Assessment of Nuclear and Mitochondrial DNA, Expression of Mitochondria-Related Genes in Different Brain Regions in Rats after Whole-Body X-ray Irradiation
Abstract
:1. Introduction
2. Results
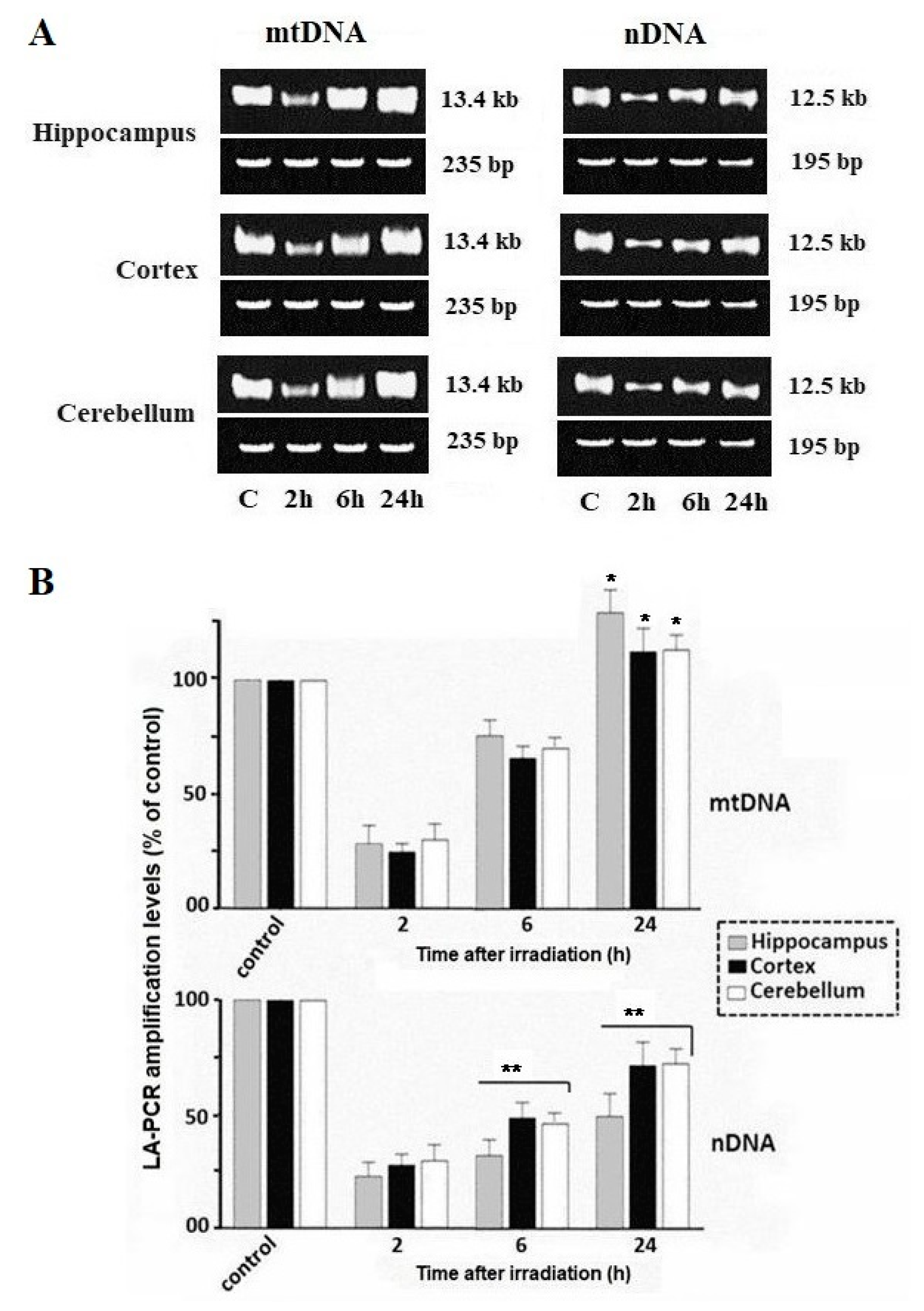
2.1. Damage and Repair of Mitochondrial DNA and Nuclear DNA
2.2. Relative Quantification of the Total Level of mtDNA Copies
2.3. Analysis of Mitochondrial DNA Mutant Copies
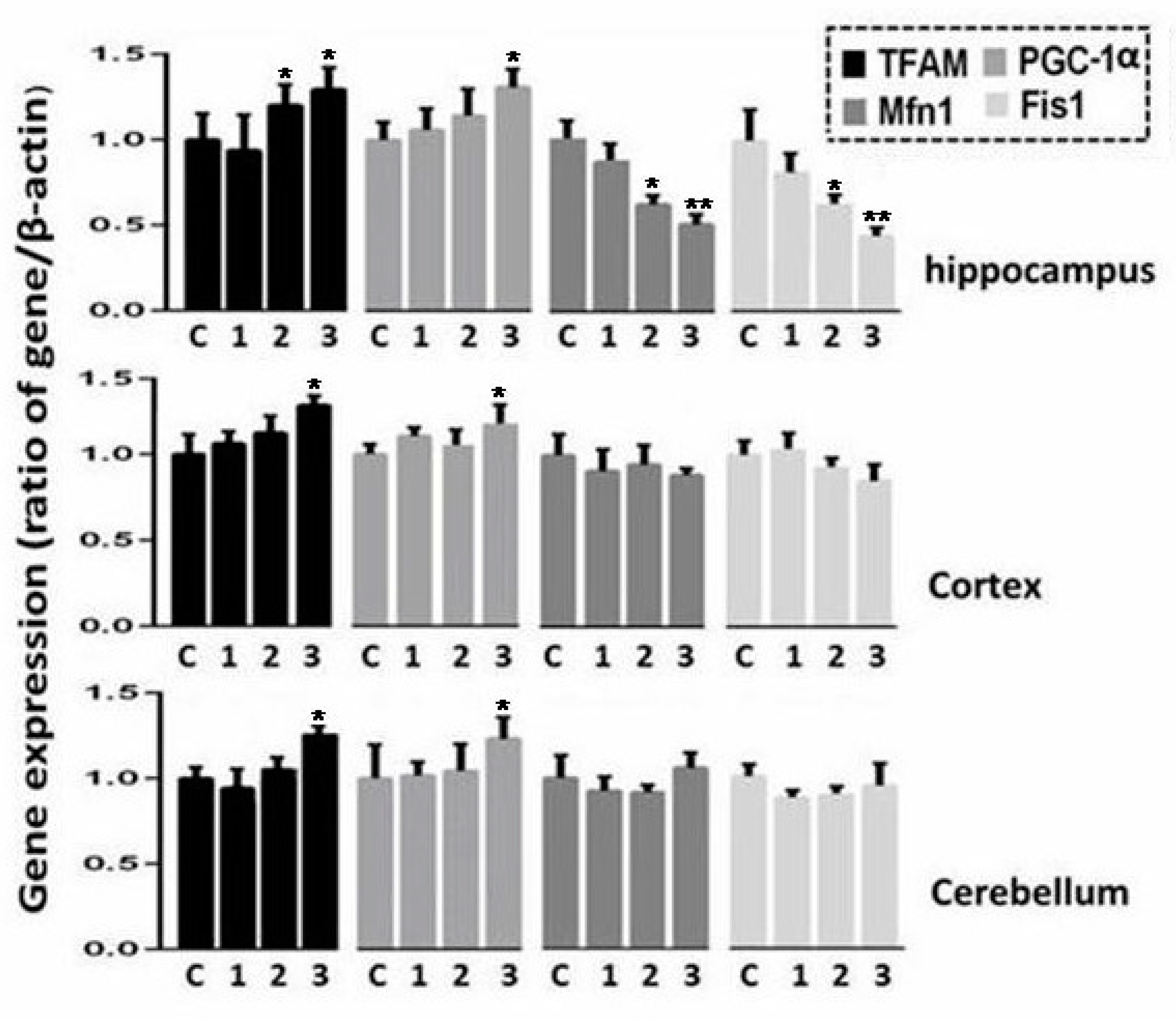
2.4. Expression Analysis of Genes Involved in Oxidative Phosphorylation, Regulation of Biogenesis, and Dynamics of Mitochondria in Three Regions of Rat Brain after Irradiation
3. Discussion
4. Materials and Methods
4.1. Animals and Their Irradiation
4.2. DNA Isolation and Purification
4.3. Analysis of Damage and Repair of Mitochondrial DNA and Nuclear DNA
4.4. Quantitative Analysis of Mitochondrial DNA Copies Relative to the Nuclear DNA
4.5. Surveyor Nuclease Assay of mtDNA Mutant Copies
4.6. RNA Isolation, Reverse Transcription and Real Time PCR
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Hladik, D.; Tapio, S. Effects of ionizing radiation on the mammalian brain. Mutat. Res. 2016, 770, 219–230. [Google Scholar] [CrossRef]
- Jacob, J.; Durand, T.; Feuvret, L.; Mazeron, J.J.; Delattre, J.Y.; Hoang-Xuan, K.; Psimaras, D.; Douzane, H.; Ribeiro, M.; Capelle, L.; et al. Cognitive impairment and morphological changes after radiation therapy in brain tumors: A review. Radiother. Oncol. 2018, 128, 221–228. [Google Scholar] [CrossRef]
- Cuccurullo, V.; Di Stasio, G.D.; Cascini, G.L.; Gatta, G.; Bianco, C. The Molecular Effects of Ionizing Radiations on Brain Cells: Radiation Necrosis vs. Tumor Recurrence. Diagnostics 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucinotta, F.A.; Cacao, E. Risks of cognitive detriments after low dose heavy ion and proton exposures. Int. J. Radiat. Biol. 2019, 95, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Hladik, D.; Dalke, C.; von Toerne, C.; Hauck, S.M.; Azimzadeh, O.; Philipp, J.; Ung, M.C.; Schlattl, H.; Rößler, U.; Graw, J.; et al. CREB Signaling Mediates Dose-dependent Radiation Response in the Murine Hippocampus Two Years after Total Body Exposure. J. Proteome Res. 2019. [Google Scholar] [CrossRef]
- Schöllnberger, H.; Eidemüller, M.; Cullings, H.M.; Simonetto, C.; Neff, F.; Kaiser, J.C. Dose-responses for mortality from cerebrovascular and heart diseases in atomic bomb survivors: 1950–2003. Radiat. Environ. Biophys. 2018, 57, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, H.; Tanaka, K.; Nakao, R.; Iso, F.; Honda, S.; Tanaka, G.; Nakane, H. Comparison of mental cognitive function of A-bomb survivors and non-A-bomb survivors in Nagasaki. Psychiatry Clin. Neurosci. 2019, 73, 594. [Google Scholar] [CrossRef]
- Azizova, T.V.; Haylock, R.G.; Moseeva, M.B.; Bannikova, M.V.; Grigoryeva, E.S. Cerebrovascular diseases incidence and mortality in an extended Mayak Worker Cohort 1948–1982. Radiat. Res. 2014, 182, 529–544. [Google Scholar] [CrossRef]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, J.; Baryawno, N.; Nayyar, N.; Valtis, Y.K.; Yang, B.; Ly, I.; Besnard, A.; Severe, N.; Gustafsson, K.U.; Andronesi, O.C.; et al. Bone marrow drives central nervous system regeneration after radiation injury. J. Clin. Invest. 2018, 128, 281–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyama, T.; Wilson, D.M., III. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.H.; Qiao, H.; Du, F.; Xiong, Q.; Liu, X.; Zhang, X.; Ugurbil, K.; Chen, W. Quantitative imaging of energy expenditure in human brain. Neuroimage 2012, 60, 2107–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, R.; Harper, M.E. Mitochondrial stress controls the radiosensitivity of the oxygen effect: Implications for radiotherapy. Oncotarget 2016, 7, 21469–21483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- DeBalsi, K.L.; Hoff, K.E.; Copeland, W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017, 33, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Wisnovsky, S.; Sack, T.; Pagliarini, D.J.; Laposa, R.R.; Kelley, S.O. DNA polymerase θ increases mutational rates in mitochondrial DNA. ACS Chem. Biol. 2018, 13, 900–908. [Google Scholar] [CrossRef]
- Schmal, Z.; Isermann, A.; Hladik, D.; von Toerne, C.; Tapio, S.; Rübe, C.E. DNA damage accumulation during fractionated low-dose radiation compromises hippocampal neurogenesis. Radiother. Oncol. 2019, 137, 45–54. [Google Scholar] [CrossRef]
- Mata-Garrido, J.; Tapia, O.; Casafont, I.; Berciano, M.T.; Cuadrado, A.; Lafarga, M. Persistent accumulation of unrepaired DNA damage in rat cortical neurons: Nuclear organization and ChIP-seq analysis of damaged DNA. Acta Neuropathol. Commun. 2018, 6, 68. [Google Scholar] [CrossRef]
- Ambrosio, S.; Palo, G.D.; Napolitano, G.; Amente, S.; Dellino, G.I.; Faretta, M.; Pelicci, P.G.; Lania, L.; Majello, B. Repair pathway choices and consequences at the double strand break. Oncotarget 2015, 7, 4949–4959. [Google Scholar]
- Zhang, L.Y.; Chen, L.S.; Sun, R.; Ji, S.J.; Ding, Y.Y.; Wu, J.; Tian, Y. Effects of expression level of DNA repair-related genes involved in the NHEJ pathway on radiation-induced cognitive impairment. J. Radiat. Res. 2013, 54, 235–242. [Google Scholar] [CrossRef]
- Kim, G.J.; Fiskum, G.M.; Morgan, W.F. A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res. 2006, 66, 10377–10383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Houten, B.; Hunter, S.E.; Meyer, J.N. Mitochondrial DNA damage induced autophagy, cell death, and disease. Front. Biosci. 2016, 21, 42–54. [Google Scholar] [CrossRef]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef] [Green Version]
- Moretton, A.; Morel, F.; Macao, B.; Lachaume, P.; Ishak, L.; Lefebvre, M.; Garreau-Balandier, I.; Vernet, P.; Falkenberg, M.; Farge, G. Selective mitochondrial DNA degradation following double strand breaks. PLoS ONE 2017, 12, e0176795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeva, V.; Blei, D.; Trombly, G.; Corsi, S.; Szukszto, M.J.; Rebelo-Guiomar, P.; Gammage, P.A.; Kudin, A.P.; Becker, C.; Altmüller, J.; et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018, 9, 1727. [Google Scholar] [CrossRef] [Green Version]
- Nissanka, N.; Bacman, S.R.; Plastini, M.J.; Moraes, C.T. The mitochondrial DNA polymerase gamma degrades linear DNA fragments precluding the formation of deletions. Nat. Commun. 2018, 9, 2491. [Google Scholar] [CrossRef]
- Dahal, S.; Dubey, S.; Raghavan, S.C. Homologous recombination-mediated repair of DNA double-strand breaks operates in mammalian mitochondria. Cell Mol. Life Sci. 2018, 75, 1641–1655. [Google Scholar] [CrossRef]
- Malakhova, L.; Bezlepkin, V.; Antipova, V.; Ushakova, T.; Fomenko, L.; Sirota, N.; Gaziev, A.I. The increase in mitochondrial DNA copy number in the tissues of γ-irradiated mice. Cell Mol. Biol. Lett. 2005, 10, 721–732. [Google Scholar]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald 3rd, E.R.; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. γH2AX and Cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.J.; Bateman, J.M. Mitochondrial retrograde signaling in the nervous system. FEBS Lett. 2018, 592, 663–678. [Google Scholar] [CrossRef] [Green Version]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Invest. 2013, 123, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Wallace, D.C.; Burelle, Y. The rise of mitochondria in medicine. Mitochondrion 2016, 30, 105–116. [Google Scholar] [CrossRef]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.G. Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Cline, S.D. Mitochondrial DNA Damage and its consequences for mitochondrial gene expression. Biochim. Biophys. Acta 2012, 1819, 979–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, L.; Chen, W.K.; Liu, S.T.; Chang, C.R.; Kao, M.C.; Chen, K.W.; Chiu, S.C.; Hsu, M.L.; Hsiang, I.C.; Chen, Y.J.; et al. Low-dose ionizing radiation induces mitochondrial fusion and increases expression of mitochondrial complexes I and III in hippocampal neurons. Oncotarget 2015, 6, 30628–30639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, E.; Nelson, D.O.; Coleman, M.A.; Peterson, L.E.; Wyrobek, A.J. Gene expression changes in mouse brain after exposure to low-dose ionizing radiation. Int. J. Radiat. Biol. 2003, 79, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud-Ahmed, A.S.; Atkinson, S.; Wong, C.S. Early gene expression profile in mouse brain after exposure to ionizing radiation. Radiat. Res. 2006, 165, 142–154. [Google Scholar] [CrossRef]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef] [Green Version]
- Villena, J.A. New insights into PGC-1 coactivators: Redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015, 282, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.M.; Jung, Y.K. A molecular approach to mitophagy and mitochondrial dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [PubMed]
- Gao, J.; Wang, L.; Liu, J.; Xie, F.; Su, B.; Wang, X. Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants 2017, 6, 25. [Google Scholar] [CrossRef]
- Tada, E.; Parent, J.M.; Lowenstein, D.H.; Fike, J.R. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 2000, 99, 33–41. [Google Scholar] [CrossRef]
- Williams, J.P.; Brown, S.L.; Georges, G.E.; Hauer-Jensen, M.; Hill, R.P.; Huser, A.K.; Kirsch, D.G.; MacVittie, T.J.; Mason, K.A.; Medhora, M.M.; et al. Animal models for medical countermeasures to radiation exposure (Meeting report). Radiat. Res. 2010, 173, 557–578. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Hunt, C.P.; Rooney, J.P.; Ryde, I.T.; Anbalagan, C.; Joglekar, R.; Meyer, J.N. PCR-based analysis of mitochondrial DNA copy number, mitochondrial DNA damage, and nuclear DNA damage. Curr. Protoc. Toxicol. 2016, 67, 1–34. [Google Scholar] [CrossRef]
- Furda, A.; Santos, J.H.; Meyer, J.N.; Van Houten, B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol. 2014, 1105, 419–437. [Google Scholar]
- Sanders, L.H.; Rouanet, J.P.; Howlett, E.H.; Leuthner, T.C.; Rooney, J.P.; Greenamyre, J.T.; Meyer, J.N. Newly revised protocol for quantitative PCR-based assay to measure mitochondrial and nuclear DNA damage. Curr. Protoc. Toxicol. 2018, 76, e50. [Google Scholar] [CrossRef]
- Rooney, J.P.; Ryde, I.T.; Sanders, L.H.; Howlett, E.H.; Colton, M.D.; Germ, K.E.; Mayer, G.D.; Greenamyre, J.T.; Meyer, J.N. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol. Biol. 2015, 1241, 23–38. [Google Scholar] [PubMed] [Green Version]
- Bannwarth, S.; Procaccio, V.; Paquis-Flucklinger, V. Rapid identification of unknown heteroplasmic mitochondrial DNA mutations with mismatch-specific surveyor nuclease. Methods Mol. Biol. 2009, 554, 301–313. [Google Scholar] [PubMed]




| Locus | Primers, Probes | 5′→3′ sequence | Size, bp |
|---|---|---|---|
| Primers for quantitative analysis of mtDNA/nDNA | |||
| mt-tRNA | for | AATGGTTCGTTTGTTCAACGATT | |
| rev | AGAAACCGACCTGGATTGCTC | ||
| probe | R6G-AAGTCCTACGTGATCTGAGTT-RHQ1 | 73 | |
| GAPDH | for | TGGCCTCCAAGGAGTAAGAAAC | |
| rev | GGCTCTCTCCTTGCTCTCAGTATC | ||
| probe | FAM-CTGGACCACCCAGCCCAGCAA-RTQ1 | 80 | |
| Primers for LA-QPCR | |||
| mtDNA | for | AAAATCCCCGCAAACAATGACCACCC | |
| rev | GGCAATTAAGAGTGGGATGGAGCCAA | 13.4 kb | |
| nDNA | for | AGACGGGTGAGACAGCTGCACCTTTTC | |
| rev | CGAGAGCATCAAGTGCAGGCATTAGAG | 12.5 kb | |
| mtDNA | for | CCTCCCATTCATTATCGCCGCCCTTGC | |
| rev | GTCTGGGTCTCCTAGTAGGTCTGGGAA | 235 | |
| nDNA | for | GGTGTACTTGAGCAGAGCGCTATAAAT | |
| rev | CACTTACCCACGGCAGCTCTCTAC | 195 | |
| Primers for mtDNA mutant copies | |||
| mt-tRNA | for | CACACTCTCACTCGCATGAA | |
| rev | TCCTTCCAATCTAGTTGAGG | 507 | |
| Primers for gene transcripts (RT-PCR) | |||
| ND2 | for | ATGGCCTTCCTCACCCTAGT | |
| rev | GTTAGGGGGCGTATGGGTTC | 146 | |
| CytB | for | CACGCTTCTTCGCATTCCAC | |
| rev | GGGATTTTGTCTGCGTCGGA | 130 | |
| ATP5O | for | GCTGAAAATGGTCGCCTAGG | |
| rev | AGGAAACGCTGTGGTCAC | 110 | |
| Mfn1 | for | CGCCTGTCTGTTTTGGTTGA | |
| rev | GCATTGACTTCACTGGTGCA | 146 | |
| Fis1 | for | AAAGAGGAGCAGCGGGATTA | |
| rev | TGGGGCTCAGTCTGTAACAG | 110 | |
| PGC-1α | for | GCACCAGAAAACAGCTCCAA | |
| rev | TTGCCATCCCGTAGTTCACT | 121 | |
| TFAM | for | ATCAAGACTGTGCGTGCATC | |
| rev | AGAACTTCACAAACCCGCAC | 115 | |
| β-Actin | for | TCTTCCAGCCTTCCTTCCTG | |
| rev | CAATGCCTGGGTACATGGTG | 147 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullaev, S.; Gubina, N.; Bulanova, T.; Gaziev, A. Assessment of Nuclear and Mitochondrial DNA, Expression of Mitochondria-Related Genes in Different Brain Regions in Rats after Whole-Body X-ray Irradiation. Int. J. Mol. Sci. 2020, 21, 1196. https://doi.org/10.3390/ijms21041196
Abdullaev S, Gubina N, Bulanova T, Gaziev A. Assessment of Nuclear and Mitochondrial DNA, Expression of Mitochondria-Related Genes in Different Brain Regions in Rats after Whole-Body X-ray Irradiation. International Journal of Molecular Sciences. 2020; 21(4):1196. https://doi.org/10.3390/ijms21041196
Chicago/Turabian StyleAbdullaev, Serazhutdin, Nina Gubina, Tatiana Bulanova, and Azhub Gaziev. 2020. "Assessment of Nuclear and Mitochondrial DNA, Expression of Mitochondria-Related Genes in Different Brain Regions in Rats after Whole-Body X-ray Irradiation" International Journal of Molecular Sciences 21, no. 4: 1196. https://doi.org/10.3390/ijms21041196
APA StyleAbdullaev, S., Gubina, N., Bulanova, T., & Gaziev, A. (2020). Assessment of Nuclear and Mitochondrial DNA, Expression of Mitochondria-Related Genes in Different Brain Regions in Rats after Whole-Body X-ray Irradiation. International Journal of Molecular Sciences, 21(4), 1196. https://doi.org/10.3390/ijms21041196







