The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury
Abstract
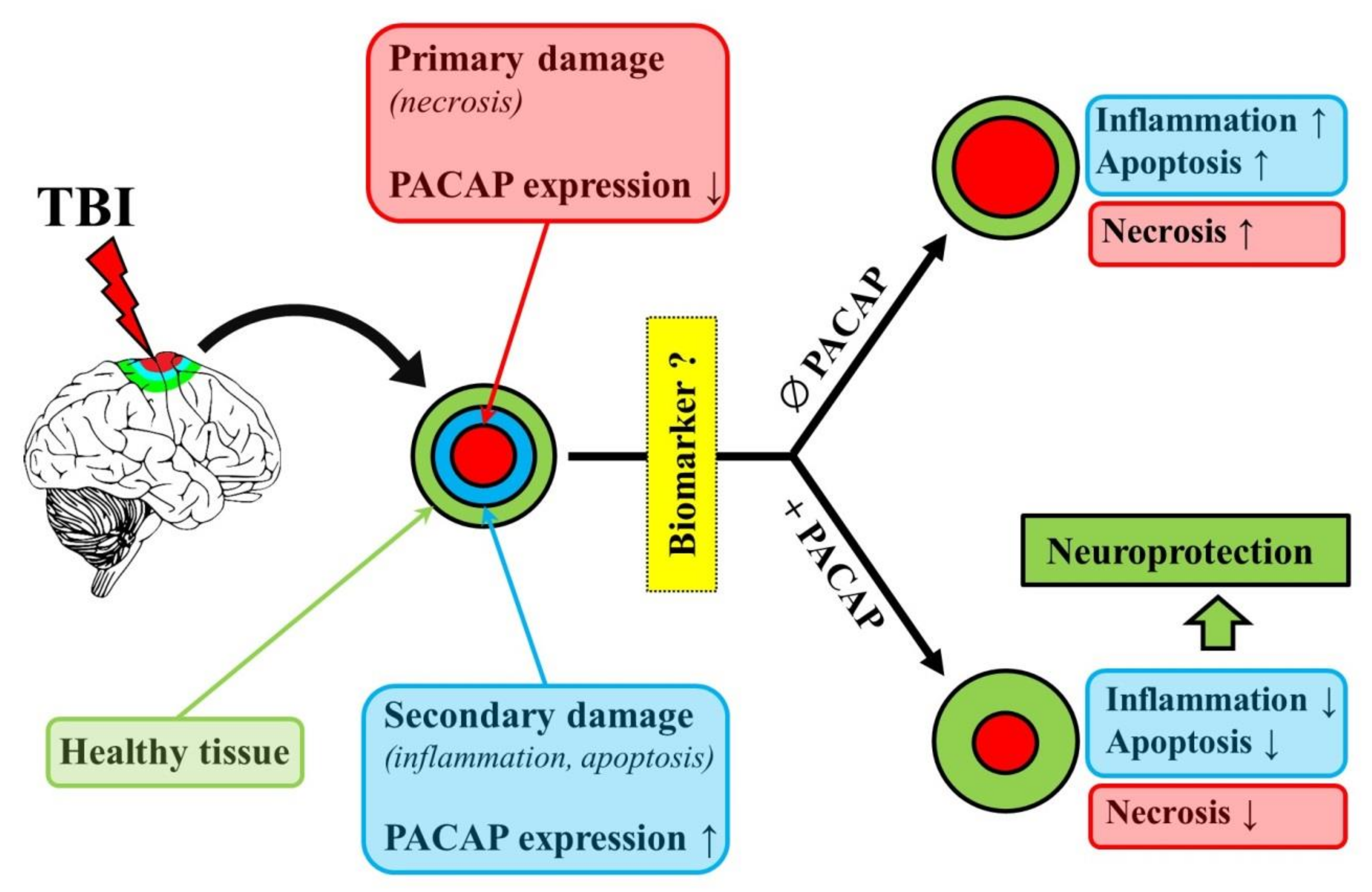
:1. Introduction
2. General Overview
3. Protective Effects of PACAP in Animal Models of TBI
4. PACAP Levels in the Brain After TBI in Animal Models
5. PACAP Levels in the Brain, CSF and Serum in TBI Patients
6. Concluding Remarks
Funding
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, M. Giving voice to a silent epidemic. Nat. Rev. Neurol. 2013, 9, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. JNS 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [Green Version]
- Mondello, S.; Sorinola, A.; Czeiter, E.; Vámos, Z.; Amrein, K.; Synnot, A.; Donoghue, E.; Sándor, J.; Wang, K.K.W.; Diaz-Arrastia, R.; et al. Blood-Based Protein Biomarkers for the Management of Traumatic Brain Injuries in Adults Presenting to Emergency Departments with Mild Brain Injury: A Living Systematic Review and Meta-Analysis. J. Neurotr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Tomar, G.S.; Singh, G.P.; Lahkar, D.; Sengar, K.; Nigam, R.; Mohan, M.; Anindya, R. New biomarkers in brain trauma. Clin. Chim. Acta 2018, 487, 325–329. [Google Scholar] [CrossRef]
- Gan, Z.S.; Stein, S.C.; Swanson, R.; Guan, S.; Garcia, L.; Mehta, D.; Smith, D.H. Blood biomarkers for traumatic brain injury: A quantitative assessment of diagnostic and prognostic accuracy. Front. Neurol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Reglodi, D.; Helyes, Z.; Nemeth, J.; Vass, R.; Tamas, A. PACAP as a Potential Biomarker: Alterations of PACAP Levels in Human Physiological and Pathological Conditions. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 815–832. [Google Scholar] [CrossRef] [Green Version]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Yon, L.; Fournier, A.; Vaudry, H. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: From Structure to Functions. Pharmacol. Rev. 2000, 52, 269–324. [Google Scholar]
- Watanabe, J.; Seki, T.; Shioda, S. PACAP and Neural Development. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 65–82. [Google Scholar] [CrossRef]
- Miyata, A.; Jiang, L.; Dahl, R.D.; Kitada, C.; Kubo, K.; Fujino, M.; Minamino, N.; Arimura, A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990, 170, 643–648. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Basille, M.; Vaudry, D.; Coulouarn, Y.; Jegou, S.; Lihrmann, I.; Fournier, A.; Vaudry, H.; Gonzalez, B. Comparative distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) binding sites and PACAP receptor mRNAs in the rat brain during development. J. Comp. Neurol. 2000, 425, 495–509. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Jolivel, V.; Basille, M.; Aubert, N.; de Jouffrey, S.; Ancian, P.; Le Bigot, J.-F.; Noack, P.; Massonneau, M.; Fournier, A.; Vaudry, H.; et al. Distribution and functional characterization of pituitary adenylate cyclase–activating polypeptide receptors in the brain of non-human primates. Neuroscience 2009, 160, 434–451. [Google Scholar] [CrossRef]
- Reglodi, D.; Atlasz, T.; Szabo, E.; Jungling, A.; Tamas, A.; Juhasz, T.; Fulop, B.D.; Bardosi, A. PACAP deficiency as a model of aging. GeroScience 2018, 40, 437–452. [Google Scholar] [CrossRef]
- Johanson, C.; Stopa, E.; Baird, A.; Sharma, H. Traumatic brain injury and recovery mechanisms: Peptide modulation of periventricular neurogenic regions by the choroid plexus-CSF nexus. J. Neural. Transm. 2011, 118, 115–133. [Google Scholar] [CrossRef] [Green Version]
- Frechilla, D.; Garcia-Osta, A.; Palacios, S.; Cenarruzabeitia, E.; Del Rio, J. BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. Neuroreport 2001, 12, 919–923. [Google Scholar] [CrossRef]
- Somogyvari-Vigh, A.; Reglodi, D. Pituitary Adenylate Cyclase Activating Polypeptide: A Potential Neuroprotective Peptide. Curr. Pharm. Des. 2004, 10, 2861–2889. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A.; Jungling, A.; Vaczy, A.; Rivnyak, A.; Fulop, B.D.; Szabo, E.; Lubics, A.; Atlasz, T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology 2018, 66, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Nakamachi, T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Toth, D.; Vicena, V.; Manavalan, S.; Brown, D.; Getachew, B.; Tizabi, Y. Therapeutic potential of PACAP in alcohol toxicity. Neurochem. Int. 2019, 124, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Rivnyak, A.; Kiss, P.; Tamas, A.; Balogh, D.; Reglodi, D. Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair. Int. J. Mol. Sci. 2018, 19, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reglodi, D.; Vaczy, A.; Rubio-Beltran, E.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache. Pain 2018, 19, 19. [Google Scholar] [CrossRef] [Green Version]
- Atlasz, T.; Vaczy, A.; Werling, D.; Kiss, P.; Tamas, A.; Kovacs, K.; Fabian, E.; Kvarik, T.; Mammel, B.; Danyadi, B.; et al. Protective Effects of PACAP in the Retina. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 501–527. [Google Scholar] [CrossRef] [Green Version]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Van, C.; Condro, M.C.; Lov, K.; Zhu, R.; Ricaflanca, P.T.; Ko, H.H.; Diep, A.L.; Hoang, A.Q.; Pisegna, J.; Rohrer, H.; et al. PACAP/PAC1 Regulation of Inflammation via Catecholaminergic Neurons in a Model of Multiple Sclerosis. J. Mol. Neurosci. 2019, 68, 439–451. [Google Scholar] [CrossRef]
- Reglodi, D.; Renaud, J.; Tamas, A.; Tizabi, Y.; Socías, S.B.; Del-Bel, E.; Raisman-Vozari, R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 2017, 155, 120–148. [Google Scholar] [CrossRef]
- Maasz, G.; Zrinyi, Z.; Reglodi, D.; Petrovics, D.; Rivnyak, A.; Kiss, T.; Jungling, A.; Tamas, A.; Pirger, Z. Pituitary adenylate cyclase-activating polypeptide (PACAP) has a neuroprotective function in dopamine-based neurodegeneration in rat and snail parkinsonian models. Dis. Model. Mech. 2017, 10, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Reglodi, D.; Atlasz, T.; Jungling, A.; Szabo, E.; Kovari, P.; Manavalan, S.; Tamas, A. Alternative Routes of Administration of the Neuroprotective Pituitary Adenylate Cyclase Activating Polypeptide. Curr. Pharm. Des. 2018, 24, 3892–3904. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Federico, C.; Saccone, S.; Morello, G.; La Cognata, V.; Cavallaro, S.; D’Agata, V. Molecular mechanisms involved in the protective effect of pituitary adenylate cyclase-activating polypeptide in an in vitro model of amyotrophic lateral sclerosis. J. Cell. Physiol. 2019, 234, 5203–5214. [Google Scholar] [CrossRef]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effect of PACAP in central and peripheral nerve injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferencz, S.; Reglodi, D.; Kaszas, B.; Bardosi, A.; Toth, D.; Vekony, Z.; Vicena, V.; Karadi, O.; Kelemen, D. PACAP and PAC1 receptor expression in pancreatic ductal carcinoma. Oncol. Lett. 2019, 18, 5725–5730. [Google Scholar] [CrossRef] [Green Version]
- Bardosi, S.; Bardosi, A.; Nagy, Z.; Reglodi, D. Expression of PACAP and PAC1 Receptor in Normal Human Thyroid Gland and in Thyroid Papillary Carcinoma. J. Mol. Neurosci. 2016, 60, 171–178. [Google Scholar] [CrossRef]
- Szanto, Z.; Sarszegi, Z.; Reglodi, D.; Nemeth, J.; Szabadfi, K.; Kiss, P.; Varga, A.; Banki, E.; Csanaky, K.; Gaszner, B.; et al. PACAP immunoreactivity in human malignant tumor samples and cardiac diseases. J. Mol. Neurosci. 2012, 48, 667–673. [Google Scholar] [CrossRef]
- Horvath, G.; Illes, A.; Heimesaat, M.M.; Bardosi, A.; Bardosi, S.; Tamas, A.; Fulop, B.D.; Opper, B.; Nemeth, J.; Ferencz, A.; et al. Protective Intestinal Effects of Pituitary Adenylate Cyclase Activating Polypeptide. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer Nature: New York, NY, USA, 2016; pp. 271–288. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Liang, W.; Baxter, L.C.; Yin, J.; Tang, Z.; Beach, T.G.; Caselli, R.J.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase–activating polypeptide is reduced in Alzheimer disease. Neurology 2014, 82, 1724–1728. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Caselli, R.J.; Baxter, L.; Serrano, G.; Yin, J.; Beach, T.G.; Reiman, E.M.; Shi, J. Association of Pituitary Adenylate Cyclase–Activating Polypeptide With Cognitive Decline in Mild Cognitive Impairment Due to Alzheimer Disease. JAMA Neurol. 2015, 72, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Brubel, R.; Reglodi, D.; Jambor, E.; Koppan, M.; Varnagy, A.; Biro, Z.; Kiss, P.; Gaal, V.; Matkovits, A.; Farkas, J.; et al. Investigation of pituitary adenylate cyclase activating polypeptide in human gynecological and other biological fluids by using MALDI TOF mass spectrometry. J. Mass Spectrom. 2011, 46, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Koppan, M.; Varnagy, A.; Reglodi, D.; Brubel, R.; Nemeth, J.; Tamas, A.; Mark, L.; Bodis, J. Correlation between oocyte number and follicular fluid concentration of pituitary adenylate cyclase-activating polypeptide (PACAP) in women after superovulation treatment. J. Mol. Neurosci. 2012, 48, 617–622. [Google Scholar] [CrossRef]
- Borzsei, R.; Mark, L.; Tamas, A.; Bagoly, T.; Bay, C.; Csanaky, K.; Banki, E.; Kiss, P.; Vaczy, A.; Horvath, G.; et al. Presence of pituitary adenylate cyclase activating polypeptide-38 in human plasma and milk. Eur. J. Endocrinol. 2009, 160, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Csanaky, K.; Banki, E.; Szabadfi, K.; Reglodi, D.; Tarcai, I.; Czegledi, L.; Helyes, Z.; Ertl, T.; Gyarmati, J.; Szanto, Z.; et al. Changes in PACAP immunoreactivity in human milk and presence of PAC1 receptor in mammary gland during lactation. J. Mol. Neurosci. 2012, 48, 631–637. [Google Scholar] [CrossRef]
- Sun, B.-Y.; Sun, Z.-P.; Pang, Z.-C.; Huang, W.-T.; Wu, S.-P. Decreased synovial fluid pituitary adenylate cyclase-activating polypeptide (PACAP) levels may reflect disease severity in post-traumatic knee osteoarthritis after anterior cruciate ligament injury. Peptides 2019, 116, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.; Sitarz, J.T.; Petersen, K.A.; Oturai, P.S.; Kruuse, C.; Fahrenkrug, J.; Olesen, J. The effect of intravenous PACAP38 on cerebral hemodynamics in healthy volunteers. Regul. Pept. 2007, 140, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Maderdrut, J.L.; Lertora, J.J.L.; Batuman, V. Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: A case study. Peptides 2007, 28, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Eneman, B.; Freson, K.; van den Heuvel, L.; van Hoyweghen, E.; Collard, L.; Vande Walle, J.; van Geet, C.; Levtchenko, E. Pituitary adenylate cyclase-activating polypeptide deficiency associated with increased platelet count and aggregability in nephrotic syndrome. J. Thromb. Haemost. 2015, 13, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Sarszegi, Z.; Szabo, D.; Gaszner, B.; Konyi, A.; Reglodi, D.; Nemeth, J.; Lelesz, B.; Polgar, B.; Jungling, A.; Tamas, A. Examination of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) as a Potential Biomarker in Heart Failure Patients. J. Mol. Neurosci. 2019, 68, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.-H.; Dong, X.-Q.; Yu, W.-H.; Du, Q.; Yang, D.-B.; Wang, H.; Shen, Y.-F. The change of plasma pituitary adenylate cyclase-activating polypeptide levels after aneurysmal subarachnoid hemorrhage. Acta Neurol. Scand. 2016, 134, 131–139. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Zhang, M.; Ba, L. Plasma pituitary adenylate cyclase-activating polypeptide concentrations and mortality after acute spontaneous basal ganglia hemorrhage. Clin. Chim. Acta. 2015, 439, 102–106. [Google Scholar] [CrossRef]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Szolcsanyi, J.; Szabo, N.; Toth, E.; Kincses, Z.T.; Vecsei, L.; Tajti, J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013, 33, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Amin, F.M.; Hougaard, A.; Schytz, H.W.; Asghar, M.S.; Lundholm, E.; Parvaiz, A.I.; de Koning, P.J.H.; Andersen, M.R.; Larsson, H.B.W.; Fahrenkrug, J.; et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014, 137, 779–794. [Google Scholar] [CrossRef]
- Tajti, J.; Tuka, B.; Botz, B.; Helyes, Z.; Vecsei, L. Role of pituitary adenylate cyclase-activating polypeptide in nociception and migraine. CNS Neurol. Disord. Drug Targets 2015, 14, 540–553. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Dong, Z.; Hou, L.; Wan, D.; Chen, M.; Tang, W.; Yu, S. Interictal plasma pituitary adenylate cyclase-activating polypeptide levels are decreased in migraineurs but remain unchanged in patients with tension-type headache. Clin. Chim. Acta. 2015, 450, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J.; et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011, 470, 492–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska-Bik, A.; Kochanowski, J.; Uchman, D.; Wolinska-Witort, E.; Kalisz, M.; Martynska, L.; Baranowska, B.; Bik, W. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) in humans with multiple sclerosis. J. Neuroimmunol. 2013, 263, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Van Landeghem, F.K.H.; Weiss, T.; Oehmichen, M.; von Deimling, A. Cellular localization of pituitary adenylate cyclase-activating peptide (PACAP) following traumatic brain injury in humans. Acta Neuropathol. 2007, 113, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Bukovics, P.; Czeiter, E.; Amrein, K.; Kovacs, N.; Pal, J.; Tamas, A.; Bagoly, T.; Helyes, Z.; Buki, A.; Reglodi, D. Changes of PACAP level in cerebrospinal fluid and plasma of patients with severe traumatic brain injury. Peptides 2014, 60, 18–22. [Google Scholar] [CrossRef]
- Farkas, O.; Tamás, A.; Zsombok, A.; Reglődi, D.; Pál, J.; Büki, A.; Lengvári, I.; Povlishock, J.T.; Dóczi, T. Effects of pituitary adenylate cyclase activating polypeptide in a rat model of traumatic brain injury. Regul. Pept. 2004, 123, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tamás, A.; Zsombok, A.; Farkas, O.; Reglödi, D.; Pál, J.; Büki, A.; Lengvári, I.; Povlishock, J.T.; Dóczi, T. Postinjury Administration of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Attenuates Traumatically Induced Axonal Injury in Rats. J. Neurotrauma 2006, 23, 686–695. [Google Scholar] [CrossRef]
- Miyamoto, K.; Tsumuraya, T.; Ohtaki, H.; Dohi, K.; Satoh, K.; Xu, Z.; Tanaka, S.; Murai, N.; Watanabe, J.; Sugiyama, K.; et al. PACAP38 suppresses cortical damage in mice with traumatic brain injury by enhancing antioxidant activity. J. Mol. Neurosci. 2014, 54, 370–379. [Google Scholar] [CrossRef]
- Kovesdi, E.; Tamas, A.; Reglodi, D.; Farkas, O.; Pal, J.; Toth, G.; Bukovics, P.; Doczi, T.; Buki, A. Posttraumatic administration of pituitary adenylate cyclase activating polypeptide in central fluid percussion injury in rats. Neurotox. Res. 2008, 13, 71–78. [Google Scholar] [CrossRef]
- Mao, S.-S.; Hua, R.; Zhao, X.-P.; Qin, X.; Sun, Z.-Q.; Zhang, Y.; Wu, Y.-Q.; Jia, M.-X.; Cao, J.-L.; Zhang, Y.-M. Exogenous Administration of PACAP Alleviates Traumatic Brain Injury in Rats through a Mechanism Involving the TLR4/MyD88/NF-κB Pathway. J. Neurotrauma 2012, 29, 1941–1959. [Google Scholar] [CrossRef]
- Hua, R.; Mao, S.-S.; Zhang, Y.-M.; Chen, F.-X.; Zhou, Z.-H.; Liu, J.-Q. Effects of pituitary adenylate cyclase activating polypeptide on CD4(+)/CD8(+) T cell levels after traumatic brain injury in a rat model. World J. Emerg. Med. 2012, 3, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Skoglosa, Y.; Lewen, A.; Takei, N.; Hillered, L.; Lindholm, D. Regulation of pituitary adenylate cyclase activating polypeptide and its receptor type 1 after traumatic brain injury: comparison with brain-derived neurotrophic factor and the induction of neuronal cell death. Neuroscience 1999, 90, 235–247. [Google Scholar] [CrossRef]
- Jaworski, D.M. Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) and the PACAP-selective receptor in cultured rat astrocytes, human brain tumors, and in response to acute intracranial injury. Cell Tissue Res. 2000, 300, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Dohi, K.; Yofu, S.; Mihara, Y.; Nakamachi, T.; Ohtaki, H.; Shioda, S.; Aruga, T. Expression and localization of pituitary adenylate cyclase-activating polypeptide (PACAP) specific receptor (PAC1R) after traumatic brain injury in mice. In Transmitters and Modulators in Health and Disease; Shioda, S., Homma, I., Kato, N., Eds.; Springer: Tokyo, Japan, 2009; pp. 207–210. [Google Scholar] [CrossRef]
- Suzuki, R.; Arata, S.; Nakajo, S.; Ikenaka, K.; Kikuyama, S.; Shioda, S. Expression of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC1-R) in reactive astrocytes. Brain Res. Mol. Brain Res. 2003, 115, 10–20. [Google Scholar] [CrossRef]
- Rhea, E.M.; Bullock, K.M.; Banks, W.A. Effect of controlled cortical impact on the passage of pituitary adenylate cyclase activating polypeptide (PACAP) across the blood-brain barrier. Peptides 2018, 99, 8–13. [Google Scholar] [CrossRef]
- Ye, D.; Yang, Y.; Lu, X.; Xu, Y.; Shi, Y.; Chen, H.; Huang, J. Spatiotemporal Expression Changes of PACAP and Its Receptors in Retinal Ganglion Cells After Optic Nerve Crush. J. Mol. Neurosci. 2019, 68, 465–474. [Google Scholar] [CrossRef]
- Reglodi, D.; Kiss, P.; Szabadfi, K.; Atlasz, T.; Gabriel, R.; Horvath, G.; Szakaly, P.; Sandor, B.; Lubics, A.; Laszlo, E.; et al. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J. Mol. Neurosci. 2012, 48, 482–492. [Google Scholar] [CrossRef]
- Tsuchikawa, D.; Nakamachi, T.; Tsuchida, M.; Wada, Y.; Hori, M.; Farkas, J.; Yoshikawa, A.; Kagami, N.; Imai, N.; Shintani, N.; et al. Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J. Mol. Neurosci. 2012, 48, 508–517. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toth, D.; Tamas, A.; Reglodi, D. The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 827. https://doi.org/10.3390/ijms21030827
Toth D, Tamas A, Reglodi D. The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury. International Journal of Molecular Sciences. 2020; 21(3):827. https://doi.org/10.3390/ijms21030827
Chicago/Turabian StyleToth, Denes, Andrea Tamas, and Dora Reglodi. 2020. "The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury" International Journal of Molecular Sciences 21, no. 3: 827. https://doi.org/10.3390/ijms21030827





