Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury
Abstract
:1. Introduction
2. Results
2.1. Lack of PKCθ in Mdx Mice Boosts Muscle Regeneration While Reducing Muscle Fiber Degeneration
2.2. Dystrophic Muscle Repair After Injury is Enhanced in The Absence of PKCθ
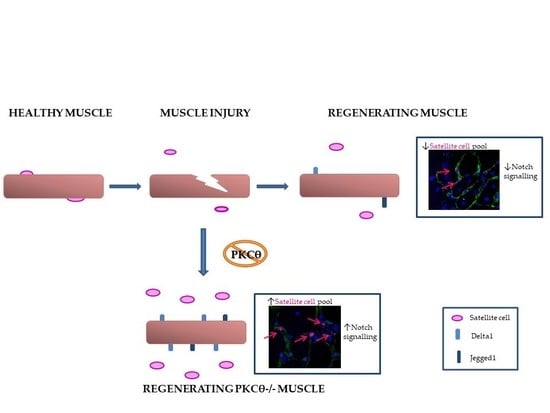
2.3. Lack of PKCθ in Mdx Mice Preserves the Self-Renewal Ability of Satellite Cells
2.4. The Self-Renewal Ability of Satellite Cells in mdxθ−/− Muscle is Maintained and Supported by Up-Regulation of Notch-Signaling
2.5. Lack of PKCθ Supports Transplanted Stem Cells Survival and Differentiation in Injured and Dystrophic Muscle
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Muscle Injury Procedure
4.3. Mesangioblast Transplantation
4.4. Histochemistry and Immunofluorescence Analyses
4.5. Cell Cultures
4.6. RNA Extraction, cDNA Synthesis and qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMD | Duchenne muscular Dystrophy |
| SCs | Satellite Cells |
| PKCθ | Protein Kinase C θ |
| Pax7 | Paired box 7 |
| MyoD | Myogenic Differentiation1 |
| GRMD | Golden Retriever Muscular Dystrophy |
| eMyHC | embryonic Myosin Heavy Chain |
| TA | Tibialis Anterior |
| ECM | ExtraCellular Matrix |
| CTX | Cardiotoxin |
| MABs | Mesoangioblasts |
References
- Christov, C.; Chretien, F.; Abou-Khalil, R.; Bassez, G.; Vallet, G.; Authier, F.J.; Bassaglia, Y.; Shinin, V.; Tajbakhsh, S.; Chazaud, B.; et al. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol. Biol. Cell 2007, 18, 1397–1409. [Google Scholar] [CrossRef] [Green Version]
- Madaro, L.; Bouche, M. From innate to adaptive immune response in muscular dystrophies and skeletal muscle regeneration: The role of lymphocytes. BioMed Res. Int. 2014, 2014, 438675. [Google Scholar] [CrossRef] [PubMed]
- Pessina, P.; Kharraz, Y.; Jardi, M.; Fukada, S.; Serrano, A.L.; Perdiguero, E.; Munoz-Canoves, P. Fibrogenic Cell Plasticity Blunts Tissue Regeneration and Aggravates Muscular Dystrophy. Stem Cell Rep. 2015, 4, 1046–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Wen, Y.; Kuroda, K.; Hannon, K.; Rudnicki, M.A.; Kuang, S. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis. Models Mech. 2014, 7, 997–1004. [Google Scholar] [CrossRef] [Green Version]
- Delaporte, C.; Dehaupas, M.; Fardeau, M. Comparison between the growth pattern of cell cultures from normal and Duchenne dystrophy muscle. J. Neurol. Sci. 1984, 64, 149–160. [Google Scholar] [CrossRef]
- Sacco, A.; Mourkioti, F.; Tran, R.; Choi, J.; Llewellyn, M.; Kraft, P.; Shkreli, M.; Delp, S.; Pomerantz, J.H.; Artandi, S.E.; et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 2010, 143, 1059–1071. [Google Scholar] [CrossRef] [Green Version]
- Reimann, J.; Irintchev, A.; Wernig, A. Regenerative capacity and the number of satellite cells in soleus muscles of normal and mdx mice. Neuromuscul. Disord. 2000, 10, 276–282. [Google Scholar] [CrossRef]
- Mourikis, P.; Tajbakhsh, S. Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev. Biol. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasut, A.; Chang, N.C.; Gurriaran-Rodriguez, U.; Faulkes, S.; Yin, H.; Lacaria, M.; Ming, H.; Rudnicki, M.A. Notch Signaling Rescues Loss of Satellite Cells Lacking Pax7 and Promotes Brown Adipogenic Differentiation. Cell Rep. 2016, 16, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, S.; Barnes, J.L.; Zammit, P.S.; Beauchamp, J.R. Delta-Like 4 Activates Notch 3 to Regulate Self-Renewal in Skeletal Muscle Stem Cells. Stem Cells 2018, 36, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Bi, P.; Liu, W.; Asakura, A.; Keller, C.; Kuang, S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 2012, 32, 2300–2311. [Google Scholar] [CrossRef] [Green Version]
- Fujimaki, S.; Seko, D.; Kitajima, Y.; Yoshioka, K.; Tsuchiya, Y.; Masuda, S.; Ono, Y. Notch1 and Notch2 Coordinately Regulate Stem Cell Function in the Quiescent and Activated States of Muscle Satellite Cells. Stem Cells 2018, 36, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Bjornson, C.R.; Cheung, T.H.; Liu, L.; Tripathi, P.V.; Steeper, K.M.; Rando, T.A. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 2012, 30, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Vieira, N.M.; Elvers, I.; Alexander, M.S.; Moreira, Y.B.; Eran, A.; Gomes, J.P.; Marshall, J.L.; Karlsson, E.K.; Verjovski-Almeida, S.; Lindblad-Toh, K.; et al. Jagged 1 Rescues the Duchenne Muscular Dystrophy Phenotype. Cell 2015, 163, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Madaro, L.; Pelle, A.; Nicoletti, C.; Crupi, A.; Marrocco, V.; Bossi, G.; Soddu, S.; Bouche, M. PKC theta ablation improves healing in a mouse model of muscular dystrophy. PLoS ONE 2012, 7, e31515. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, V.; Fiore, P.; Benedetti, A.; Pisu, S.; Rizzuto, E.; Musaro, A.; Madaro, L.; Lozanoska-Ochser, B.; Bouche, M. Pharmacological Inhibition of PKCtheta Counteracts Muscle Disease in a Mouse Model of Duchenne Muscular Dystrophy. EBioMedicine 2017, 16, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Lozanoska-Ochser, B.; Benedetti, A.; Rizzo, G.; Marrocco, V.; Di Maggio, R.; Fiore, P.; Bouche, M. Targeting early PKCtheta-dependent T-cell infiltration of dystrophic muscle reduces disease severity in a mouse model of muscular dystrophy. J. Pathol. 2018, 244, 323–333. [Google Scholar] [CrossRef]
- Madaro, L.; Marrocco, V.; Fiore, P.; Aulino, P.; Smeriglio, P.; Adamo, S.; Molinaro, M.; Bouche, M. PKCtheta signaling is required for myoblast fusion by regulating the expression of caveolin-3 and beta1D integrin upstream focal adhesion kinase. Mol. Biol. Cell 2011, 22, 1409–1419. [Google Scholar] [CrossRef]
- Messina, G.; Biressi, S.; Monteverde, S.; Magli, A.; Cassano, M.; Perani, L.; Roncaglia, E.; Tagliafico, E.; Starnes, L.; Campbell, C.E.; et al. Nfix regulates fetal-specific transcription in developing skeletal muscle. Cell 2010, 140, 554–566. [Google Scholar] [CrossRef] [Green Version]
- Zappelli, F.; Willems, D.; Osada, S.; Ohno, S.; Wetsel, W.C.; Molinaro, M.; Cossu, G.; Bouche, M. The inhibition of differentiation caused by TGFbeta in fetal myoblasts is dependent upon selective expression of PKCtheta: A possible molecular basis for myoblast diversification during limb histogenesis. Dev. Biol. 1996, 180, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.P.; Misyak, S.A.; Robertson, J.L.; Bassaganya-Riera, J.; Grange, R.W. Dysregulated intracellular signaling and inflammatory gene expression during initial disease onset in Duchenne muscular dystrophy. Am. J. Phys. Med. Rehabil. 2009, 88, 502–522. [Google Scholar] [CrossRef]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal models of Duchenne muscular dystrophy: From basic mechanisms to gene therapy. Dis. Models Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Irintchev, A.; Zweyer, M.; Wernig, A. Impaired functional and structural recovery after muscle injury in dystrophic mdx mice. Neuromuscul. Disord 1997, 7, 117–125. [Google Scholar] [CrossRef]
- Garg, K.; Corona, B.T.; Walters, T.J. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front. Pharmacol. 2015, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Zammit, P.S.; Heslop, L.; Hudon, V.; Rosenblatt, J.D.; Tajbakhsh, S.; Buckingham, M.E.; Beauchamp, J.R.; Partridge, T.A. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 2002, 281, 39–49. [Google Scholar] [CrossRef]
- Halevy, O.; Piestun, Y.; Allouh, M.Z.; Rosser, B.W.; Rinkevich, Y.; Reshef, R.; Rozenboim, I.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004, 231, 489–502. [Google Scholar] [CrossRef]
- Day, K.; Shefer, G.; Richardson, J.B.; Enikolopov, G.; Yablonka-Reuveni, Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev. Biol. 2007, 304, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Science 2006, 119, 1824–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeriglio, P.; Alonso-Martin, S.; Masciarelli, S.; Madaro, L.; Iosue, I.; Marrocco, V.; Relaix, F.; Fazi, F.; Marazzi, G.; Sassoon, D.A.; et al. Phosphotyrosine phosphatase inhibitor bisperoxovanadium endows myogenic cells with enhanced muscle stem cell functions via epigenetic modulation of Sca-1 and Pw1 promoters. FASEB J. 2016, 30, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Costamagna, D.; Giacomazzi, G.; Camps, J.; Sampaolesi, M. Notch signaling regulates myogenic regenerative capacity of murine and human mesoangioblasts. Cell Death Dis. 2014, 5, e1448. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, C.; Salvatori, M.L.; Biondo, A.; Shapira-Schweitzer, K.; Santoleri, S.; Antonini, S.; Bernardini, S.; Tedesco, F.S.; Cannata, S.; Seliktar, D.; et al. Injectable polyethylene glycol-fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal-muscle degeneration. Skelet. Muscle 2012, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Arendt, C.W.; Ellmeier, W.; Schaeffer, E.M.; Sunshine, M.J.; Gandhi, L.; Annes, J.; Petrzilka, D.; Kupfer, A.; Schwartzberg, P.L.; et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 2000, 404, 402–407. [Google Scholar] [CrossRef]
- Saccone, V.; Consalvi, S.; Giordani, L.; Mozzetta, C.; Barozzi, I.; Sandona, M.; Ryan, T.; Rojas-Munoz, A.; Madaro, L.; Fasanaro, P.; et al. HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes Dev. 2014, 28, 841–857. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, P.F.; Benedetti, A.; Sandonà, M.; Madaro, L.; De Bardi, M.; Saccone, V.; Puri, P.L.; Gargioli, C.; Lozanoska-Ochser, B.; Bouché, M. Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury. Int. J. Mol. Sci. 2020, 21, 932. https://doi.org/10.3390/ijms21030932
Fiore PF, Benedetti A, Sandonà M, Madaro L, De Bardi M, Saccone V, Puri PL, Gargioli C, Lozanoska-Ochser B, Bouché M. Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury. International Journal of Molecular Sciences. 2020; 21(3):932. https://doi.org/10.3390/ijms21030932
Chicago/Turabian StyleFiore, Piera Filomena, Anna Benedetti, Martina Sandonà, Luca Madaro, Marco De Bardi, Valentina Saccone, Pier Lorenzo Puri, Cesare Gargioli, Biliana Lozanoska-Ochser, and Marina Bouché. 2020. "Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury" International Journal of Molecular Sciences 21, no. 3: 932. https://doi.org/10.3390/ijms21030932
APA StyleFiore, P. F., Benedetti, A., Sandonà, M., Madaro, L., De Bardi, M., Saccone, V., Puri, P. L., Gargioli, C., Lozanoska-Ochser, B., & Bouché, M. (2020). Lack of PKCθ Promotes Regenerative Ability of Muscle Stem Cells in Chronic Muscle Injury. International Journal of Molecular Sciences, 21(3), 932. https://doi.org/10.3390/ijms21030932