Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. Study Selection and Characteristics
2.2. Increased Abundance and Altered Morphology of OSCC-Derived EVs
2.3. Differential Expression of OSCC-Derived EV Surface and Cargo Proteins
2.4. Directed microRNA Cargo of OSCC-Derived EVs
2.5. Selective microRNA Cargo Loading into EVs in OC and Oral Dysplasia
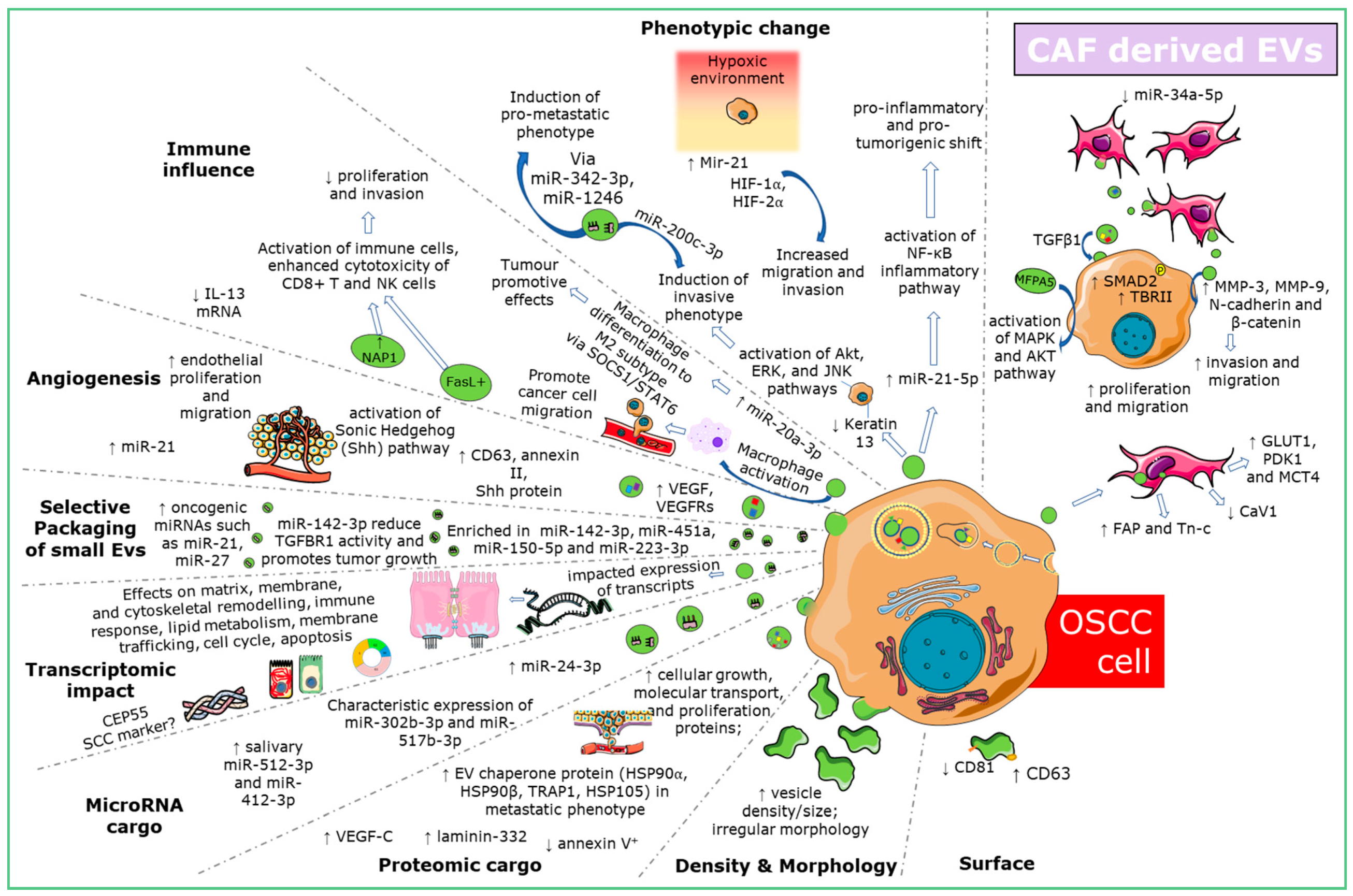
2.6. OSCC-Derived EVs Influence Phenotypic Change and Confer Tumorigenicity
2.7. EV Mediated Fibroblast Interaction in OSCC
2.8. OSCC-Derived EVs Stimulate Changes in Endothelial Cells Consistent with Angiogenesis and Lymphangiogenesis
2.9. Immune Influence of OSCC Derived EVs
2.10. Modulating OSCC-EV Production and Packaging
2.11. Therapeutic Influence on OSCC-EVs
2.12. OSCC-EV Modulated Influence on Therapeutic Response
2.13. EVs for Therapeutic Delivery in OSCC
2.14. EVs and OPMDs
2.14.1. Oral Leukoplakia and Dysplasia
2.14.2. Oral Lichen Planus
3. Discussion
4. Materials and Methods
4.1. Selection Criteria
4.2. Study Selection
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seoane-Romero, J.M.; Vazquez-Mahia, I.; Seoane, J.; Varela-Centelles, P.; Tomas, I.; Lopez-Cedrun, J.L. Factors related to late stage diagnosis of oral squamous cell carcinoma. Med. Oralpatologia Oral Y Cir. Bucal 2012, 17, e35–e40. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Pigati, L.; Yaddanapudi, S.C.; Iyengar, R.; Kim, D.J.; Hearn, S.A.; Danforth, D.; Hastings, M.L.; Duelli, D.M. Selective release of microrna species from normal and malignant mammary epithelial cells. PLoS ONE 2010, 5, e13515. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113 Pt 19, 3365–3374. [Google Scholar]
- Stein, J.M.; Luzio, J.P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem. J. 1991, 274 Pt 2, 381–386. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Prada, I.; Meldolesi, J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Wieckowski, E.; Taylor, D.D.; Reichert, T.E.; Watkins, S.; Whiteside, T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated t lymphocytes. Clin. Cancer Res. 2005, 11, 1010–1020. [Google Scholar] [PubMed]
- Sharma, S.; Gillespie, B.M.; Palanisamy, V.; Gimzewski, J.K. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir 2011, 27, 14394–14400. [Google Scholar] [CrossRef]
- Winck, F.V.; Prado Ribeiro, A.C.; Ramos Domingues, R.; Ling, L.Y.; Riano-Pachon, D.M.; Rivera, C.; Brandao, T.B.; Gouvea, A.F.; Santos-Silva, A.R.; Coletta, R.D.; et al. Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci. Rep. 2015, 5, 16305. [Google Scholar] [CrossRef] [PubMed]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Morphological and molecular features of oral fluid-derived exosomes: Oral cancer patients versus healthy individuals. J. Cancer Res. Clin. Oncol. 2016, 142, 101–110. [Google Scholar] [CrossRef]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated mirnas as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18(1), 439. [Google Scholar] [CrossRef] [PubMed]
- 17/ Zlotogorski-Hurvitz, A.; Dekel, B.Z.; Malonek, D.; Yahalom, R.; Vered, M. FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 685–694. [Google Scholar] [CrossRef]
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharm. 2020, 121. [Google Scholar] [CrossRef]
- Zhong, W.Q.; Ren, J.G.; Xiong, X.P.; Man, Q.W.; Zhang, W.; Gao, L.; Li, C.; Liu, B.; Sun, Z.J.; Jia, J.; et al. Increased salivary microvesicles are associated with the prognosis of patients with oral squamous cell carcinoma. J. Cell. Mol. Med. 2019, 23, 4054–4062. [Google Scholar] [CrossRef]
- Rabinowits, G.; Bowden, M.; Flores, L.M.; Verselis, S.; Vergara, V.; Jo, V.Y.; Chau, N.; Lorch, J.; Hammerman, P.S.; Thomas, T.; et al. Comparative analysis of microrna expression among benign and malignant tongue tissue and plasma of patients with tongue cancer. Front. Oncol. 2017, 7, 191. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S. Extracellular vesicles in oral squamous carcinoma carry oncogenic miRNA profile and reprogramme monocytes via NF-kappaB pathway. Oncotarget 2018, 9, 34838–34854. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, S.; Pérez-Sayans, M.; Fais, S.; Logozzi, M.; Torreira, M.G.; Garcia, A.G. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Xu, Z.; Wang, M.; Yan, T.; Huang, C.; Zhou, X.; Liu, Q.; Wang, L.; Chen, Y.; Wang, H.; et al. Tumoral microvesicle-activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. FASEB J. 2019, 33, 5690–5703. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Hong, S.H.; Choi, J.K.; Jung, J.K.; Lee, H.J. Diagnostic profiling of salivary exosomal micrornas in oral lichen planus patients. Oral Dis. 2015, 21, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, J.; Zhou, G. Differentially circulating exosomal microRNAs expression profiling in oral lichen planus. Am. J. Transl. Res. 2018, 10, 2848–2858. [Google Scholar] [PubMed]
- Sento, S.; Sasabe, E.; Yamamoto, T. Application of a persistent heparin treatment inhibits the malignant potential of oral squamous carcinoma cells induced by tumor cell-derived exosomes. PLoS ONE 2016, 11, e0148454. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hayashi, Y.; Nishinakagawa, T.; Hazekawa, M.; Kawano, S.; Nakamura, S.; Nakashima, M. The squu-b cell line spreads its metastatic properties to nonmetastatic clone squu-a from the same patient through exosomes. J. Oral Biosci. 2016, 58, 33–38. [Google Scholar] [CrossRef]
- Sakha, S.; Muramatsu, T.; Ueda, K.; Inazawa, J. Exosomal microrna mir-1246 induces cell motility and invasion through the regulation of dennd2d in oral squamous cell carcinoma. Sci. Rep. 2016, 6, 38750. [Google Scholar] [CrossRef]
- Liu, C.; Gao, H.; Lv, P.; Liu, J.; Liu, G. Extracellular vesicles as an efficient nanoplatform for the delivery of therapeutics. Hum. Vaccines Immunother. 2017, 13, 2678–2687. [Google Scholar] [CrossRef]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.I.; Calderwood, S.K.; Okamoto, K.; Kozaki, K.I. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-egfr antibody cetuximab. Oral Oncol. 2018, 86, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Qadir, F.; Aziz, M.A.; Sari, C.P.; Ma, H.; Dai, H.; Wang, X.; Raithatha, D.; Da Silva, L.G.L.; Hussain, M.; Poorkasreiy, S.P.; et al. Transcriptome reprogramming by cancer exosomes: Identification of novel molecular targets in matrix and immune modulation. Mol. Cancer 2018, 17(1), 97. [Google Scholar] [CrossRef]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Hu, Q.; Wang, H.; Song, Y.; Ni, Y.; Hou, Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis 2018, 39, 397–406. [Google Scholar] [CrossRef]
- Languino, L.R.; Singh, A.; Prisco, M.; Inman, G.J.; Luginbuhl, A.; Curry, J.M.; South, A.P. Exosome-mediated transfer from the tumor microenvironment increases tgfbeta signaling in squamous cell carcinoma. Am. J. Transl. Res. 2016, 8, 2432–2437. [Google Scholar] [PubMed]
- Principe, S.; Mejia-Guerrero, S.; Ignatchenko, V.; Sinha, A.; Ignatchenko, A.; Shi, W.; Pereira, K.; Su, S.S.; Huang, S.H.; O’Sullivan, B.; et al. Proteomic analysis of cancer-associated fibroblasts reveals a paracrine role for mfap5 in human oral tongue squamous cell carcinoma. J. Proteome Res. 2018, 17, 2045–2059. [Google Scholar] [CrossRef]
- Li, Y.Y.; Tao, Y.W.; Gao, S.; Li, P.; Zheng, J.M.; Zhang, S.E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine mir-34a-5p. Ebiomedicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.R.; Korvala, J.; Astrom, P.; De Oliveira, C.E.; Cervigne, N.K.; Mofatto, L.S.; Campanella Bastos, D.; Pereira Messetti, A.C.; Graner, E.; Paes Leme, A.F.; et al. Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. J. Extracell. Vesicles 2019, 8, 1578525. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Xu, K.; Cui, J.; Yuan, D.Y.; Zou, B.; Li, J.; Liu, J.L.; Li, K.Y.; Meng, Z.; Zhang, B. Cancer-associated fibroblast-derived exosomal miR-382-5p promotes the migration and invasion of oral squamous cell carcinoma. Oncol. Rep. 2019, 42, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Sjoqvist, S.; Kasai, Y.; Shimura, D.; Ishikawa, T.; Ali, N.; Iwata, T.; Kanai, N. Oral keratinocyte-derived exosomes regulate proliferation of fibroblasts and epithelial cells. Biochem. Biophys. Res. Commun. 2019, 514, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Morioka, M.; Kawakubo-Yasukochi, T.; Hayashi, Y.; Hazekawa, M.; Nishinakagawa, T.; Ono, K.; Kawano, S.; Nakamura, S.; Nakashima, M. Exosomes from oral squamous carcinoma cell lines, squu-a and squu-b, define the tropism of lymphatic dissemination. J. Oral Biosci. 2016, 58, 180–184. [Google Scholar] [CrossRef]
- Xiao, H.T.; Feng, Y.Y.; Tao, Y.Q.; Zhao, P.; Shang, W.; Song, K. Microvesicles releasing by oral cancer cells enhance endothelial cell angiogenesis via shh/rhoa signaling pathway. Cancer Biol. Ther. 2017, 18, 783–791. [Google Scholar]
- De Andrade, A.; de Oliveira, C.E.; Dourado, M.R.; Macedo, C.; Winck, F.V.; Paes Leme, A.F.; Salo, T.; Coletta, R.D.; de Almeida Freitas, R.; Galvao, H.C. Extracellular vesicles from oral squamous carcinoma cells display pro- and anti-angiogenic properties. Oral Dis. 2018, 24, 725–731. [Google Scholar] [CrossRef]
- Ludwig, N.; Yerneni, S.S.; Razzo, B.M.; Whiteside, T.L. Exosomes from hnscc promote angiogenesis through reprogramming of endothelial cells. Mol. Cancer Res. 2018, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Liou, G.G.; Liu, S.H.; Chang, J.S.; Hsiao, J.R.; Yen, Y.C.; Chen, Y.L.; Wu, W.L.; Chang, J.Y.; Chen, Y.W. Laminin gamma2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin alpha3-dependent uptake by lymphatic endothelial cells. Int. J. Cancer 2019, 144, 2795–2810. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, X.; Zhu, X.; Zhang, J.; Chen, W. Oral cancer-derived exosomal NAP1 enhances cytotoxicity of natural killer cells via the IRF-3 pathway. Oral Oncol. 2018, 76, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, J.; Chen, W.; Chen, W. M1-like tumor-associated macrophages activated by exosome-transferred thbs1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 143. [Google Scholar] [CrossRef]
- Li, L.; Cao, B.; Liang, X.; Lu, S.; Luo, H.; Wang, Z.; Wang, S.; Jiang, J.; Lang, J.; Zhu, G. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral gammadelta T cell equilibrium via tumor-derived exosomes. Oncogene 2019, 38, 2830–2843. [Google Scholar] [CrossRef]
- Cai, J.; Qiao, B.; Gao, N.; Lin, N.; He, W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol.-Cell Physiol. 2019, 316, C731–C740. [Google Scholar] [CrossRef]
- Li, W.; Hang, Y.; Zhao, Z.; Ji, X.; Wang, X.; Jin, J.; Wang, Q.; Guo, X.; Cheng, Z.; Lu, M.; et al. Oral mucosal mesenchymal stem cell-derived exosomes: A potential therapeutic target in oral premalignant lesions. Int. J. Oncol. 2019, 54, 1567–1578. [Google Scholar] [CrossRef]
- Xie, C.; Du, L.Y.; Guo, F.; Li, X.; Cheng, B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell. Biochem. 2019, 458, 11–26. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, J.; Zhou, G. Circulating exosomes regulate T-cell-mediated inflammatory response in oral lichen planus. J. Oral Pathol. Med. 2019, 48, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-Y.; Chen, C. Toward characterizing extracellular vesicles at a single-particle level. J. Biomed. Sci. 2019, 26, 9. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K.I. Hsp-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018, 119, 7350–7362. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Liu, J.; Su, X.; Qin, H.; Huang, S.; Huang, X.; Zhou, N. Potential markers from serum-purified exosomes for detecting oral squamous cell carcinoma metastasis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1668–1681. [Google Scholar] [CrossRef]
- Miyazaki, K. Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006, 97, 91–98. [Google Scholar] [CrossRef]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.X.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microrna-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef]
- Dickman, C.T.; Lawson, J.; Jabalee, J.; MacLellan, S.A.; LePard, N.E.; Bennewith, K.L.; Garnis, C. Selective extracellular vesicle exclusion of mir-142-3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget 2017, 8, 15252–15266. [Google Scholar] [CrossRef]
- Morifuji, M.; Taniguchi, S.; Sakai, H.; Nakabeppu, Y.; Ohishi, M. Differential expression of cytokeratin after orthotopic implantation of newly established human tongue cancer cell lines of defined metastatic ability. Am. J. Pathol. 2000, 156, 1317–1326. [Google Scholar] [CrossRef][Green Version]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef]
- Franzen, C.A.; Simms, P.E.; Van Huis, A.F.; Foreman, K.E.; Kuo, P.C.; Gupta, G.N. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res. Int. 2014, 2014, 619829. [Google Scholar] [CrossRef]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hazekawa, M.; Yasukochi, A.; Nishinakagawa, T.; Ono, K.; Kawano, S.; Nakamura, S.; Nakashima, M. Mir-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment. Mol. Carcinog. 2018, 57, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Razzo, B.M.; Ludwig, N.; Hong, C.S.; Sharma, P.; Fabian, K.P.; Fecek, R.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma. Carcinogenesis 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Bhome, R.; Goh, R.W.; Bullock, M.D.; Pillar, N.; Thirdborough, S.M.; Mellone, M.; Mirnezami, R.; Galea, D.; Veselkov, K.; Gu, Q.; et al. Exosomal micrornas derived from colorectal cancer-associated fibroblasts: Role in driving cancer progression. Aging 2017, 9, 2666–2694. [Google Scholar] [CrossRef] [PubMed]
- Bockmuhl, U.; Schluns, K.; Kuchler, I.; Petersen, S.; Petersen, I. Genetic imbalances with impact on survival in head and neck cancer patients. Am. J. Pathol. 2000, 157, 369–375. [Google Scholar] [CrossRef]
- Kanojia, D.; Vaidya, M.M. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Reichert, T.E.; Strauss, L.; Wagner, E.M.; Gooding, W.; Whiteside, T.L. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin. Cancer Res. 2002, 8, 3137–3145. [Google Scholar]
- Gastman, B.R.; Atarshi, Y.; Reichert, T.E.; Saito, T.; Balkir, L.; Rabinowich, H.; Whiteside, T.L. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of t lymphocytes. Cancer Res. 1999, 59, 5356–5364. [Google Scholar]
- Al-Samadi, A.; Awad, S.A.; Tuomainen, K.; Zhao, Y.; Salem, A.; Parikka, M.; Salo, T. Crosstalk between tongue carcinoma cells, extracellular vesicles, and immune cells in in vitro and in vivo models. Oncotarget 2017, 8, 60123–60134. [Google Scholar] [CrossRef][Green Version]
- Wang, J.P.; Tang, Y.Y.; Fan, C.M.; Guo, C.; Zhou, Y.H.; Li, Z.; Li, X.L.; Li, Y.; Li, G.Y.; Xiong, W.; et al. The role of exosomal non-coding rnas in cancer metastasis. Oncotarget 2018, 9, 12487–12502. [Google Scholar] [CrossRef]
- Mori, K.; Hiroi, M.; Shimada, J.; Ohmori, Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers (Basel) 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Raulf, N.; Lucarelli, P.; Thavaraj, S.; Brown, S.; Vicencio, J.M.; Sauter, T.; Tavassoli, M. Annexin a1 regulates egfr activity and alters egfr-containing tumour-derived exosomes in head and neck cancers. Eur. J. Cancer 2018, 102, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Zhong, L.P.; Zhou, X.J.; Pan, H.Y.; Wei, K.J.; Li, J.; Chen, W.T.; Zhang, Z.Y. Decreased expression of Annexin A1 correlates with pathologic differentiation grade in oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.P.; Wei, K.J.; Yang, X.; Zhang, L.; Zhou, X.J.; Pan, H.Y.; Li, J.; Chen, W.T.; Zhang, Z.Y. Increased expression of annexin a2 in oral squamous cell carcinoma. Arch. Oral. Biol. 2009, 54, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, H.L.; Deng, M.J.; Wen, X.J.; Mo, Y.Y.; Chen, F.M.; Zou, C.L.; Duan, W.F.; Li, L.; Nie, X. Melatonin inhibits reactive oxygen species-driven proliferation, epithelial-mesenchymal transition, and vasculogenic mimicry in oral cancer. Oxidative Med. Cell. Longev. 2018, 2018, 3510970. [Google Scholar] [CrossRef]
- Hunsaker, M.; Barba, G.; Kingsley, K.; Howard, K.M. Differential MicroRNA Expression of miR-21 and miR-155 within Oral Cancer Extracellular Vesicles in Response to Melatonin. Dent. J. (Basel) 2019, 7, 48. [Google Scholar] [CrossRef]
- Baba, O.; Hasegawa, S.; Nagai, H.; Uchida, F.; Yamatoji, M.; Kanno, N.I.; Yamagata, K.; Sakai, S.; Yanagawa, T.; Bukawa, H. MicroRNA-155-5p is associated with oral squamous cell carcinoma metastasis and poor prognosis. J. Oral. Pathol. Med. 2016, 45, 248–255. [Google Scholar] [CrossRef]
- He, B.; Lin, X.; Tian, F.; Yu, W.; Qiao, B. Mir-133a-3p inhibits oral squamous cell carcinoma (oscc) proliferation and invasion by suppressing col1a1. J Cell Biochem. 2018, 119, 338–346. [Google Scholar] [CrossRef]
- Liu, T.; Chen, G.; Sun, D.; Lei, M.; Li, Y.; Zhou, C.; Li, X.; Xue, W.; Wang, H.; Liu, C.; et al. Exosomes containing mir-21 transfer the characteristic of cisplatin resistance by targeting pten and pdcd4 in oral squamous cell carcinoma. Acta Biochim. Et Biophys. Sin. 2017, 49, 808–816. [Google Scholar] [CrossRef]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.I.; Okamoto, K.; Calderwood, S.K.; Kozaki, K.I. Anti-egfr antibody cetuximab is secreted by oral squamous cell carcinoma and alters egf-driven mesenchymal transition. Biochem. Biophys. Res. Commun. 2018, 503, 1267–1272. [Google Scholar] [CrossRef]
- Khoo, X.H.; Paterson, I.C.; Goh, B.H.; Lee, W.L. Cisplatin-resistance in oral squamous cell carcinoma: Regulation by tumor cell-derived extracellular vesicles. Cancers 2019, 11, 1166. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zhang, W.; Zhao, J.Y.; Zhong, W.Q.; Ren, J.G.; Wu, M.; Zhang, Z.L.; Pang, D.W.; Zhao, Y.F.; Chen, G. Development of a dual-modally traceable nanoplatform for cancer theranostics using natural circulating cell-derived microparticles in oral cancer patients. Adv. Funct. Mater. 2017, 27, 15. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Hoornstra, D.; Vesterlin, J.; Parnanen, P.; Al-Samadi, A.; Zlotogorski-Hurvitz, A.; Vered, M.; Salo, T. Fermented lingonberry juice inhibits oral tongue squamous cell carcinoma invasion in vitro similarly to curcumin. In Vivo 2018, 32, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.B.; Prendes, M.J.; Sternberg, H.; Kidd, J.L.; Funk, W.D.; Wagner, J.; West, M.D. Col10a1 expression is elevated in diverse solid tumor types and is associated with tumor vasculature. Future Oncol. (Lond. Engl.) 2012, 8, 1031–1040. [Google Scholar] [CrossRef]
- Wei, J.; Nduom, E.K.; Kong, L.-Y.; Hashimoto, Y.; Xu, S.; Gabrusiewicz, K.; Ling, X.; Huang, N.; Qiao, W.; Zhou, S.; et al. Mir-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro. Oncol. 2015, 18, 639–648. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liang, X.; Cao, B.; Wang, S.; Jiang, J.; Luo, H.; He, S.; Lang, J.; Zhu, G. Gammadeltatdes: An Efficient Delivery System for miR-138 with Anti-tumoral and Immunostimulatory Roles on Oral Squamous Cell Carcinoma. Mol. Ther. Nucleic. Acids 2019, 14, 101–113. [Google Scholar] [CrossRef]
- Luo, Y.; He, J.; Yang, C.; Orange, M.; Ren, X.; Blair, N.; Tan, T.; Yang, J.M.; Zhu, H. Uch-l1 promotes invasion of breast cancer cells through activating akt signaling pathway. J. Cell Biochem. 2018, 119, 691–700. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.M.; Lim, S.; Nam, Y.K.; Jeong, J.; Kim, H.J.; Lee, K.J. Ubiquitin c-terminal hydrolase-l1 is a key regulator of tumor cell invasion and metastasis. Oncogene 2009, 28, 117–127. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hwang, D.; Bheda-Malge, A.; Whitehurst, C.B.; Kabanov, A.V.; Kondo, S.; Aga, M.; Yoshizaki, T.; Pagano, J.S.; Sokolsky, M.; et al. Inhibition of UCH-L1 deubiquitinating activity with two forms of LDN-57444 has anti-invasive effects in metastatic carcinoma cells. Int. J. Mol. Sci. 2019, 20, 3733. [Google Scholar] [CrossRef]
- Vagner, T.; Spinelli, C.; Minciacchi, V.R.; Balaj, L.; Zandian, M.; Conley, A.; Zijlstra, A.; Freeman, M.R.; Demichelis, F.; De, S.; et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles 2018, 7, 1505403. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]

| Study | Specimen Type | Markers | Assay | Findings |
|---|---|---|---|---|
| [13] | Salivary EVs | quantification morphology CD63 surface density | AFM, SMFS-CD63 mapping, Western blot analysis |
|
| [14] | Salivary EVs | shotgun protein analysis | MS |
|
| [15] | Salivary exosomes | quantification CD81, CD9, CD63 | NTA, AFM, TEM, ELISA, Western blotting |
|
| [16] | Salivary EVs | microRNA | qPCR array; qPCR |
|
| [17] | Salivary exosomes | spectroscopy intensity ratios | Fourier-transform IR spectroscopy |
|
| [18] | Salivary exosomes | microRNA | microarray; qPCR |
|
| [19] | Salivary MVs and circulating MVs | Quantification; Annexin V | TEM; dynamic light scattering; CFSE labelling; flow cytometry |
|
| [20] | Plasma EVs | microRNA | microarray |
|
| [21] | Plasma EVs | Quantification; microRNA | NTA; qPCR |
|
| [22] | Plasma EVs | CD63, Cav-1 | immunocapture |
|
| [23] | Serum exosomes | protein | LC-MS; MS; qPCR |
|
| Oral Lichen Planus | ||||
| Sample Type | Markers | Assay | Findings | |
| [24] | Salivary exosomes | microRNA | microarray; qPCR |
|
| [25] | Plasma exosomes | microRNA | microarray |
|
| OSCC | |||
|---|---|---|---|
| Study | Cell Type | Main Findings | |
| EVs Derived from | EVs Studied on | ||
| OSCC and Keratinocytes | |||
| [26] | OSCC lines (OSC-3, OSC-4) | OSCC lines (OSC-3, OSC-4) | OSCC cell-derived exosomes promote source cell line proliferation, migration, and invasion in a dose dependent manner |
| [27] | OSCC lines (Cal-27, SCC9) | OSCC lines (Cal-27, SCC9) | OSCC—EVs produced under hypoxic conditions increased the migration and invasion of normoxic OSCC cells in a hypoxia-inducible factors—HIF1α and HIF-2α–dependent manner which was abrogated by miR-21 depletion |
| [28] | OSCC line (SQUU-B (metastatic)) | OSCC line (SQUU-A (non-metastatic) | SQUU-B- exosomes s conferred metastatic ability to non-metastatic SQUU-A cells and reduced mRNA expression of cytokeratin 13 |
| [29] | OSCC line (HOC313-LM (highly metastatic sub line)) | OSCC line (HOC313-P (parent cell line)) | HOC313-LM exosomes transferred oncogenic miR-343-3p and miR-1246 to HOC313-P cells and resulted in increase in cell motility and invasive ability |
| [30] | Cisplatin resistant OSCC cell lines (HSC3, SCC9) | Parental OSCC (HSC3 and SCC9) | EVs released from cisplatin-resistant OSCC cells transmit miR-21 to induce cisplatin resistance of OSCC cells |
| [31] | OSCC line (HSC3) | Oral keratinocytes (RT7) | OSCC derived EGFR-containing EVs were able to transform RT7 cells, effects of which were largely blocked by cetumixab |
| [32] | OSCC lines (Ca1 CALH2, SQCC/Y1) Premalignant buccal oral keratinocyte (SVpgC2a) Transformed malignant (SVFN9) normal oral keratinocyte lines (OH113, NK4, NOK368) | Primary normal oral keratinocytes | Exposure to OSCC-derived exosomes specifically modulated mRNA transcripts associated with matrix remodeling, cell cycle, differentiation, apoptosis, transcription and translation |
| OSCC & Fibroblasts | |||
| [33] | OSCC line (HSC3) | NOF | HSC3-exosomes lead to uptake by NOFs with resulting upregulation of expression of non-coding RNA Lnc-CAF |
| [23] | OSCC line (Cal-27) | HGFs (human gingival fibroblasts) | Cal-27 MVs were internalized increased levels of CAF markers (FAP and Tn-c) were isolated from HCFs, Cal-27 MV treated HGFs also showed increased glucose and lactate production with an increased expression of GLUT1, PDK1 and MCT4 but a decrease in CaV1 |
| [34] | Primary OSCC CAFs | Primary OSCC keratinocytes | Exosome transfer from TGFβ signalling-competent fibroblasts increased transforming growth factor-beta receptor II (TBRII) levels and the TBRII signal transducing protein SMAD2 (but not SMAD3) phosphorylation in OSCC keratinocytes |
| [35] | Primary OSCC CAFs and adjacent tissue fibroblasts (AF) | OSCC lines (SCC25, SCC4) | CAF derived EVs increased C25(OTSCC) cell proliferation and migration compared with exosome depleted media or controls |
| [36] | Primary OSCC CAFs and matched NOFs | OSCC lines (Cal-27, SCC15) | CAF derived exosomes containing low or lentiviral plasmid restored expression of miR-34a-5p are transferred to OSCC cells |
| [37] | Primary CAFs and NOFs, | OSCC lines (HSC3, SAS, SCC15, SCC25) | CAF-EVS significantly increased the invasive, migration and apoptosis rate of HSC-3, SAS and SCC-15 but not SCC-25 with HSC-3 the most was most responsive to pooled CAF-EVs with deeper invasion, small tumour islands |
| [38] | Primary cancer associated fibroblasts; normal fibroblasts | OSCC line (Cal-27) | Significantly increased Cal-27 migration and invasion |
| [39] | Human dermal fibroblasts; normal keratinocytes | OSCC line (TR146) | Normal fibroblast and keratinocyte derived EVs suppressed OSCC proliferation but only at particular doses |
| OSCC & Endothelial Cells | |||
| [40] | OSCC cell lines (SQUU-A (non-metastatic), SQUU-B (metastatic)) | endothelial cells (HUVECs, HDLECs) | Both OSCC-derived exosomes increased VEGFR2 expression in HUVECS; SQUU-B exosomes increased tube formation in HDLECs, both OSCC cell line derived exosomes stimulate expression of HDLEC mRNA expression of VEGFRs1-3 but only SQUU-B exosomes increased expression of VEFG-A,-C and -D |
| [41] | OSCC lines (Cal-27) | Endothelial cells (HUVECs) | Cal-27-MVs carrying Sonic hedgehog (Shh) protein significantly induce tube formation in HUVECS |
| [42] | OSCC lines (SCC15 AND HSC3) | Endothelial cells (HUVECs) | SSC15-EVs showed significant HUVEC tube formation, migration and increased apoptotic bodies vs. HSC3—EVs which significantly inhibited tube formation and proliferation; EVs derived from different OSCC cell lines are either pro-or anti angiogenic |
| [43] | OSCC lines (PCI-13, UMSCC47) | Endothelial cells (HUVECs) | PCI-13– exosomes caused significant increase in VEGF mRNA levels and IGFBP-3 mRNA expression levels in the recipient cells; no significant changes after co-incubation of HUVECs with UMSCC47-derived exosomes |
| [44] | Metastatic OSCC subline (LN1-1) and parent line (OEC-M1) | Human dermal lymphatic endothelial cells (LECs) | LN1-1 derived EVs significantly increased migration and tube formation compared to incubation with parent cell |
| OSCC & Immune Cells | |||
| [12] | OSCC patient sera; T cells (Jurkat) and OSCC line (PCI-13) | T-blast cells, T cells (Jurkat) | OSCC serum MV fractions were FasL positive and induced DNA fragmentation, decreased the MMP potential or induced apoptosis of Jurkat cells, T blast cells or activated T lymphocytes |
| [21] | OSCC line (Cal-27) derived EVs | THP1 monocytes | Increase in miR-21-5p and activation of NF- κB suggesting pro-inflammatory, pro-tumorigenic shift |
| [45] | OSCC cell lines (SCC-25, Cal27) | NK cells | OSCC exosomes enhanced cytotoxicity of NK cells via the interferon regulatory factor 3 (IRF-3) pathway by delivery of that NF-κB-activating kinase-associated protein 1 (NAP1) |
| [46] | immortalized keratinocytes (HIOEC) leukoplakia cell line (Leuk1) OSCC cell lines (SCC25, Cal27) | Macrophages (THP-1 derived); healthy donor PBMCs | OSCC—exosomes but not HIOEC- or Leuk1- exosomes THP-1 and PBMCs derived macrophages into a M1 phenotype associated with tumor suppression |
| [47] | OSCC lines (Cal-27; SCC-29) | Primary γδ T cells | OSCC derived exosomes produced under normoxic conditions activated cytotoxicity of γδ T cells against these same oral cancer cell lines |
| [48] | OSCC line (SCC9, Cal-27), immortalized keratinocytes (HIOEC) | Macrophages (THP-1 derived), HBMCs | OSCC- exosome co-cultured macrophages showed higher expression levels of protein markers of M2 macrophage subtype: CD163, CD206, Arg-1, and IL-10; media of above cultured macrophages increased proliferation and invasive ability of OSCC cell lines with this effect abrogated by inhibition of miR-29a-3p |
| OSCC and Mesenchymal Stem Cells | |||
| [49] | Primary mesenchymal stem cell (MSCs) from normal oral mucosa, dysplastic leukoplakia (LK) and OSCC | OSCC line (SCC-15); oral dysplasia line (DOK) | LK and OSCC mesenchymal stem cell derived exosomes both accelerated proliferation, invasion and migration of both SCC-15 and DOK cells |
| [50] | Primary human bone marrow mesenchymal stem cells | OSCC line (TCA 8113) | hBMSCs transfected with miR-101-3p-Cy3-derived exosomes donated miR-101-3p to OSCC cells repressing invasion and migration and reducing colony forming ability |
| OPMD | |||
| Study | Cell Type | Main Findings | |
| EVS Derived from | EVs Studied on | ||
| [51] | OLPPlasma-derived exosome from OLP patients | T lymphocytes (Jurkat) | T-cell proliferation and migration significantly increased with erosive LP-derived exosomes but not non-erosive LP exosomes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, T.; Pruthi, N.; Seers, C.; Belobrov, S.; McCullough, M.; Celentano, A. Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 1197. https://doi.org/10.3390/ijms21041197
Yap T, Pruthi N, Seers C, Belobrov S, McCullough M, Celentano A. Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. International Journal of Molecular Sciences. 2020; 21(4):1197. https://doi.org/10.3390/ijms21041197
Chicago/Turabian StyleYap, Tami, Neha Pruthi, Christine Seers, Simone Belobrov, Michael McCullough, and Antonio Celentano. 2020. "Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review" International Journal of Molecular Sciences 21, no. 4: 1197. https://doi.org/10.3390/ijms21041197
APA StyleYap, T., Pruthi, N., Seers, C., Belobrov, S., McCullough, M., & Celentano, A. (2020). Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. International Journal of Molecular Sciences, 21(4), 1197. https://doi.org/10.3390/ijms21041197






