Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway
Abstract
1. Introduction
2. Results
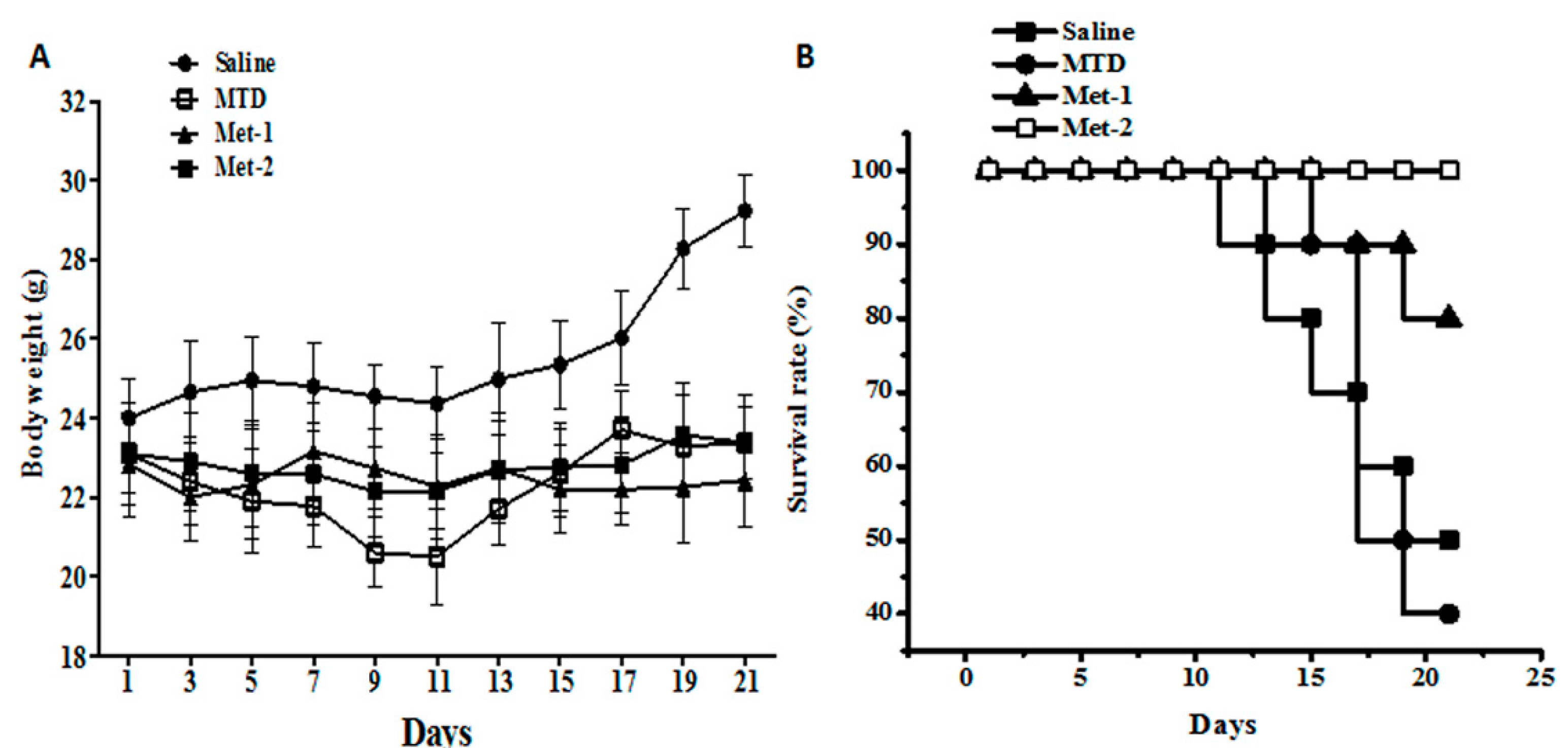
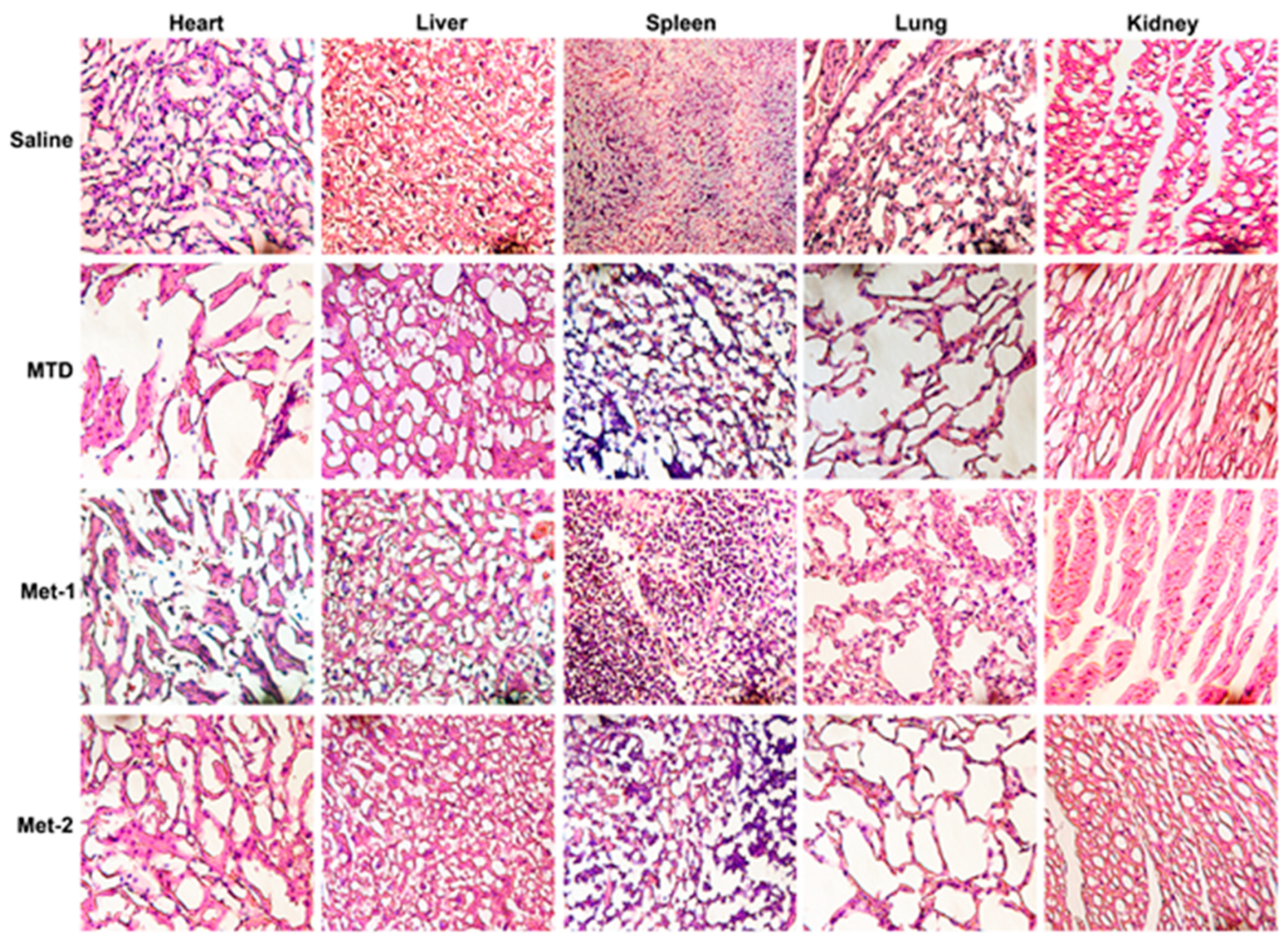
2.1. Toxicity Evaluation of Different Regimens
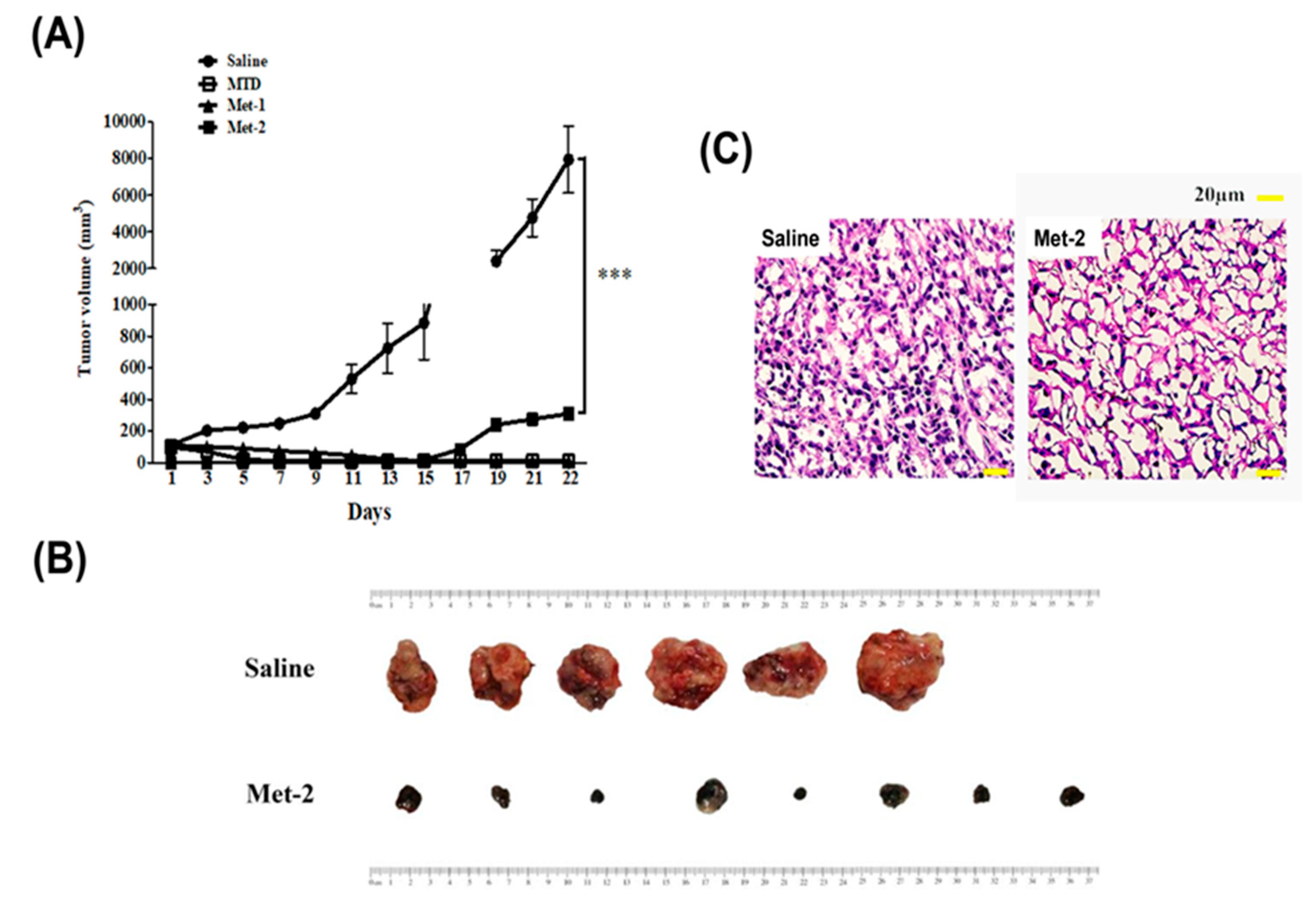
2.2. The Antitumor Effect of Different Schedules
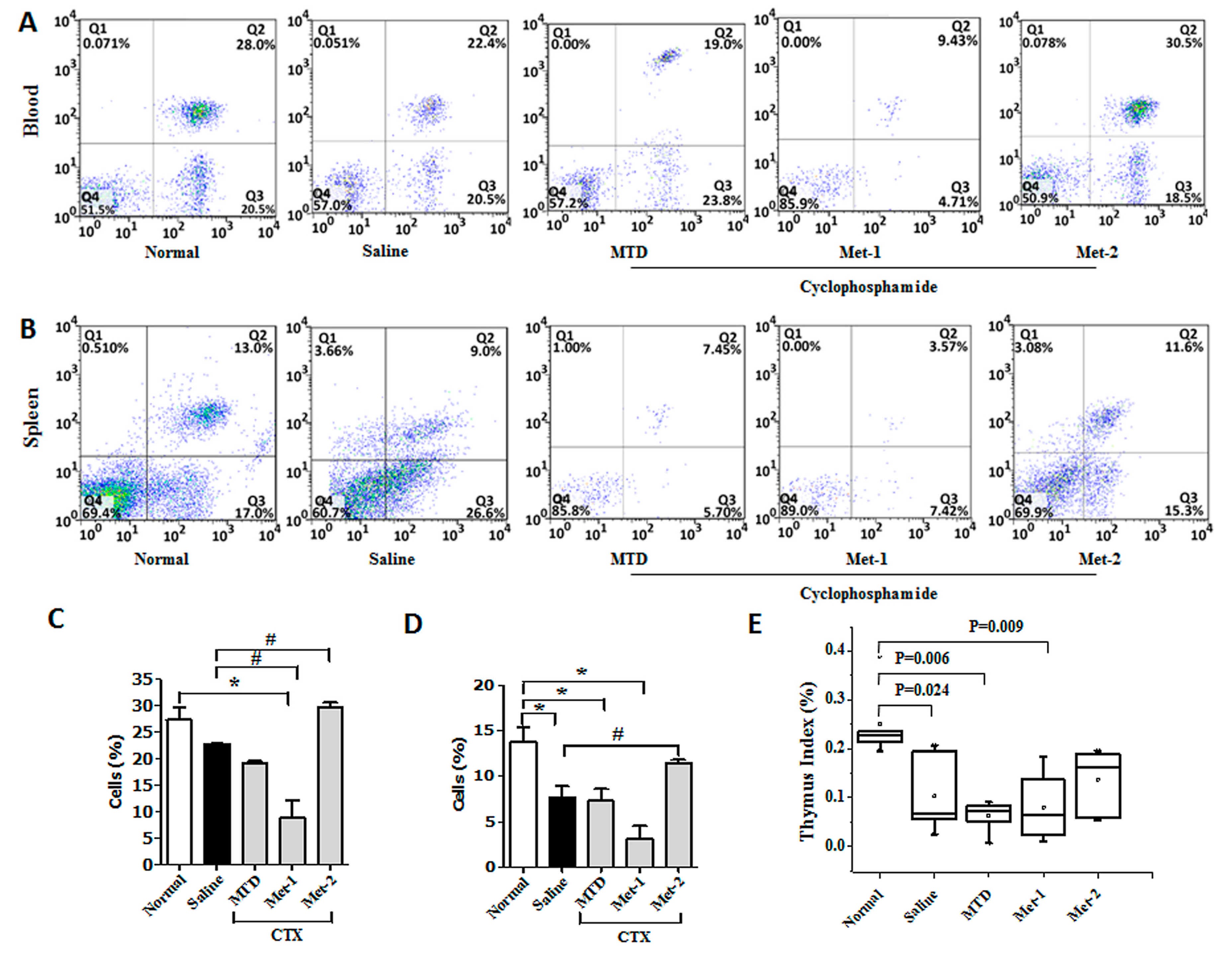
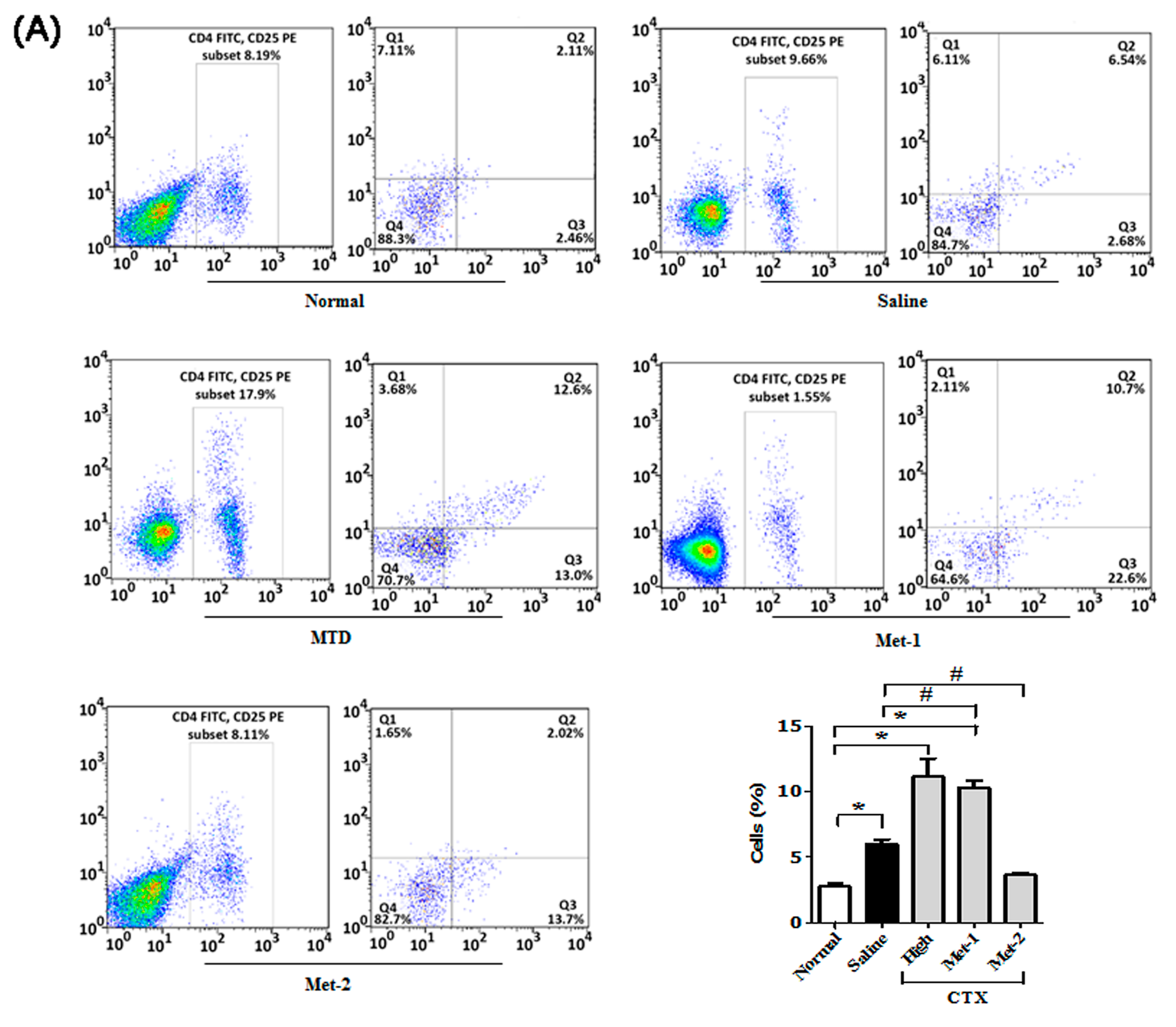
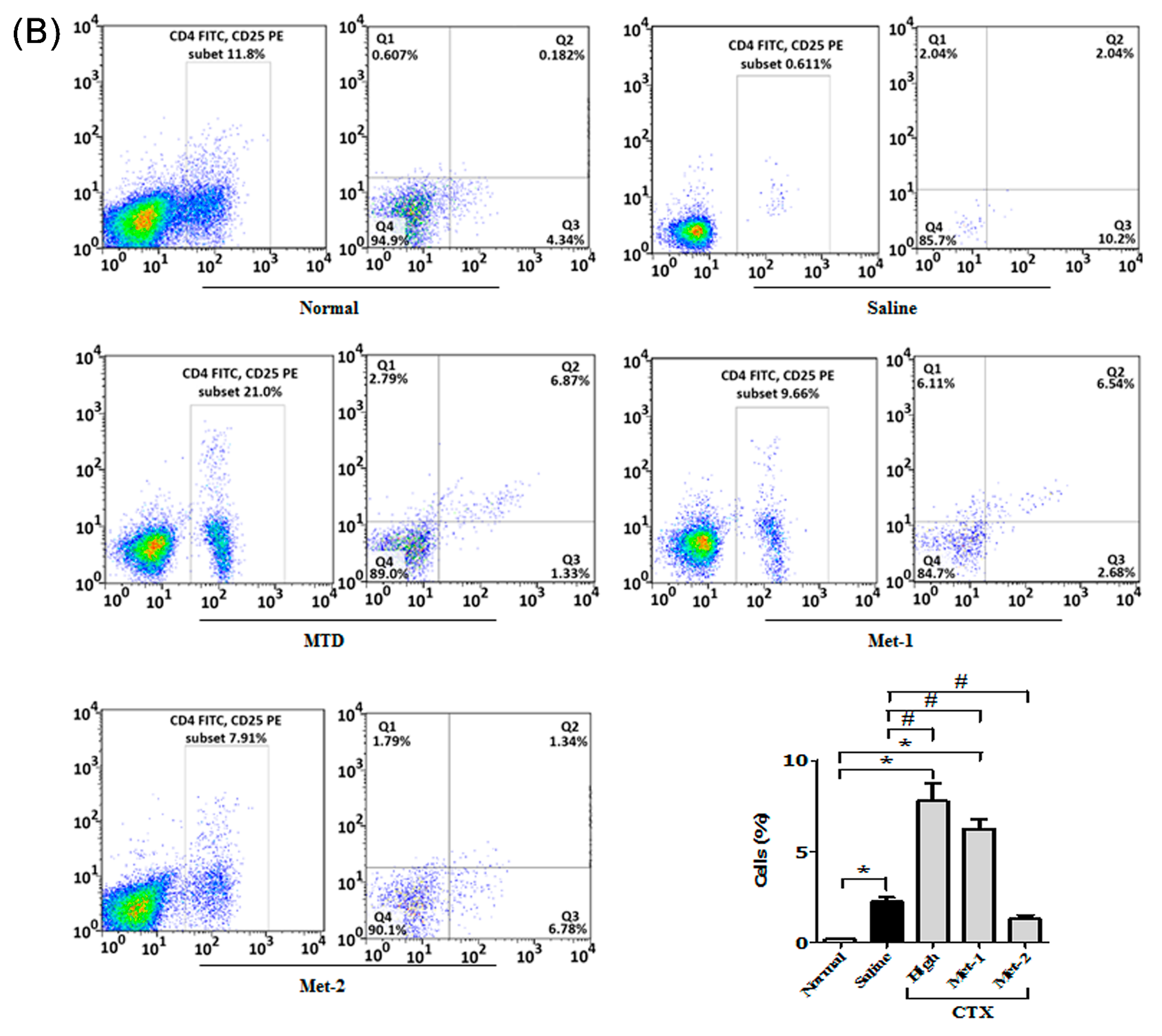
2.3. The Immunomodulatory Effect of CTX Regimens
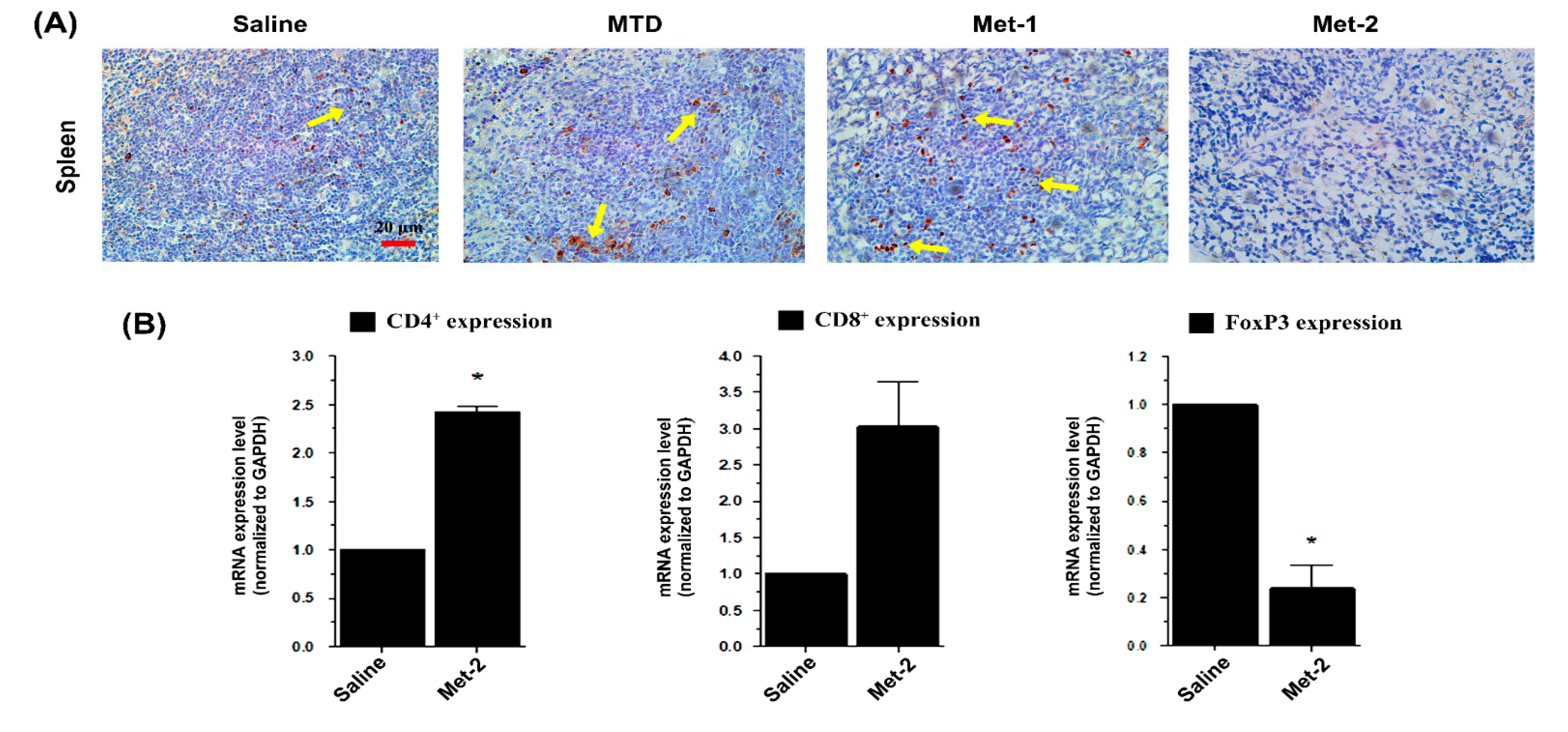
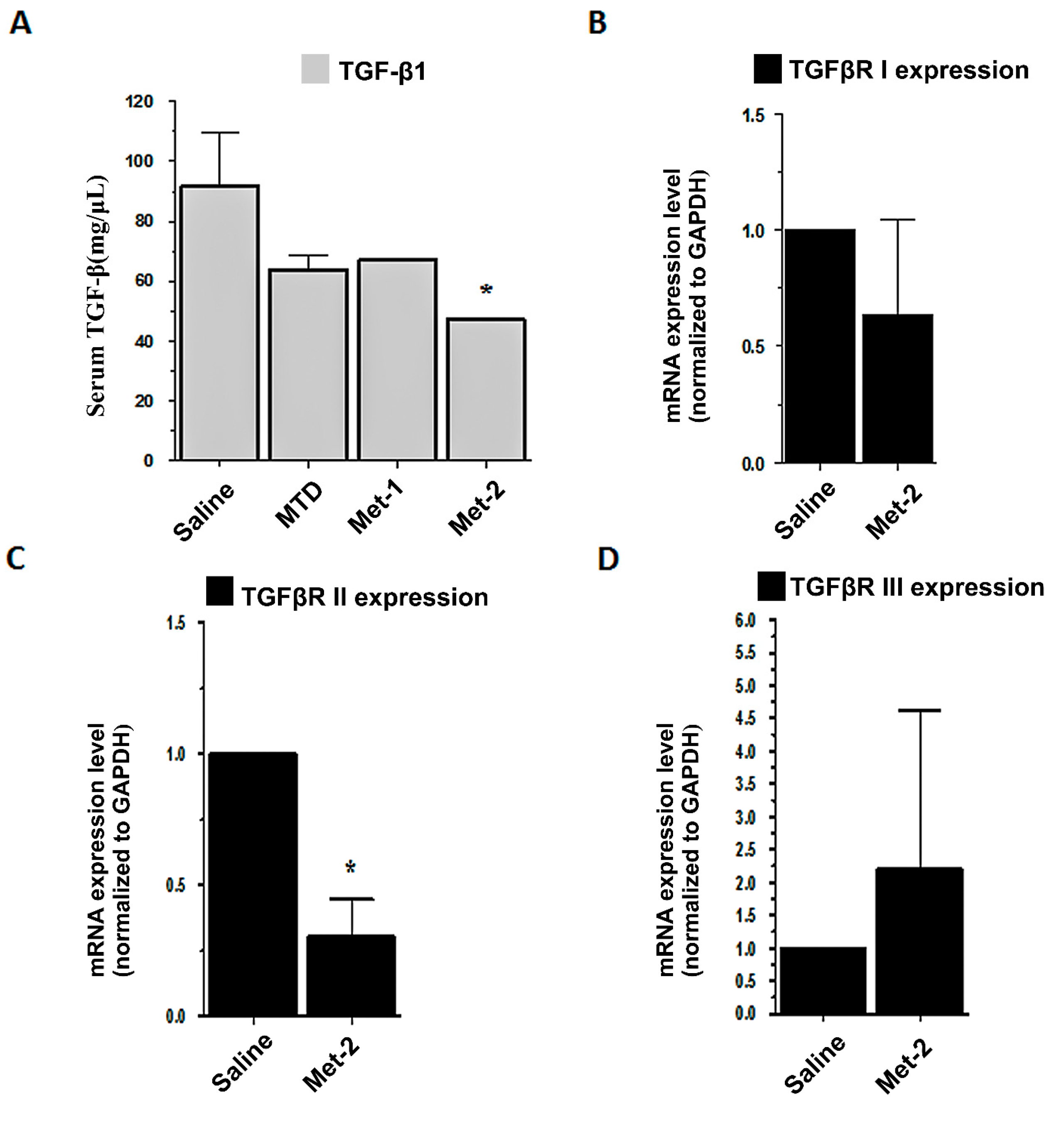
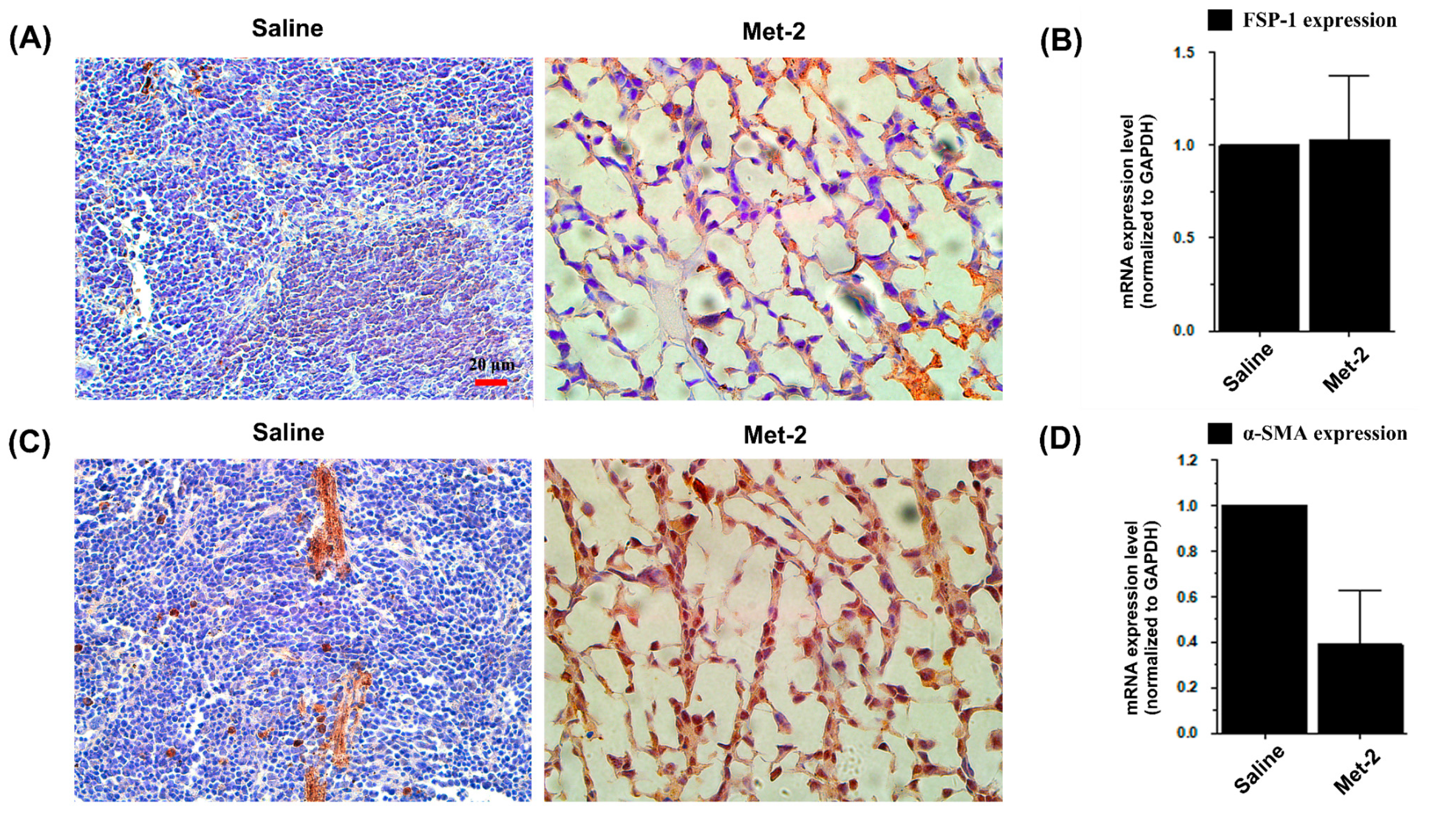
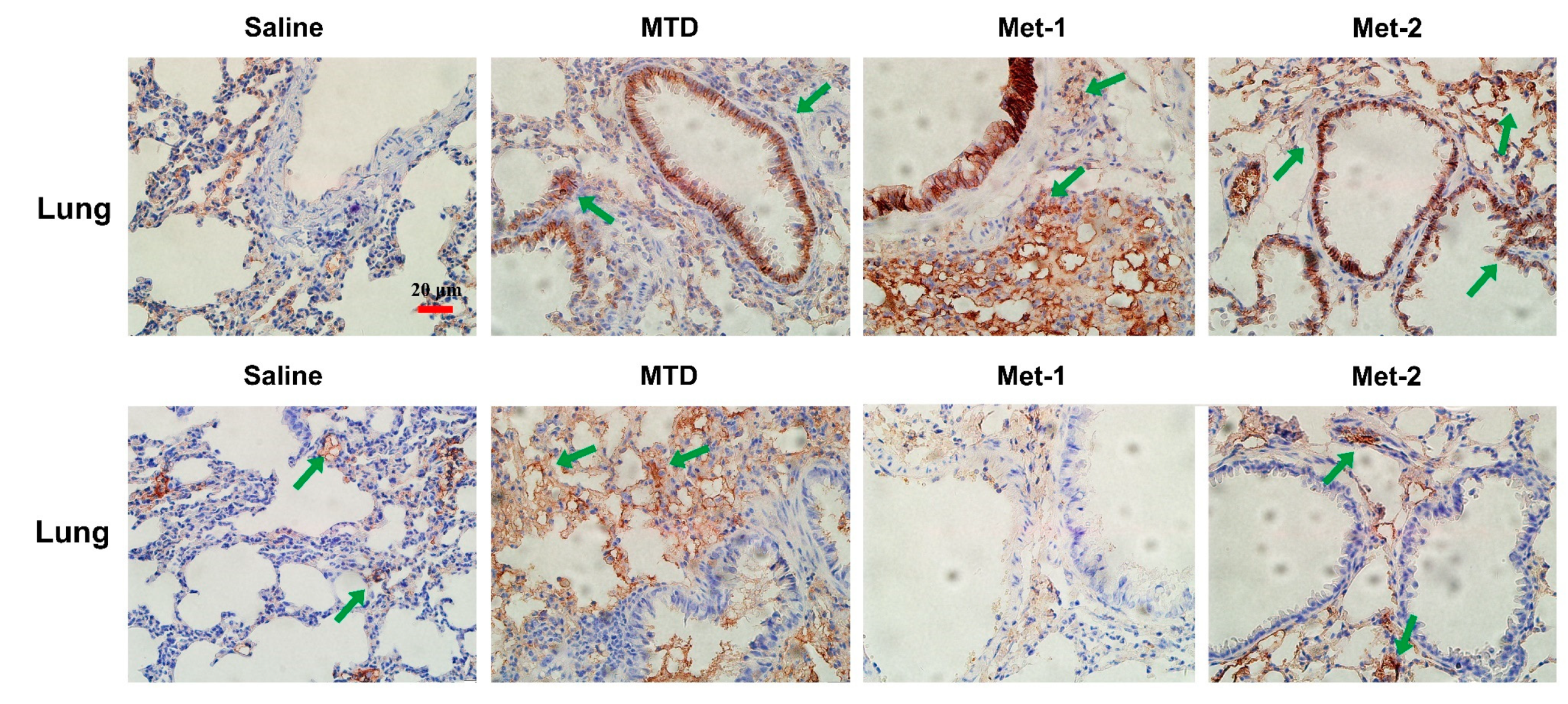
2.4. The Effect of Low-Dose CTX on Tumor Microenvironment and Its Relationship With TGF-β Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Mouse LLC Lung Cancer Model
4.4. Flow Cytomerty Analysis
4.5. Histopathological Analysis
4.6. Immunohistochemistry
4.7. qPCR Analysis
| Mouse GAPDH, (NCBI RefSeq: NM_008084.2): Forward: 5’-CTTTGGCATTGTGGAAGGGCTC-3’ Reverse: 5’-GGCATGGACTGTGGTCATGA-3’ |
| Mouse CD4, (NCBI RefSeq: NM_008084.2): Forward: 5’-GAGAACAGGAAAGAGGAGGTGGAGT-3’ Reverse: 5’-GGAGAGAACTTTGGAACCACTGACA-3’ |
| Mouse CD8, (NCBI RefSeq: NM_008084.2): Forward: 5’-AAGCCCAGACCTTCAGAGAAAATT-3’ Reverse: 5’-CCCATCACACCCCTACTAAAACAA-3’ |
| Mouse Foxp3, (NCBI RefSeq: NM_001199347.1): Forward: 5’-ACCTATGCCACCCTTATCCGA-3’ Reverse: 5’-CGAACATGCGAGTAAACCAATG-3’ |
| Mouse alpha smooth muscle actin (α-SMA), (NCBI RefSeq: NM_007392.3): Forward: 5’-CCACTGAACCCTAAGGCCAAC-3’ Reverse: 5’-CTCCAGAGTCCAGCACAATACCA-3’ |
| Mouse fibroblast specific protein (FSP-1) (NCBI RefSeq: NM_011311.2): Forward: 5’-GTGTCCACCTTCCACAAATACTCAG-3’ Reverse: 5’-AATGCAGCTTCATCTGTCCTTTTC-3’ |
| Mouse transforming growth factor, beta receptor I (Tgfbr1) (NCBI RefSeq: NM_009370.2): Forward: 5’-CTCTGTTTTTCCCACTCTGCC-3’ Reverse: 5’-GCTTCCATCAATCACTTCATTTTAG-3’ |
| Mouse transforming growth factor, beta receptor II (Tgfbr2) (NCBI RefSeq: NM_009371.3): Forward: 5’-TCCAGACTTCCCATTACTCACACC-3’ Reverse: 5’-TCTCCATGCTCACGAACGCAC-3’ |
| Mouse transforming growth factor, beta receptor III (Tgfbr3) (NCBI RefSeq: NM_011578.3): Forward: 5’-GTCCCTGTGTTTGTCCTGATGAG-3’ Reverse: 5’-CCAGACAGAACGGTGAAGCTCTC-3’ |
4.8. Mouse TGF-β1 ELISA Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, D.A.; Gini, B.; Mottahedeh, J.; Visnyei, K.; Koga, T.; Gomez, G.; Eskin, A.; Hwang, K.; Wang, J.; Masui, K.; et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 2014, 343, 72–76. [Google Scholar] [CrossRef]
- Kumar, V. Adenosine as an endogenous immunoregulator in cancer pathogenesis: Where to go? Purinergic Signal 2013, 9, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Devaud, C.; John, L.B.; Westwood, J.A.; Darcy, P.K. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology 2013, 2, e25962. [Google Scholar] [CrossRef]
- Poggi, A.; Musso, A.; Dapino, I.; Zocchi, M.R. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunol. Lett. 2014, 159, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Shirakihara, T.; Horiguchi, K.; Miyazawa, K.; Ehata, S.; Shibata, T.; Morita, I.; Miyazono, K.; Saitoh, M. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011, 30, 783–795. [Google Scholar] [CrossRef]
- Matar, P.; Rozados, V.R.; Gervasoni, S.I.; Scharovsky, G.O. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol. Immunother. 2002, 50, 588–596. [Google Scholar] [CrossRef]
- Browder, T.; Butterfield, C.E.; Kräling, B.M.; Shi, B.; Marshall, B.; O’Reilly, M.S.; Folkman, J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000, 60, 1878–1886. [Google Scholar]
- Hicklin, D.J.; Bohlen, P.; Kerbel, R.S. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin. Invest. 2000, 105, R15–R24. [Google Scholar] [CrossRef]
- Hanahan, D.; Bergers, G.; Bergsland, E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J. Clin. Invest. 2000, 105, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Loven, D.; Hasnis, E.; Bertolini, F.; Shaked, Y. Low-dose metronomic chemotherapy: From past experience to new paradigms in the treatment of cancer. Drug Discov. Today 2013, 18, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Tongu, M.; Harashima, N.; Yamada, T.; Harada, T.; Harada, M. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer Immunol. Immunother. 2010, 59, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Tongu, M.; Harashima, N.; Monma, H.; Inao, T.; Yamada, T.; Kawauchi, H.; Harada, M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol. Immunother. 2013, 62, 383–391. [Google Scholar] [CrossRef]
- Matar, P.; Rozados, V.R.; González, A.D.; Dlugovitzky, D.G.; Bonfil, R.D.; Scharovsky, O.G. Mechanism of antimetastatic immunopotentiation by low-dose cyclophosphamide. Eur. J. Cancer 2000, 36, 1060–1066. [Google Scholar] [CrossRef]
- Mandal, D.; Bhattacharyya, A.; Lahiry, L.; Choudhuri, T.; Sa, G.; Das, T. Failure in peripheral immuno-surveillance due to thymic atrophy: Importance of thymocyte maturation and apoptosis in adult tumor-bearer. Life Sci. 2005, 77, 2703–2716. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Chang, C.L.; Hsu, Y.T.; Wu, C.C.; Lai, Y.Z.; Wang, C.; Yang, Y.C.; Wu, T.C.; Hung, C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013, 73, 119–127. [Google Scholar] [CrossRef]
- Cerullo, V.; Diaconu, I.; Kangasniemi, L.; Rajecki, M.; Escutenaire, S.; Koski, A.; Romano, V.; Rouvinen, N.; Tuuminen, T.; Laasonen, L.; et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol. Ther. 2011, 19, 1737–1746. [Google Scholar] [CrossRef]
- Ha, T.Y. The role of regulatory T cells in cancer. Immune Netw. 2009, 9, 209–235. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Larmonier, N.; Schmitt, E.; Parcellier, A.; Cathelin, D.; Garrido, C.; Chauffert, B.; Solary, E.; Bonnotte, B.; Martin, F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004, 34, 336–344. [Google Scholar] [CrossRef]
- Lutsiak, M.E.C.; Semnani, R.T.; de Pascalis, R.; Kashmiri, S.V.S.; Schlom, J.; Sabzevari, H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005, 105, 2862–2868. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef]
- Liu, C.; Billadeau, D.D.; Abdelhakim, H.; Leof, E.; Kaibuchi, K.; Bernabeu, C.; Bloom, G.S.; Yang, L.; Boardman, L.; Shah, V.H.; et al. IQGAP1 suppresses TβRII-mediated myofibroblastic activation and metastatic growth in liver. J. Clin. Invest. 2013, 123, 1138–1156. [Google Scholar] [CrossRef]
- Janik, P.; Assaf, A.; Bertram, J.S. Inhibition of growth of primary and metastatic lewis lung carcinoma cells by the phosphodiesterase inhibitor isobutylmethylxanthine. Cancer Res. 1980, 40, 1950–1954. [Google Scholar]
- Genin, O.; Rechavi, G.; Nagler, A.; Ben-Itzhak, O.; Nazemi, K.J.; Pines, M. Myofibroblasts in pulmonary and brain metastases of alveolar soft-part sarcoma: A novel target for treatment? Neoplasia 2008, 10, 940–948. [Google Scholar] [CrossRef]
- Gravdal, K.; Halvorsen, O.J.; Haukaas, S.A.; Akslen, L.A. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 2007, 13, 7003–7011. [Google Scholar] [CrossRef]
- Scharovsky, O.G.; Mainetti, L.E.; Rozados, V.R. Metronomic chemotherapy: Changing the paradigm that more is better. Curr. Oncol. 2009, 16, 7–15. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Kerbel, R.S. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays 1991, 13, 31–36. [Google Scholar] [CrossRef]
- Lien, K.; Georgsdottir, S.; Sivanathan, L.; Chan, K.; Emmenegger, U. Low-dose metronomic chemotherapy: A systematic literature analysis. Eur. J. Cancer 2013, 49, 3387–3395. [Google Scholar] [CrossRef]
- Kono, K.; Kawaida, H.; Takahashi, A.; Sugai, H.; Mimura, K.; Miyagawa, N.; Omata, H.; Fujii, H. CD4(+)CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother. 2006, 55, 1064–1071. [Google Scholar] [CrossRef]
- Motoyoshi, Y.; Kaminoda, K.; Saitoh, O.; Hamasaki, K.; Nakao, K.; Ishii, N.; Nagayama, Y.; Eguchi, K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol. Rep. 2006. [Google Scholar] [CrossRef]
- Ge, Y.; Domschke, C.; Stoiber, N.; Schott, S.; Heil, J.; Rom, J.; Blumenstein, M.; Thum, J.; Sohn, C.; Schneeweiss, A.; et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: Immunological effects and clinical outcome. Cancer Immunol. Immunother. 2012, 61, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. Regulatory T cells, transforming growth factor-beta, and immune suppression. Proc. Am. Thorac. Soc. 2007, 4, 271–276. [Google Scholar] [CrossRef]
- Tran, D.Q. TGF-β: The sword, the wand, and the shield of FOXP3(+) regulatory T cells. J. Mol. Cell Biol. 2012, 4, 29–37. [Google Scholar] [CrossRef]
- Malvicini, M.; Alaniz, L.; Bayo, J.; Garcia, M.; Piccioni, F.; Fiore, E.; Atorrasagasti, C.; Aquino, J.B.; Matar, P.; Mazzolini, G. Single low-dose cyclophosphamide combined with interleukin-12 gene therapy is superior to a metronomic schedule in inducing immunity against colorectal carcinoma in mice. Oncoimmunology 2012, 1, 1038–1047. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 957. https://doi.org/10.3390/ijms21030957
Zhong H, Lai Y, Zhang R, Daoud A, Feng Q, Zhou J, Shang J. Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. International Journal of Molecular Sciences. 2020; 21(3):957. https://doi.org/10.3390/ijms21030957
Chicago/Turabian StyleZhong, Hui, Yifan Lai, Rui Zhang, Abdelkader Daoud, Qingyuan Feng, Jia Zhou, and Jing Shang. 2020. "Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway" International Journal of Molecular Sciences 21, no. 3: 957. https://doi.org/10.3390/ijms21030957
APA StyleZhong, H., Lai, Y., Zhang, R., Daoud, A., Feng, Q., Zhou, J., & Shang, J. (2020). Low Dose Cyclophosphamide Modulates Tumor Microenvironment by TGF-β Signaling Pathway. International Journal of Molecular Sciences, 21(3), 957. https://doi.org/10.3390/ijms21030957



