Synaptotagmin 1 Is Involved in Neuropathic Pain and Electroacupuncture-Mediated Analgesic Effect
Abstract
:1. Introduction
2. Results
2.1. Syt-1 Is Up-Regulated Mainly in Spinal Lamina I-II after Neuropathic Pain
2.2. Syt-1 Antibody Relieved SNI-Induced Neuropathic Pain
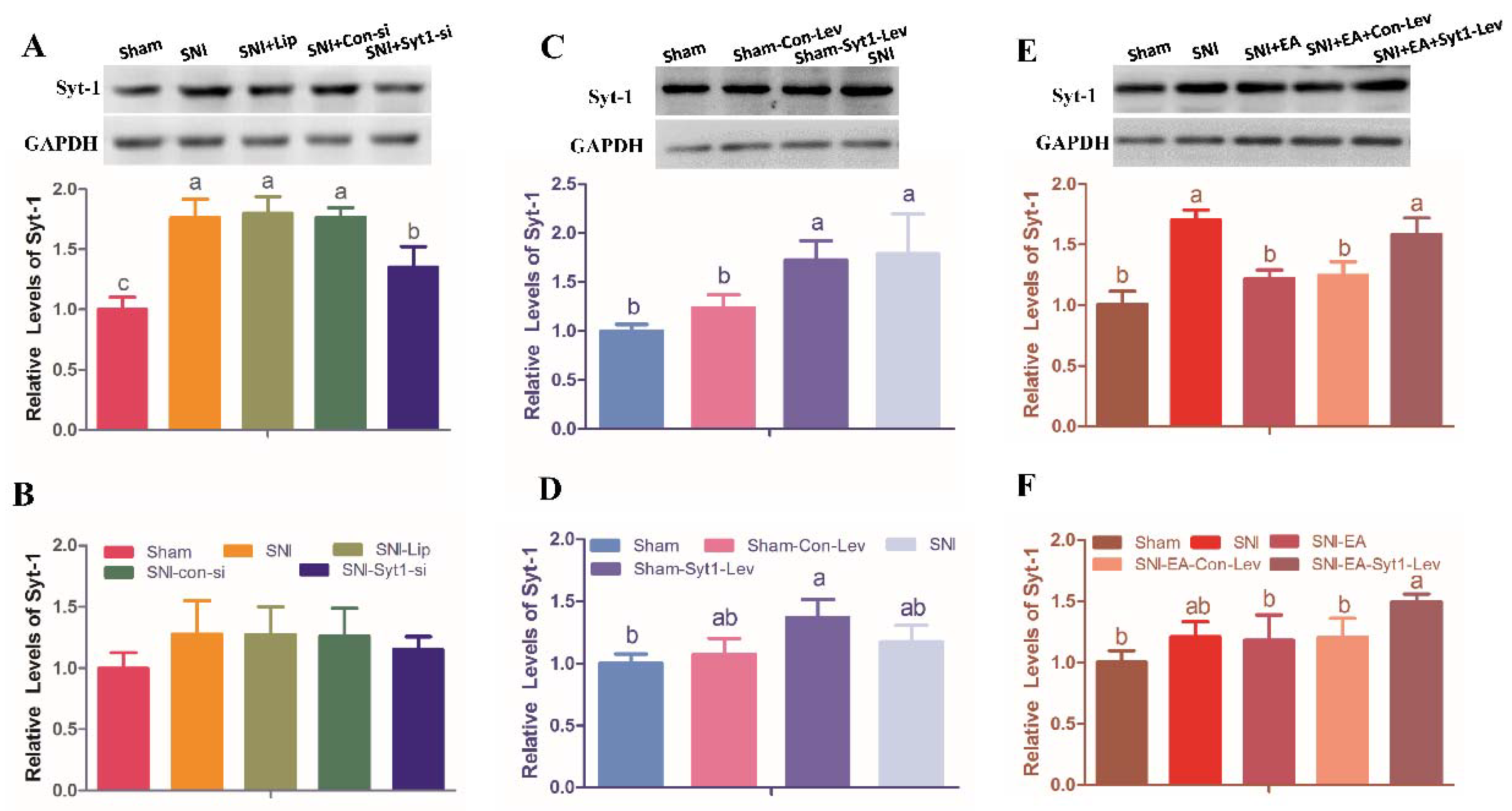
2.3. Syt-1 Knockdown Attenuated Neuropathic Pain
2.4. Syt-1 Overexpression Causes Pain Hypersensitivity
2.5. EA Attenuated Neuropathic Pain via Down-Regulating Syt-1
2.6. Syt-1 Was Expressed in Neurons and Glial Cells in the Spinal Cord Dorsal Horn
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Groups and Design
4.3. SNI Rat Preparation
4.4. Intrathecal Cannulation
4.5. Mechanical Hypersensitivity
4.6. Cold and Hot Hypersensitivities
4.7. Electroacupuncture Application
4.8. Sample Collection
4.9. Immunohistochemistry
4.10. Western Blotting
4.11. QRT-PCR
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EA | Electroacupuncture |
| Syt-1 | Synaptotagmin 1 |
| SNI | Spared nerve injury |
| PWT | Paw withdrawal threshold |
| PWL | Paw withdrawal latency |
| EAAs | Excitatory amino acids |
| ST36 | Zusanli |
| SP6 | Sanyinjiao |
| SCDH | Spinal cord dorsal horn |
| IHC | Immunohistochemistry |
| IR | Immunoreactive |
| PBS | Phosphate buffer saline |
References
- Kajander, K.C.; Bennett, G.J. Onset of a painful peripheral neuropathy in rat: A partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J. Neurophysiol. 1992, 68, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Butera, J.A. Current and emerging targets to treat neuropathic pain. J. Med. Chem. 2007, 50, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, V.L.; Nutile-McMenemy, N.; LaCroix-Fralish, M.L.; Deleo, J.A. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav. Immun. 2007, 21, 238–246. [Google Scholar] [CrossRef]
- Muthuraman, A.; Singh, N.; Jaggi, A.S.; Ramesh, M. Drug Therapy of Neuropathic Pain: Current Developments and Future Perspectives. Curr. Drug Targets 2014, 15, 210–253. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zhou, F.-Y.; Liu, J.-J.; Ding, Y.; Zhong, J.-M.; Ding, M.-X. Electroacupuncture Inhibits the Activation of p38MAPK in the Central Descending Facilitatory Pathway in Rats with Inflammatory Pain. Evid.-Based Complementary Altern. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.Y.; Hsieh, C.L.; Huang, C.P.; Lin, Y.W. Electroacupuncture Attenuates Induction of Inflammatory Pain by Regulating Opioid and Adenosine Pathways in Mice. Sci. Rep. 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Omana, I.; Olvera, V.; Santos, P.; Calderon, J.L. Naloxone prevents reduction of pain responses evoked by acupuncture in neuropathic rats. Proc. West. Pharmacol. Soc. 1994, 37, 135–136. [Google Scholar]
- Zeng, J.; Cui, L.Y.; Feng, Y.; Ding, M.X. Electroacupuncture relieves neuropathic pain via upregulation of glutamate transporters in the spinal cord of rats. Neurosci. Lett 2016, 620, 38–42. [Google Scholar] [CrossRef]
- Dai, Y.; Kondo, E.; Fukuoka, T.; Tokunaga, A.; Miki, K.; Noguchi, K. The effect of electroacupuncture on pain behaviors and noxious stimulus-evoked Fos expression in a rat model of neuropathic pain. J. Pain Off. J. Am. Pain Soc. 2001, 2, 151–159. [Google Scholar] [CrossRef]
- Dong, Z.Q.; Ma, F.; Xie, H.; Wang, Y.Q.; Wu, G.C. Down-regulation of GFRalpha-1 expression by antisense oligodeoxynucleotide attenuates electroacupuncture analgesia on heat hyperalgesia in a rat model of neuropathic pain. Brain Res. Bull. 2006, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, H.T.; Shi, Y.S.; Han, J.S.; Wan, Y. Ketamine potentiates the effect of electroacupuncture on mechanical allodynia in a rat model of neuropathic pain. Neurosci. Lett. 2004, 368, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.G.; Min, B.I.; Kim, J.H.; Na, H.S.; Park, D.S. Effects of electroacupuncture on the mechanical allodynia in the rat model of neuropathic pain. Neurosci. Lett. 2002, 320, 49–52. [Google Scholar] [CrossRef]
- Wan, J.; Ding, Y.; Tahir, A.H.; Shah, M.K.; Janyaro, H.; Li, X.; Zhong, J.; Vodyanoy, V.; Ding, M. Electroacupuncture Attenuates Visceral Hypersensitivity by Inhibiting JAK2/STAT3 Signaling Pathway in the Descending Pain Modulation System. Front. Neurosci. 2017, 11, 644. [Google Scholar] [CrossRef] [Green Version]
- Kawamata, M.; Omote, K. Involvement of increased excitatory amino acids and intracellular Ca2+ concentration in the spinal dorsal horn in an animal model of neuropathic pain. Pain 1996, 68, 85–96. [Google Scholar] [CrossRef]
- Davar, G.; Hama, A.; Deykin, A.; Vos, B.; Maciewicz, R. MK-801 blocks the development of thermal hyperalgesia in a rat model of experimental painful neuropathy. Brain Res. 1991, 553, 327–330. [Google Scholar] [CrossRef]
- Sudhof, T.C. Synaptotagmins: Why so many? J. Biol. Chem. 2002, 277, 7629–7632. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Chacón, R.; Königstorfer, A.; Gerber, S.H.; García, J.; Matos, M.F.; Stevens, C.F.; Brose, N.; Rizo, J.; Rosenmund, C.; Südhof, T.C. Synaptotagmin I functions as a calcium regulator of release probability. Nature 2001, 410, 41–49. [Google Scholar] [CrossRef]
- Geppert, M.; Goda, Y.; Hammer, R.E.; Li, C.; Rosahl, T.W.; Stevens, C.F.; Südhof, T.C. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell 1994, 79, 717–727. [Google Scholar] [CrossRef]
- Nishiki, T.; Augustine, G.J. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J. Neurosci. 2004, 24, 6127–6132. [Google Scholar] [CrossRef]
- Cui, L.; Ding, Y.; Zeng, J.; Feng, Y.; Li, M.; Ding, M. Spinal Glutamate Transporters Are Involved in the Development of Electroacupuncture Tolerance. Int. J. Mol. Sci. 2016, 17, 357. [Google Scholar] [CrossRef] [Green Version]
- al-Ghoul, W.M.; Li Volsi, G.; Weinberg, R.J.; Rustioni, A. Glutamate immunocytochemistry in the dorsal horn after injury or stimulation of the sciatic nerve of rats. Brain Res. Bull. 1993, 30, 453–459. [Google Scholar] [CrossRef]
- Sung, B.K.; Lim, G.; Mao, J.R. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 2003, 23, 2899–2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.L.; Zhu, H.M.; Zhang, Q.L.; Liu, J.J.; Ding, Y.; Zhong, J.M.; Vodyanoy, V.; Ding, M.X. Exploring the Mechanisms of Electroacupuncture-Induced Analgesia through RNA Sequencing of the Periaqueductal Gray. Int. J. Mol. Sci. 2018, 19, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Tucker, W.C.; Chapman, E.R. Role of synaptotagmin in Ca(2+)-triggered exocytosis. Biochem. J. 2002, 366, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, A.; Chicka, M.C.; Tucker, W.C.; Chapman, E.R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 2006, 13, 323–330. [Google Scholar] [CrossRef]
- Dai, H.; Shen, N.; Arac, D.; Rizo, J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 2007, 367, 848–863. [Google Scholar] [CrossRef] [Green Version]
- Jia, N.; Yang, K.; Sun, Q.; Cai, Q.; Li, H.; Cheng, D.; Fan, X.; Zhu, Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev. Neurobiol. 2010, 70, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Gong, Y.; Wang, X.F.; Xiao, F.; Xi, Z.Q.; Lu, Y.; Sun, H.B. Altered expression of synaptotagmin I in temporal lobe tissue of patients with refractory epilepsy. J. Mol. Neurosci. Mn 2009, 38, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Proper, E.A.; Hoogland, G.; Kappen, S.M.; Jansen, G.H.; Rensen, M.G.A.; Schrama, L.H.; van Veelen, C.W.M.; van Rijen, P.C.; van Nieuwenhuizen, O.; Gispen, W.H.; et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 2002, 125, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W. Glutamate: A major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology 1995, 61, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Liaw, W.J.; Stephens, R.L.; Binns, B.C.; Chu, Y.C.; Sepkuty, J.P.; Johns, R.A.; Rothstein, J.D.; Tao, Y.X. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain 2005, 115, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-p.; Wu, X.-t.; Yin, Z.-y.; Ma, C. Effect of electroacupuncture on the levels of amino acid neurotransmitters in the spinal cord in rats with chronic constrictive injury. Zhen Ci Yan Jiu = Acupunct. Res. 2011, 36, 353–356, 379. [Google Scholar]
- Cui, L.-Y.; Guo, N.-N.; Li, Y.-L.; Li, M.; Ding, M.-X. Analgesic and physiological effect of electroacupuncture combined with epidural lidocaine in goats. Vet. Anaesth. Analg. 2017, 44, 959–967. [Google Scholar] [CrossRef]
- Hu, M.L.; Qiu, Z.Y.; Hu, K.; Ding, M.X. Analgesic Neural Circuits Are Activated by Electroacupuncture at Two Sets of Acupoints. Evid.-Based Complementary Altern. Med. Ecam 2016, 2016, 3840202. [Google Scholar] [CrossRef] [Green Version]
- Shah, Z.; Hu, M.L.; Qiu, Z.Y.; Zhou, F.Y.; Zeng, J.; Wan, J.; Wang, S.W.; Zhang, W.; Ding, M.X. Physiologic and biochemical effects of electroacupuncture combined with intramuscular administration of dexmedetomidine to provide analgesia in goats. Am. J. Vet. Res. 2016, 77, 252–259. [Google Scholar] [CrossRef]
- Liu, D.-M.; Zhou, Z.-Y.; Ding, Y.; Chen, J.-G.; Hu, C.-M.; Chen, X.; Ding, M.-X. Physiologic effects of electroacupuncture combined with intramuscular administration of xylazine to provide analgesia in goats. Am. J. Vet. Res. 2009, 70, 1326–1332. [Google Scholar] [CrossRef]
- Lau, W.K.; Lau, Y.M.; Zhang, H.Q.; Wong, S.C.; Bian, Z.X. Electroacupuncture versus celecoxib for neuropathic pain in rat SNL model. Neuroscience 2010, 170, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-L.; Ding, M.-X.; Xiong, C.; Zhou, M.-Y.; Qiu, Z.-Y.; Wang, Q. Effects of Electroacupuncture of Different Frequencies on the Release Profile of Endogenous Opioid Peptides in the Central Nerve System of Goats. Evid.-Based Complementary Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, R.; Taguchi, T.; Kitakoji, H. Involvement of peripheral opioid receptors in electroacupuncture analgesia for carrageenan-induced hyperalgesia. Brain Res. 2010, 1355, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-L.; Ding, M.-X.; Wei, J.; Wu, Y.-Q.; Qiu, Z.-Y.; Chen, J.-G.; Liu, D.-M.; Hu, C.-M.; Hu, M.-L.; Shah, Z.; et al. Electroacupuncture-induced dynamic processes of gene expression levels of endogenous opioid Peptide precursors and opioid receptors in the CNS of goats. Evid.-Based Complementary Altern. Med. Ecam 2013, 2013, 257682. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Du, M.Y.; Wu, W.Y.; Jiang, Y.; Han, J.S. Effect of intracerebral microinjection of naloxone on acupuncture- and morphine-analgesia in the rabbit. Sci. Sin. 1981, 24, 1166–1178. [Google Scholar]
- Ma, C.; Li, C.-x.; Yi, J.-l.; Yan, L.-p. Effects of electroacupuncture on glutamate and aspartic acid contents in the dorsal root ganglion and spinal cord in rats with neuropathic pain. Zhen Ci Yan Jiu = Acupunct. Res. 2008, 33, 250–254. [Google Scholar]
- Cui, L.; Ding, Y.; Feng, Y.; Chen, S.; Xu, Y.; Li, M.; Hu, M.; Qiu, Z.; Ding, M. MiRNAs are involved in chronic electroacupuncture tolerance in the rat hypothalamus. Mol. Neurobiol. 2017, 54, 1429–1439. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Ding, Y.; Cui, L.Y.; Hu, M.L.; Ding, M.X. The Expression Patterns of c-Fos and c-Jun Induced by Different Frequencies of Electroacupuncture in the Brain. Evid.-Based Complementary Altern. Med. 2015, 2015, 343682. [Google Scholar] [CrossRef] [Green Version]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef]
- Storkson, R.V.; Kjorsvik, A.; Tjolsen, A.; Hole, K. Lumbar catheterization of the spinal subarachnoid space in the rat. J. Neurosci. Methods 1996, 65, 167–172. [Google Scholar] [CrossRef]
- Yamada, M.; Fujita, Y.; Hayano, Y.; Hayakawa, H.; Baba, K.; Mochizuki, H.; Yamashita, T. Increased Expression of Fibronectin Leucine-Rich Transmembrane Protein 3 in the Dorsal Root Ganglion Induces Neuropathic Pain in Rats. J. Neurosci. 2019, 39, 7615–7627. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kim, Y.H.; Wang, X.; Liu, D.; Zhang, Z.-J.; Bey, A.L.; Lay, M.; Chang, W.; Berta, T.; Zhang, Y. SHANK3 Deficiency Impairs Heat Hyperalgesia and TRPV1 Signaling in Primary Sensory Neurons. Neuron 2016, 92, 1279–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minfeng, R.; Jisheng, H. Rat tail flick acupuncture analgesia model. Chin. Med. J. 1979, 92, 576–582. [Google Scholar] [PubMed]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, J.; Nan, S.; Liu, J.; Ding, M.; Zhu, H.; Suo, C.; Wang, Z.; Hu, M.; Wang, D.; Ding, Y. Synaptotagmin 1 Is Involved in Neuropathic Pain and Electroacupuncture-Mediated Analgesic Effect. Int. J. Mol. Sci. 2020, 21, 968. https://doi.org/10.3390/ijms21030968
Wan J, Nan S, Liu J, Ding M, Zhu H, Suo C, Wang Z, Hu M, Wang D, Ding Y. Synaptotagmin 1 Is Involved in Neuropathic Pain and Electroacupuncture-Mediated Analgesic Effect. International Journal of Molecular Sciences. 2020; 21(3):968. https://doi.org/10.3390/ijms21030968
Chicago/Turabian StyleWan, Juan, Sha Nan, Jingjing Liu, Mingxing Ding, Hongmei Zhu, Chuanguang Suo, Zhuole Wang, Manli Hu, Dehai Wang, and Yi Ding. 2020. "Synaptotagmin 1 Is Involved in Neuropathic Pain and Electroacupuncture-Mediated Analgesic Effect" International Journal of Molecular Sciences 21, no. 3: 968. https://doi.org/10.3390/ijms21030968




