006, 31, 2778–2782. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Beirith, A.; Mori, M.A.; Araujo, R.C.; Bader, M.; Pesquero, J.B.; Calixto, J.B. Reduced nerve injury-induced neuropathic pain in kinin B1 receptor knock-out mice. J. Neurosci. 2005, 25, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Gabra, B.H.; Merino, V.F.; Bader, M.; Pesquero, J.B.; Sirois, P. Absence of diabetic hyperalgesia in bradykinin B1 receptor-knockout mice. Regul. Pept. 2005, 127, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Pesquero, J.B.; Araujo, R.C.; Heppenstall, P.A.; Stucky, C.L.; Silva, J.A., Jr.; Walther, T.; Oliveira, S.M.; Pesquero, J.L.; Paiva, A.C.; Calixto, J.B.; et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 8140–8145. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Dias, J.P.; Lahjouji, K.; Bogo, M.R.; Campos, M.M.; Gaudreau, P.; Couture, R. Activation of TRPV1 by capsaicin induces functional kinin B(1) receptor in rat spinal cord microglia. J. Neuroinflamm. 2012, 9, 16. [Google Scholar] [CrossRef]
- Huang, W.X.; Yu, F.; Sanchez, R.M.; Liu, Y.Q.; Min, J.W.; Hu, J.J.; Bsoul, N.B.; Han, S.; Yin, J.; Liu, W.H.; et al. TRPV1 promotes repetitive febrile seizures by pro-inflammatory cytokines in immature brain. Brain Behav. Immun. 2015, 48, 68–77. [Google Scholar] [CrossRef]
- Phagoo, S.B.; Reddi, K.; Anderson, K.D.; Leeb-Lundberg, L.M.F.; Warburton, D. Bradykinin B1 Receptor Up-Regulation by Interleukin-1β and B1 Agonist Occurs through Independent and Synergistic Intracellular Signaling Mechanisms in Human Lung Fibroblasts. J. Pharmacol. Exp. Ther. 2001, 298, 77. [Google Scholar]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef]
- Khan, A.A.; Diogenes, A.; Jeske, N.A.; Henry, M.A.; Akopian, A.; Hargreaves, K.M. Tumor necrosis factor α enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience 2008, 155, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, H.; Watanabe, M.; Shimizu, K.; Zou, S.; LaGraize, S.C.; Wei, F.; Dubner, R.; Ren, K. Glial—Cytokine—Neuronal Interactions Underlying the Mechanisms of Persistent Pain. J. Neurosci. 2007, 27, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krady, J.K.; Levison, S.W. Interleukin-1: A master regulator of neuroinflammation. J. Neurosci. Res. 2004, 78, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yuan, H. Role of glia in neuropathic pain. Front. Biosci. 2014, 19, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Chahmi, E. Rôle et Localisation Intraspinale Du Récepteur B1 Des Kinines Dans La Douleur Neuropathique. Master’s Thesis, in Physiology. Faculty of Medicine, Université de Montréal, Montreal, QC, Canada, 2011. [Google Scholar]
- Cayla, C.; Labuz, D.; Machelska, H.; Bader, M.; Schafer, M.; Stein, C. Impaired nociception and peripheral opioid antinociception in mice lacking both kinin B1 and B2 receptors. Anesthesiology 2012, 116, 448–457. [Google Scholar] [CrossRef]
- Porreca, F.; Vanderah, T.W.; Guo, W.; Barth, M.; Dodey, P.; Peyrou, V.; Luccarini, J.M.; Junien, J.L.; Pruneau, D. Antinociceptive pharmacology of N-[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]methyl]-2-[2-[[(4-methoxy-2,6-dimethyl phenyl) sulfonyl]methylamino]ethoxy]-N-methylacetamide, fumarate (LF22-0542), a novel nonpeptidic bradykinin B1 receptor antagonist. J. Pharmacol. Exp. Ther. 2006, 318, 195–205. [Google Scholar] [CrossRef]
- Rashid, M.H.; Inoue, M.; Matsumoto, M.; Ueda, H. Switching of bradykinin-mediated nociception following partial sciatic nerve injury in mice. J. Pharmacol. Exp. Ther. 2004, 308, 1158–1164. [Google Scholar] [CrossRef]
- Passos, G.F.; Fernandes, E.S.; Campos, M.M.; Araujo, J.G.; Pesquero, J.L.; Souza, G.E.; Avellar, M.C.; Teixeira, M.M.; Calixto, J.B. Kinin B1 receptor up-regulation after lipopolysaccharide administration: Role of proinflammatory cytokines and neutrophil influx. J. Immunol. 2004, 172, 1839–1847. [Google Scholar] [CrossRef]
- Cunha, J.M.; Cunha, F.Q.; Poole, S.; Ferreira, S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br. J. Pharmacol. 2000, 130, 1418–1424. [Google Scholar] [CrossRef]
- Liberto, C.M.; Albrecht, P.J.; Herx, L.M.; Yong, V.W.; Levison, S.W. Pro-regenerative properties of cytokine-activated astrocytes. J. Neurochem. 2004, 89, 1092–1100. [Google Scholar] [CrossRef]
- Doly, S.; Fischer, J.; Salio, C.; Conrath, M. The vanilloid receptor-1 is expressed in rat spinal dorsal horn astrocytes. Neurosci. Lett. 2004, 357, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tidjane, N.; Gaboury, L.; Couture, R. Cellular localisation of the kinin B1R in the pancreas of streptozotocin-treated rat and the anti-diabetic effect of the antagonist SSR240612. Biol. Chem. 2016, 397, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, M.; Farber, K.; Okuno, Y.; Yamakawa, Y.; Miyamoto, T.; Nolte, C.; Merrino, V.F.; Kita, S.; Iwamoto, T.; Komuro, I.; et al. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J. Neurosci. 2007, 27, 13065–13073. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R64–R76. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Honda, K.; Shinoda, M.; Furukawa, A.; Kita, K.; Noma, N.; Iwata, K. TRPA1 contributes to capsaicin-induced facial cold hyperalgesia in rats. Eur. J. Oral Sci. 2014, 122, 391–396. [Google Scholar] [CrossRef]
- Honda, K.; Shinoda, M.; Kondo, M.; Shimizu, K.; Yonemoto, H.; Otsuki, K.; Akasaka, R.; Furukawa, A.; Iwata, K. Sensitization of TRPV1 and TRPA1 via peripheral mGluR5 signaling contributes to thermal and mechanical hypersensitivity. Pain 2017, 158, 1754–1764. [Google Scholar] [CrossRef]
- Labuz, D.; Celik, M.O.; Zimmer, A.; Machelska, H. Distinct roles of exogenous opioid agonists and endogenous opioid peptides in the peripheral control of neuropathy-triggered heat pain. Sci. Rep. 2016, 6, 32799. [Google Scholar] [CrossRef][Green Version]
- Meng, J.; Wang, J.; Steinhoff, M.; Dolly, J.O. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18–1/syntaxin1/SNAP-25 mediated fusion. Sci. Rep. 2016, 6, 21226. [Google Scholar] [CrossRef]
- Seltzer, Z.; Dubner, R.; Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990, 43, 205–218. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Choi, Y.; Yoon, Y.W.; Na, H.S.; Kim, S.H.; Chung, J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994, 59, 369–376. [Google Scholar] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Chizh, B.A. Clinical development of TRPV1 antagonists: Targeting a pivotal point in the pain pathway. Drug Discov. Today 2009, 14, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, Y.; Kawamata, T.; Yamamoto, J.; Furuse, S.; Namiki, A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br. J. Anaesth. 2009, 102, 251–258. [Google Scholar] [CrossRef]
- Haddad, Y.; Couture, R. Kininase 1 As a Preclinical Therapeutic Target for Kinin B1 Receptor in Insulin Resistance. Front. Pharmacol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Katikireddy, K.R.; O’Sullivan, F. Immunohistochemical and Immunofluorescence Procedures for Protein Analysis. In Gene Expression Profiling: Methods and Protocols; O’Driscoll, L., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 155–167. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Rami, H.K.; Jerman, J.C.; Smart, D.; Gill, C.H.; Soffin, E.M.; Luis Hannan, S.; Lappin, S.C.; Egerton, J.; Smith, G.D.; et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 2004, 46, 133–149. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S. GFAP and astrogliosis. Brain Pathol. 1994, 4, 229–237. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef]
- Silverman, J.D.; Kruger, L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: Relation of lectin reactivity to peptide and enzyme markers. J. Neurocytol. 1990, 19, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, G.; Alarcon, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepulveda, M.; et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, B.; Tong, X.K.; Lahjouji, K.; Couture, R.; Hamel, E. Cognitive and cerebrovascular improvements following kinin B1 receptor blockade in Alzheimer’s disease mice. J. Neuroinflamm. 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Hachana, S.; Bhat, M.; Sénécal, J.; Huppé-Gourgues, F.; Couture, R.; Vaucher, E. Expression, distribution and function of kinin B1 receptor in the rat diabetic retina. Br. J. Pharmacol. 2018, 175, 968–983. [Google Scholar] [CrossRef]
- Winkler, D.F.; McGeer, P.L. Protein labeling and biotinylation of peptides during spot synthesis using biotin p-nitrophenyl ester (biotin-ONp). Proteomics 2008, 8, 961–967. [Google Scholar] [CrossRef]
- Peretti-Renucci, R.; Feuerstein, C.; Manier, M.; Lorimier, P.; Savasta, M.; Thibault, J.; Mons, N.; Geffard, M. Quantitative image analysis with densitometry for immunohistochemistry and autoradiography of receptor binding sites--methodological considerations. J. Neurosci. Res. 1991, 28, 583–600. [Google Scholar] [CrossRef]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef]







| Side | Treatment | Spinal Cord (Fold Expression) | DRG (Fold Expression) | ||
|---|---|---|---|---|---|
| TNF-α | IL-1β | TNF-α | IL-1β | ||
| SSR240612 | |||||
| Contralateral | Control | 1 | 1 | 1 | 1 |
| Vehicle | 3.2 ± 0.1 * | 2.5 ± 0.2 | 0.6 ± 0.3 | 0.5 ± 0.3 | |
| SSR240612 | 5.5 ± 0.4 *+ | 4.7 ± 0.3 *+ | 0.9 ± 0.1 | 0.9 ± 0.2 | |
| Ipsilateral | Vehicle | 3.8 ± 0.1 * | 2.3 ± 0.1 | 3.9 ± 0.2 * | 0.2 ± 0.5 |
| SSR240612 | 5.1 ± 0.4 *+ | 5.0 ± 0.3 *+ | 3.6 ± 0.2 * | 0.4 ± 0.3 | |
| SB366791 | |||||
| Contralateral | Control | 1 | 1 | 1 | 1 |
| Vehicle | 3.1 ± 0.1 * | 2.0 ± 0.3 | 0.4 ± 0.2 | 0.2 ± 0.1 | |
| SB366791 | 3.3 ± 0.7 * | 5.0 ± 0.3 *+ | 0.1 ± 0.1 | 0.4 ± 0.3 | |
| Ipsilateral | Vehicle | 4.0 ± 0.2 * | 1.8 ± 0.2 | 3.1 ± 0.3 * | 0.3 ± 0.2 |
| SB366791 | 4.4 ± 0.3 * | 4.0 ± 0.3 *+ | 4.4 ± 0.4 * | 0.3 ± 0.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernit, V.; Sénécal, J.; Othman, R.; Couture, R. Reciprocal Regulatory Interaction between TRPV1 and Kinin B1 Receptor in a Rat Neuropathic Pain Model. Int. J. Mol. Sci. 2020, 21, 821. https://doi.org/10.3390/ijms21030821
Cernit V, Sénécal J, Othman R, Couture R. Reciprocal Regulatory Interaction between TRPV1 and Kinin B1 Receptor in a Rat Neuropathic Pain Model. International Journal of Molecular Sciences. 2020; 21(3):821. https://doi.org/10.3390/ijms21030821
Chicago/Turabian StyleCernit, Veronica, Jacques Sénécal, Rahmeh Othman, and Réjean Couture. 2020. "Reciprocal Regulatory Interaction between TRPV1 and Kinin B1 Receptor in a Rat Neuropathic Pain Model" International Journal of Molecular Sciences 21, no. 3: 821. https://doi.org/10.3390/ijms21030821
APA StyleCernit, V., Sénécal, J., Othman, R., & Couture, R. (2020). Reciprocal Regulatory Interaction between TRPV1 and Kinin B1 Receptor in a Rat Neuropathic Pain Model. International Journal of Molecular Sciences, 21(3), 821. https://doi.org/10.3390/ijms21030821





