Abstract
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and occurs mainly in patients with liver cirrhosis. The mammalian target of rapamycin (mTOR) signaling pathway is involved in many hallmarks of cancer including cell growth, metabolism re-programming, proliferation and inhibition of apoptosis. The mTOR pathway is upregulated in HCC tissue samples as compared with the surrounding liver cirrhotic tissue. In addition, the activation of mTOR is more intense in the tumor edge, thus reinforcing its role in HCC proliferation and spreading. The inhibition of the mTOR pathway by currently available pharmacological compounds (i.e., sirolimus or everolimus) is able to hamper tumor progression both in vitro and in animal models. The use of mTOR inhibitors alone or in combination with other therapies is a very attractive approach, which has been extensively investigated in humans. However, results are contradictory and there is no solid evidence suggesting a true benefit in clinical practice. As a result, neither sirolimus nor everolimus are currently approved to treat HCC or to prevent tumor recurrence after curative surgery. In the present comprehensive review, we analyzed the most recent scientific evidence while providing some insights to understand the gap between experimental and clinical studies.
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary liver malignancy and it accounts for 85–90% of all hepatic cancers. HCC is the fifth most prevalent cancer and the second cause of cancer-related death worldwide. The natural history of liver cirrhosis is tightly linked with HCC: a chronic liver insult (viral hepatitis, alcohol intake or non-alcoholic steatohepatitis (NASH) among others), which is able to produce inflammation of the liver parenchyma, which in turn triggers the activation of hepatic stellate cells and portal myofibroblasts, thus resulting in progressive liver fibrosis and, finally, in liver cirrhosis [1]. Both chronic inflammation and fibrosis are well known risk factors of cancer. NASH is also associated with an abnormal lipid metabolism, lipid accumulation within the hepatocytes, obesity and insulin resistance, with this abnormal metabolic scenario also able to promote cancer initiation and progression [2]. As a result, the vast majority of patients with HCC have an underlying chronic liver disease and indeed, HCC is the first cause of death among patients with liver cirrhosis.
Mutations in oncogenes and tumor suppressor genes are the main pathogenic mechanisms underlying hepatocarcinogenesis as they are responsible for disrupting cell signaling pathways. In HCC, the major pathways involved in the oncogenic process are WNT/b-catenin, Hedgehog, hepatocyte growth factor/c-MET, vascular endothelial growth factor (VEGF), mitogen-activated protein kinase (MAPK)/ERK (or Ras-Raf-MEK-ERK) and PI3K/AKT/mTOR [3,4]. Among all, the PI3K/AKT/mTOR pathway is particularly interesting because it is constitutively activated in a significant proportion of patients with HCC and it is associated with a more aggressive tumor progression and shorter survival [5].
In the present manuscript we aimed to review the role of the mammalian target of rapamycin (mTOR) pathway in the pathogenesis and biological aggressiveness of HCC to better understand the potential clinical impact of its inhibition by currently available drugs. In addition, we have provided some insights regarding the limitations to reproduce the beneficial effects of mTOR inhibitors observed in experimental models into human trials.
2. The mTOR Signaling Pathway
The mechanistic target of rapamycin (mTOR) is an evolutionary conserved serine/threonine kinase, which belongs to the phosphoinositide 3-kinase (PI3K)-related protein kinase family and integrates environmental information into cellular responses [6]. The main structure of the mTOR pathway and its role in liver diseases including HCC are summarized in Figure 1.
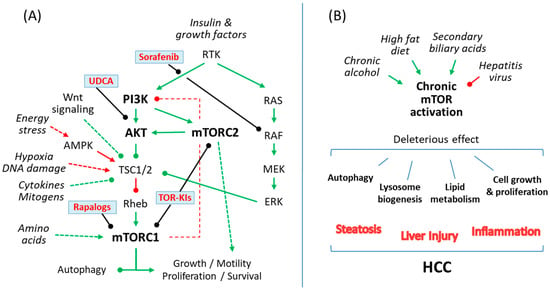
Figure 1.
(A) A simple scheme of the mammalian target of rapamycin (mTOR) signaling pathway showing action sites for rapalogs and currently available compounds for patients with chronic liver disease, like sorafenib or ursodeoxycholic acid (UDCA), which have been shown to prevent liver damage by interfering with the mTOR pathway. (B) Common causes of cirrhosis and HCC such as alcoholic liver disease, chronic viral hepatitis (B,C), non-alcoholic steatohepatitis and cholestatic liver diseases exert liver damage by causing an abnormal regulation of the mTOR signaling pathway in hepatocytes, which in turn regulates cellular lipid metabolism, autophagy and lysosome biogenesis or cellular growth and proliferation. Green, indicates mTOR pathway activation; Red, indicates mTOR pathway repression; arrowhead, activation; roundhead, inhibition.
mTOR is the keystone of at least two structurally different multiprotein complexes, known as mTOR complex 1 (mTORC1) and mTORC2 [7,8,9]. Both mTOR complexes share the mammalian lethal with SEC13 protein 8, the Tti1/Tel2 complex and the inhibitory protein DEP domain-containing mTOR-interacting protein (DEPTOR). However, while the mTORC1 contains the regulatory-associated protein of mTOR (Raptor) and the inhibitory subunit proline-rich Akt substrate of 40kDa (PRAS40), the mTORC2 exhibit the rapamycin-insensitive companion of mTOR (Rictor) and the regulatory proteins Protor1/2 and mSin1. These structural particularities of mTORC1 and mTORC2 are responsible for a different response pattern to a wide variety of upstream signals, as well as for activating different downstream effectors, thus resulting in well differentiated cellular responses [10,11,12].
mTORC1 and mTORC2 respond to insulin and related growth factors through the interconnected MAPK/ERK and PI3K/AKT/mTOR signaling pathways [13,14]. Many growth factors are able to trigger these pathways by interacting with their cell-surface receptor tyrosine kinases (RTKs). The mTORC1, which is the best characterized complex, may be activated by nutrients, cytokines, oxygen and stress signals by different routes, among which adenine monophosphate (AMP) activated protein kinase (AMPK), which is an essential regulator of protein kinase B (or AKT), seems to be particularly relevant [15]. Under favorable conditions, mTORC1 activation promotes lipid and protein biosynthesis, cell growth and proliferation, while motivating the inhibition of autophagy [16]. On the other hand, mTORC2 exert its control on cell survival, metabolism and proliferation, as well as on the organization of the cell cytoskeleton, by controlling the activity of different protein kinases [17,18,19], including also AKT [20]. In addition, both mTORC1 and mTORC2 regulate each other by controlling the activity of ribosomal protein S6 kinase (S6K) and AKT respectively, although the extent of these mechanisms is not completely understood [21].
3. Regulation of the mTOR Pathway in Liver Diseases and HCC
The mTOR signaling pathway is frequently deregulated in cancer and metabolic diseases [22]. Some metabolic diseases such as diabetes or obesity are widely known risk factors of cancer in the general population. Indeed, as part of the oncogenic process, a deep transformation of the metabolic cell program is required, with completely different bioenergetic and biosynthetic demands [23]. Alcoholic liver disease (ALD), chronic viral hepatitis (B and C), NASH and, to a lesser extent, cholestatic liver diseases are the most common causes of liver cirrhosis worldwide and they are characterized by an abnormal regulation of the mTOR pathway, although with some peculiarities for each one of them.
The liver is a paramount organ for lipid metabolism [24]. During feeding, when carbohydrates are abundant, the liver inhibits gluconeogenesis and transforms glucose into fatty acids by de novo lipogenesis, which is regulated by insulin and requires the activation of the PI3K/AKT/mTOR-signaling pathway, together with the enzymes acetyl-CoA carboxylase and fatty acid synthase. Additionally, fatty acids from gastrointestinal digestion are also transported to the liver by the portal bloodstream. Under such conditions, mTORC1 inhibits catabolism (and activates anabolism) by suppressing autophagy through the inactivation of unc-51 like autophagy activating kinase (ULK1) [25] and transcription factor EB (TFEB) [26]. Therefore, fatty acids are processed and stored as triglycerides in lipid droplets whenever needed depending on the metabolic environment. Conversely, during fasting, the liver obtains energy in the form of glucose and fatty acids by hepatic gluconeogenesis and lipolysis, respectively. In addition, the induction of autophagy by the mTOR pathway has also been associated with lipolysis and with the mobilization of intracellular lipid stores from the liver [27].
Alcohol intake may disrupt normal hepatic lipid metabolism and may lead to fatty liver or steatosis by promoting hepatic lipogenesis while hampering fatty acids transport and oxidation. This metabolic reprogramming in alcoholic liver disease requires the activation of mTORC1 by downregulating DEP domain-containing mTOR-interacting protein (DEPTOR, an upstream inhibitor of the complex) [28], and by inhibiting AMPK, which results in the upregulation of lipogenic gene sterol regulatory element binding protein-1 (SREBP1), and in the reduction of circulating adiponectin and peroxisome proliferator activated receptor-alpha (PPAR-alpha) [29]. Chronic alcohol consumption is also associated with oxidative stress, inhibition of hepatocellular autophagy and apoptosis [30,31]. Moreover, oxidative stress induced by chronic alcohol consumption inhibits AMPK and activates the mTOR signaling that, in turn, inhibits autophagy [32]. In contrast, alcohol binging promotes autophagy as a protective mechanism of hepatocytes from direct alcohol toxicity and steatosis, probably through a reactive oxygen species (ROS)-dependent inhibition of mTOR activity [33]. Therefore, acute and chronic alcohol administration has a dual and paradoxical effect on the mTOR pathway to modulate hepatic autophagy. The differential nuclear content of TFEB could be partially responsible for the observed differences [34,35]. Recent studies have shown that miR-155 targets mTOR in chronic alcoholic liver disease to impair autophagy and lysosome function [36,37]. Alcohol-induced miR-155 downregulates key proteins of the autophagy process such as lysosome-associated membrane proteins LAMP1 and LAMP2 and, indirectly, TFEB. In turn, these proteins and lysosome dysfunction exerts feedback on the mTOR pathway to maintain and amplify liver damage [38].
Chronic infection by hepatitis B (HBV) and C (HCV) viruses is still a common cause of liver cirrhosis, although their prevalence is expected to continue to decrease in the upcoming years due to advances in antiviral therapy. The role of the mTOR pathway in chronic viral hepatitis concerns the regulation of cellular homeostasis. HBV and HCV use the mTOR pathway to enhance autophagy [39] to benefit their own replication [40]. A transgenic mice model with impaired autophagy is characterized by a strong reduction in HBV replication [41]. The activation of the PI3K/AKT/mTOR signaling pathway inhibits both autophagy and HBV transcription/replication in liver cancer cells and could be responsible for the elimination of viral replication that occurs during liver oncogenesis [42]. The link between hepatitis B/C, mTOR activation and autophagy is not fully understood but the miR-99 family members, which are aberrantly expressed in the serum of HBV patients, have been proposed as key regulators in this process [43].
NASH is the liver manifestation of metabolic syndrome and it is characterized by hepatic steatosis, ballooning, inflammation and progressive fibrosis. NASH occurs as a result of an imbalance of energy intake/consumption or when there is an intrinsic problem with the lipid storing process. Within the hepatocyte, the accumulation of fatty acids leads to the production of lipid-synthesis intermediates, which causes steatosis through the mTOR pathway deregulation [44]. Thus, NASH shares many mechanisms and pathological features with ALD. In a high-fed diet mice model of NASH, the pharmacological inhibition of mTORC1 or phosphatidylinositol 3-kinase (PI3K) protects against steatosis while promoting insulin sensitivity [31]. In a different murine model, Zhang et al. showed an initial upregulation of the mTORC1, with a subsequent damping oscillation pattern in the long term, being the alteration of the mTORC1 opposite to that observed for TFEB. These findings suggested that hepatic steatosis progresses by factors other than anabolic synthesis such as inhibition of autophagy and lysosome dysfunction [38]. Perinatal exposure to bisphenol has also been associated with the development of NASH in mice offspring through the activation of the PI3K/AKT/mTOR pathway in a process that involves the overexpression of lipogenic genes, autophagy impairment and inflammatory response [45]. On the other hand, dysbiosis of the gut microbiota may play a key role in the development of NAFLD [44]. Alterations in gut microbiota are able to disturb physiological functions controlled by mTOR such as lipogenesis, gluconeogenesis or autophagy. The use of mTOR inhibitors to influence gut microbiota in order to treat NASH is an attractive research field which should be further explored [46]. Obesity, which is a major contributor to NASH, enhances the development of chemically induced HCC through the activation of mTOR and the inhibition of AKT [47].
Cholestatic liver diseases result from a repeated exposure to unconjugated primary biliary acids, which may activate mTOR through the IκB kinase β (IKKβ)/Tuberous sclerosis 1 (TSC1)/mTOR pathway as demonstrated in some precancerous conditions such as Barrett’s esophagus [48]. In the liver, chronic cholestasis is a risk factor for HCC development and conversely advanced HCC usually provokes a progressive cholestatic effect. Glycochenodeoxycholic acid, which is a glycine-conjugated form of the primary bile acid chenodeoxycholic acid, is highly cytotoxic in a dose-dependent manner and its exposure in cellular models of the disease [49] activates cellular autophagy through the AMPK/mTOR pathway to promote HCC invasiveness [50]. Secondary biliary acids produced by the metabolic activity of the gut microbiota are also able to enhance AKT-independent mTOR activity in the liver to promote hepatic steatosis and HCC, while depleting gut microbiota with antibiotics reverses these mechanisms [51].
Liver cancer development in different mouse models with genetic or dietary alterations is frequently preceded by hepatic steatosis and is associated with a deregulation of the mTOR pathway [52,53,54,55,56]. A constitutive activation of the mTOR pathway may be enough to cause fatty liver and HCC independent of the diet. Moreover, mTOR pathway deregulation has been linked to liver cancer independent of the development of liver steatosis [57]. There is evidence of mTOR pathway activation in HCC, cholangiocarcinoma and hepatoblastoma [58]. This abnormal activation involves both PI3K/AKT and MAPK/ERK [59] signaling pathways, which are frequently co-induced in liver cancer. Thus, the mTORC1 and mTORC2 pathways are upregulated in 40–50% of patients with HCC [60,61]. In addition, increased mTOR expression is found in larger tumors or in advanced stages of HCC [62,63]. In a prospective study including 49 patients with HCC who underwent liver transplantation, the explanted liver was analyzed to determine the mTOR pathway expression both in the tumor and in the surrounding non-tumoral cirrhotic tissue [64]. mTOR pathway expression was more intense at the tumor edge where cellular growth and proliferation are more intense. Noteworthy, a subanalysis of patients who had undergone locoregional ablation of the tumor prior to liver transplantation revealed a significant reduction in mTOR activity within the tumor but not in the surrounding tissue. This suggests that local ablation therapies are not able to completely suppress mTOR pathway activity, thus explaining why tumor recurrence is almost universal if no other therapy is implemented afterwards. In light of this evidence, it is not surprising that a more intense mTOR pathway deregulation is associated with increased tumor recurrence rates after surgical resection or liver transplantation, as well as with shorter survival [64].
4. Targeting mTOR Signaling in HCC: Experimental Models
As described above, the mTOR pathway is systematically deregulated among the most prevalent etiologies of liver cirrhosis and it may be found overexpressed in patients with HCC, where it confers a more aggressive biological behavior. Therefore, a pharmacological and molecular inhibition of the mTOR signaling pathway has been proposed as a therapeutic strategy for HCC [11]. Next, we have described the pharmacological compounds with anti-mTOR activity currently available for patients with chronic liver disease, including HCC (Table 1). Other drugs or strategies which also target mTOR such as miRNAs [65], antibiotics [51] or antioxidants [66,67], but are not routinely used in clinical practice, are out of the scope of this review and have not been discussed here.

Table 1.
Chemical compounds affecting the mTOR signaling pathway currently used or under investigation in chronic liver diseases and hepatocellular carcinoma (HCC).
4.1. Ursodeoxycholic Acid (UDCA)
UDCA is a cytoprotective secondary bile acid which forms the backbone of the therapeutic arsenal against cholestatic liver diseases, mainly primary biliary cholangitis. UDCA therapy improves liver biochemistry parameters and delays the progression of the disease to liver cirrhosis [68]. In a human liver cancer cell line, UDCA induced the synthesis of glutathione by activating the PI3K/AKT pathway and displayed antioxidant properties [69]. In addition, UDCA could prevent lipid accumulation and reduce liver damage by inhibiting the AKT/mTOR/SREBP1 pathway [70]. Similarly, U12, an UDCA derivative compound, is able to hamper mTOR activity in vitro and in vivo and has shown antiproliferative and proapoptotic capacities, which could be helpful to prevent cancer [71]. Nor-UDCA, a more potent homologue with anti-inflammatory, anti-cholestatic and anti-fibrotic properties, induces autophagy by activating the AMPK/mTOR/ULK1 signaling pathway (i.e., suppressing mTOR activity) and has been suggested as a novel therapeutic approach to prevent fibrosis progression and HCC in patients with alpha-1 antitrypsin deficiency [72].
4.2. Rapalogs
Rapamycin (also known as sirolimus), an antibiotic first isolated in 1972 by Sehgal and Cols from the bacterium Streptomyces hygroscopicus [73], is a potent and selective inhibitor of the mTOR protein kinase. Rapamycin exerts its activity mainly on mTORC1, although a prolonged therapy could also disrupt mTORC2 to a lesser extent [74]. Rapamycin promotes the inhibition of TFEB [38], which is associated with autophagy and catabolic processes such as fatty acid oxidation and ketogenesis. Currently, rapamycin analogues (also known as rapalogs) are widely used to modulate autophagy in experimental models. Indeed, targeting mTOR is an attractive approach for liver diseases in which autophagy has protective effects, such as storage disorders (alpha-1 antitrypsin deficiency or Wilson’s disease), acute liver injury, alcoholic liver disease, NASH or HCC [31,33,75,76,77,78]. However, since autophagy has a dual role (beneficial or detrimental) depending on the cell type and the stage of the disease, it should be considered to target specifically liver cells while considering the optimal therapeutic window for its promotion (in earlier disease stages) or inhibition (in more advanced stages or in HCC) [79].
Rapamycin has shown potent antiproliferative and immunosuppressive properties against a large variety of tumor cells in vitro and suppresses growth of cancer cells in vivo [10,80,81,82,83]. Rapalogs with an improved pharmacokinetic profile and solubility properties have been tested in clinical trials. Temsirolimus and everolimus were approved for treatment of metastatic renal carcinoma among other malignancies. However, no rapalog has been approved for HCC treatment to date (see next section), probably due to the partial inhibition of mTORC1 and the collateral hyperactivation of the MAPK/ERK pathway through a PI3K-dependent feedback loop (mTORC1-MAPK feedback loop) [84]. In this sense, everolimus inhibits both mTOR complexes more potently than sirolimus, particularly mTORC2 [85], which could be an advantage in terms of drug efficacy and safety. In any case, most studies performed in animal models as outlined above implemented a local intra-tumor administration of the drug, which does not mirror clinical practice in humans. In those studies, with oral administration of mTOR inhibitors in murine models, the dosage was up to 100-fold increased as compared with the average dose in humans. This may explain in part the difficulties to reproduce the anti-proliferative properties of mTOR inhibitors in human clinical trials (see Section 5 below).
4.3. Second-Generation mTOR Inhibitors in HCC
mTOR kinase inhibitors (TOR-KIs) are second-generation mTOR inhibitors, which emerged to solve the above referred limitations of rapalogs. TOR-KIs appear to be more potent than rapalogs because they inhibit the activity and associated functions of both mTOR complexes (related to protein and lipid biosynthesis, cell growth and proliferation). Several of these compounds are currently being tested in preclinical experiments and early clinical trials [86,87,88]. However, there is a concern that TOR-KIs also cause feedback-dependent biphasic regulation of AKT signaling, which involves RTKs and causes the reactivation of mTOR signaling. Further investigations are warranted [89].
4.4. Sorafenib
Sorafenib is a multitarget kinase (multi-kinase) inhibitor that, among its multiple action mechanisms, interferes with the AKT/mTOR pathway by targeting VEGFR, PDFGR, c-Kit, c-RAF and B-RAF [90]. Sorafenib is currently the standard of care for patients with advanced HCC and for those patients with intermediate-stage HCC who are not eligible for locoregional therapies [91]. In liver cancer cells, sorafenib disrupts lipogenesis and provokes cell death by suppressing the production of ATP, which results in AMPK activation, mTOR inhibition and SREBP1 reduction [92]. It seems that AMPK blocks mTORC1 by phosphorylating the mTOR inhibitor TSC2 [93] and the mTORC1 subunit Raptor [94]. These findings agree with the established dependence of cancer cells on de novo fatty acids biosynthesis and enhanced lipogenesis. This pathway could represent a promising target for cancer therapy. Recently, regorafenib, another multi-kinase inhibitor, was approved as a second line therapy for HCC patients who experience tumor progression regardless of sorafenib. However, the clinical benefit of these multi-kinase inhibitors is modest because patients do not respond homogeneously, which could be partially explained by the development of drug resistance through the activation of the MAPK/ERK signaling pathway [95].
4.5. Combination of mTOR Inhibitors in HCC
The oncogenic signaling pathways of HCC are dynamic and able to adapt to new situations by creating compensatory mechanisms, which provide tumor cells with the ability to evade individual drugs. Accordingly, new approaches based on the combination of different inhibitors targeting complementary critical points of these interconnected pathways have been tested. Yu et al. [88] recently published a comprehensive review, which summarized clinical trials (completed or still ongoing) involving mTOR inhibitors alone or in combination in HCC. It not only included mTOR inhibitors such as rapalogs and TOR-KIs but also PI3K, AKT and PI3K/mTOR inhibitors.
Sorafenib produces heterogeneous cellular responses depending on the genomic context or mutational profile of the tumor. When sorafenib is used in combination with specific-targeted inhibitors driven from a close analysis of the mutational cell background, the observed effect was synergic regarding the inhibition of HCC proliferation. This fact could partially explain the suboptimal results of sorafenib, alone or in combination, in clinical trials where a heterogeneous and uncharacterized group of patients was included [96]. A complete genetic characterization of HCC patients could improve the clinical use of mTOR inhibitors, alone or in combination.
Rapalogs and TOR-KIs are associated with feedback loops that promote the (re)activation of these signaling pathways, thus limiting their therapeutic use. The simultaneous inhibition of both, mTORC1 and MAPK, or mTORC1/mTORC2 and RTKs, has been shown to enhance the effectiveness of each compound separately in preclinical models (in vitro and in vivo) [84,89]. The concomitant inhibition of MEK and AKT in mice co-expressing activated forms of c-MET and AKT resulted in a stronger inhibition of tumor progression whereas neither sorafenib nor regorafenib showed any efficacy [97].
Recently, two-step senescence-focused therapies have been proposed as anti-cancer therapy [98]. In this approach, the first drug induces cell vulnerability (senescence in this case), which is exploited by the second agent. In liver cancer, Wang et al. [99] have shown that the TOR-KI AZD8055 [100] exhibits a greater activity in senescent cells as compared with proliferating cells. In this setting, XL413 acted as a second hit to inhibit the DNA-replication kinase CDC7, which selectively induces senescence in liver cancer cells with mutations in TP53 or its upstream regulators. Senescence induction by XL413 disrupted the feedback reactivation of the mTOR signaling caused by the mTOR inhibitor while increasing the apoptosis susceptibility of cancer cells to AZD8055. These observations were consistent with mouse models of HCC and revealed that a combination of XL413 and AZD8055 would be more effective than sorafenib.
Altogether, these results highlight two critical points to select the best therapeutic strategy in HCC. First, to define the components and relationships of the signaling pathways involved in tumor formation, and second, to know the genomic mutations affecting each patient. This knowledge would allow the tailoring of antiproliferative agents according to each patient and tumor characteristics, thus resulting in more effective and safer therapeutic approaches.
5. Targeting mTOR Signaling in HCC: From the Bench to the Clinic
The anti-proliferative properties of mTOR inhibitors in preclinical models would be of high value in patients with HCC as they would allow to prevent tumor recurrence after potentially curative therapies (i.e., percutaneous tumor ablation, surgical resection or liver transplantation), or to delay tumor progression in patients with a more advanced disease. Unfortunately, clinical studies are scarce and with reduced methodological quality. Most of the studies are single-centre retrospective observations and those with a prospective and/or randomized design have failed to prove any benefit in terms of recurrence free survival and/or overall survival.
In patients with unresectable or metastatic HCC, the mTOR inhibitor everolimus has been studied in combination with sorafenib. A phase I randomized trial was designed to explore the maximal dose of everolimus tolerated in combination with the standard dose of sorafenib (i.e., 400 mg bid). A total of 30 patients with compensated liver cirrhosis Child Pugh class A were included. The maximal tolerated dose of everolimus was only 2.5 mg per day. The combination of everolimus and sorafenib did not result in improved overall survival. The authors concluded that a phase II trial was not justified since a biologically active anti-proliferative dose of everolimus cannot be achieved in patients with liver cirrhosis, even in those with a preserved liver function [101]. Despite this, a phase II multicenter trial involving 106 patients with unresectable or metastatic HCC and compensated liver cirrhosis was conducted [102]. Patients were randomly assigned to receive sorafenib alone (400 mg bid) or sorafenib (400 mg bid) in combination with everolimus (5 mg od). Progression-free survival rates at 12 months were identical in patients with and without everolimus (68% vs. 70%), but severe adverse events (grade 3–4) were more frequent in the everolimus arm (86% vs. 72%). The authors confirmed that a clinically meaningful inhibition of the mTOR pathway in patients with liver cirrhosis cannot be achieved by using everolimus because of its toxicity. Finally, the sequential use of everolimus in patients with advanced HCC who experience tumor progression under sorafenib was also unable to prolong survival [103].
In earlier stages of HCC, when the tumor is confined to the liver, liver transplantation may be the best therapeutic option as it allows for curing both, the tumor and the underlying liver cirrhosis [104]. However, tumor recurrence rates after liver transplantation are 10% to 15% when HCC is under the so called Milan criteria (i.e., a single tumor less than 5 cm or up to three nodules, less than 3 cm each, without macrovascular invasion) or even higher if more nodules with increased diameter are allowed [91,105]. Since HCC recurrence after liver transplantation is associated with a dismal prognosis, the use of mTOR inhibitors as adjuvant therapy is particularly attractive. Their dual effect as immunosuppressants and antiproliferative agents would prevent graft rejection and, theoretically, would decrease the risk of HCC recurrence [106]. In liver transplantation, there are two mTOR inhibitors approved to be used as immunosuppressive agents. Sirolimus (or rapamycin) entered the market first but the initial phase II randomized trial in liver transplantation had to be prematurely stopped because the experimental arm consisting of sirolimus plus tacrolimus resulted in an increased risk of graft loss and sepsis [107]. A subsequent network meta-analysis of randomized trials have demonstrated that sirolimus-based immunosuppression is associated with increased mortality rates as compared with other protocols and therefore there are concerns about using sirolimus as a first-line therapy in patients receiving a liver transplantation [108]. Everolimus has an improved safety profile as compared with sirolimus. Several randomized trials have shown that, in combination with calcineurin inhibitors, everolimus allows for a more effective preservation of kidney function in liver transplant recipients [109,110]. Regarding prevention of HCC recurrence, several retrospective single-center studies, further meta-analyzed, have reported reduced tumor recurrence rates in patients receiving mTOR inhibitors-based immunosuppression [111]. However, the only prospective observational study (using everolimus) [112] and the only randomized trial (using sirolimus), which was sufficiently powered (n = 525) and with prolonged follow-up, have failed to demonstrate any benefit in reducing tumor recurrence rates after liver transplantation [113]. This gap between retrospective and prospective data may be explained again by safety issues [114]. Those patients with severe adverse events leading to significant dose reduction or drug withdrawal, which may account for up to 25% of patients according to the registry trial of everolimus [109], are systematically excluded from retrospective series, leading to a selection bias. In other words, retrospective studies only account for patients with adequate drug tolerance who also receive increased dosage of mTOR inhibitors whereas the results of prospective/randomized studies are diluted because of intolerant and non-compliant patients [114]. The use of everolimus-based immunosuppression would be justified in liver transplant patients with HCC showing poor prognostic histological features such as microvascular invasion in whom the risk of adverse events would be overwhelmed by a theoretical advantage of reduced tumor recurrence rates [64].
In light of the available evidence, mTOR inhibitors (i.e., sirolimus and everolimus) have not been approved to treat or to prevent HCC and they do not form part of the routine clinical armamentarium. In clinical practice, everolimus is used as an immunosuppressive agent in liver transplant patients for a more effective preservation of kidney function. Many institutions have implemented everolimus-based immunosuppression in patients with HCC undergoing liver transplantation in order to prevent tumor recurrence, even in the absence of solid scientific supporting evidence. There is a need for more potent mTOR inhibitors and with an improved safety profile. The role of second-generation TOR-KIs in HCC is still to be explored. Since HCC occurs mainly in the context of chronic liver disease, randomized controlled trials may be conducted in patients with liver cirrhosis to study efficacy and safety.
6. Conclusions
The mTOR signaling pathway is involved in cell metabolism and proliferation, which are key hallmarks of cancer and allow for HCC development, progression and spreading. The mTOR pathway inhibition exerts a pronounced antiproliferative effect in vitro and in animal models of HCC. However, clinical studies, especially those prospective and/or randomized, have failed to demonstrate any capacity of mTOR inhibitors to hamper tumor progression or to prevent tumor recurrence after potentially curative therapies such as liver transplantation. The gap between experimental and clinical observations may be partially explained by the dose-dependent toxicity of mTOR inhibitors in patients with chronic liver disease, which frequently motivates a significant dose reduction or drug withdrawal. There is a need to develop more potent mTOR inhibitors and with an improved safety profile in order to obtain a true antiproliferative (and clinically meaningful) effect in patients with HCC. A detailed molecular profiling of HCC will probably become a powerful tool to select patients with HCC to receive mTOR inhibitors as part of a true personalized medicine.
Author Contributions
Conceptualization, G.F., M.R.-P. and M.D.l.M.; literature search, G.F., M.G., V.A. and M.R.-P.; writing—original draft preparation, G.F. and M.R.-P.; writing—review and editing, G.F., M.G., V.A., M.R.-P. and M.D.l.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the research project PI18/01736 integrated into the Plan Estatal de I+D+I 2013-2016 and co-financed by the ISCIII-Subdirección General de Evaluación y Fomento de la Investigación and the Fondo Europeo de Desarrollo Regional (FEDER).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| HCC | Hepatocellular carcinoma |
| NASH | Non-alcoholic steatohepatitis |
| mTOR | Mechanistic target of rapamycin |
| PI3K mTORC DEPTOR Raptor RTKs ALD ULK1 TFEB SREBP1 HBV HCV UDCA TOR-KIs | Phosphoinositide 3-kinase mTOR complex DEP domain-containing mTOR-interacting protein Regulatory-associated protein of mTOR Receptor tyrosine kinases Alcoholic liver disease Unc-51-like kinase 1 Transcription factor EB Sterol regulatory element binding protein-1 Hepatitis B virus Hepatitis C virus Ursodeoxycholic acid mTOR kinase inhibitors |
References
- Liberal, R.; Grant, C.R. Cirrhosis and autoimmune liver disease: Current understanding. World J. Hepatol. 2016, 8, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.; Marais, R.; Zhu, A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010, 29, 4989–5005. [Google Scholar] [CrossRef] [PubMed]
- Blagotinsek, K.; Rozman, D. Targeting Signalling Pathways in Hepatocellular Carcinoma. Curr. Pharm. Des. 2017, 23, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Huang, C.; Lin, P.; Chang, H.; Ger, L.; Lin, Y.; Changchien, C.; Lee, C.; Tai, M. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003, 97, 1929–1940. [Google Scholar] [CrossRef]
- Kunz, J.; Henriquez, R.; Schneider, U.; Deuter-Reinhard, M.; Movva, N.R.; Hall, M.N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell 1993, 73, 585–596. [Google Scholar] [CrossRef]
- El Hiani, Y.; Egom, E.; Dong, X. mTOR signalling: Jack-of-all-trades. Biochem. Cell Biol. 2018, 97, 58–67. [Google Scholar] [CrossRef]
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 2002, 10, 457–468. [Google Scholar] [CrossRef]
- Sarbassov, D.; Ali, S.M.; Kim, D.; Guertin, D.; Latek, R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef]
- Caron, E.; Ghosh, S.; Matsuoka, Y.; Ashton-Beaucage, D.; Therrien, M.; Lemieux, S.; Perreault, C.; Roux, P.P.; Kitano, H. A comprehensive map of the mTOR signaling network. Mol. Syst. Biol. 2010, 6, 453. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [PubMed]
- Sangüesa, G.; Roglans, N.; Baena, M.; Velázquez, A.M.; Laguna, J.C.; Alegret, M. mTOR is a Key Protein Involved in the Metabolic Effects of Simple Sugars. Int. J. Mol. Sci. 2019, 20, 1117. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, C.; Cai, Z.; Zhang, X.; Jin, G.; Zhang, W.-N.; Xu, C.; Wang, C.-Y.; Morrow, J.; Zhang, S.; et al. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat. Commun. 2018, 9, 4728. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; Cuervo, A.M. Regulation of Liver Metabolism by Autophagy. Gastroenterology 2016, 150, 328–339. [Google Scholar] [CrossRef]
- Su, B.; Jacinto, E. Mammalian TOR signaling to the AGC kinases. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 527–547. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Jhanwar-Uniyal, M.; Wainwright, J.V.; Mohan, A.L.; Tobias, M.E.; Murali, R.; Gandhi, C.D.; Schmidt, M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019, 72, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Cantley, L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Coller, H.A. Is cancer a metabolic disease? Am. J. Pathol. 2014, 184, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 2018, 9, 145–151. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The Transcription Factor TFEB Links mTORC1 Signaling to Transcriptional Control of Lysosome Homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Chen, H.; Shen, F.; Sherban, A.; Nocon, A.; Li, Y.; Wang, H.; Xu, M.-J.; Rui, X.; Han, J.; Jiang, B.; et al. DEPTOR Suppresses Lipogenesis and Ameliorates Hepatic Steatosis and Acute-on-Chronic Liver Injury in Alcoholic Liver Disease. Hepatology 2018, 68, 496–514. [Google Scholar] [CrossRef]
- Donohue, T.J. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974–4978. [Google Scholar]
- Menk, M.; Graw, J.A.; Poyraz, D.; Möbius, N.; Spies, C.D.; von Haefen, C. Chronic Alcohol Consumption Inhibits Autophagy and Promotes Apoptosis in the Liver. Int. J. Med. Sci. 2018, 15, 682–688. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, H.; Li, M.; Xiong, X.; Chen, X.; Chen, X.; Dong, X.X.; Yin, X. Pharmacological Promotion of Autophagy Alleviates Steatosis and Injury in Alcoholic and Non-alcoholic Fatty Liver Conditions in Mice. J. Hepatol. 2013, 58, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Sid, B.; Verrax, J.; Calderon, P.B. Role of AMPK activation in oxidative cell damage: Implications for alcohol-induced liver disease. Biochem. Pharmacol. 2013, 86, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, M.; Chen, X.; Ni, H.; Lin, C.-W.; Gao, W.; Lu, B.; Stolz, D.B.; Clemens, D.L.; Yin, X. Autophagy Reduces Acute Ethanol-Induced Hepatotoxicity and Steatosis in Mice. Gastroenterology 2010, 139, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Esposito, A.; Choi, H.; Matarese, M.; Benedetti, V.; Malta, C.D.; Monfregola, J.; Medina, D.L.; Lippincott-Schwartz, J.; Ballabio, A. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.G.; Trambly, C.S.; Fox, H.S.; Tuma, D.J.; Donohue, T.M. Acute and Chronic Ethanol Administration Differentially Modulate Hepatic Autophagy and Transcription Factor EB. Alcohol. Clin. Exp. Res. 2015, 39, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Babuta, M.; Furi, I.; Bala, S.; Bukong, T.N.; Lowe, P.; Catalano, D.; Calenda, C.; Kodys, K.; Szabo, G. Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease. Hepatology 2019, 70, 2123–2141. [Google Scholar] [CrossRef]
- Wan, G.; Xie, W.; Liu, Z.; Xu, W.; Lao, Y.; Huang, N.; Cui, K.; Liao, M.; He, J.; Jiang, Y.; et al. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy 2014, 10, 70–79. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, S.; Khambu, B.; Ma, F.; Li, Y.; Chen, X.; Martina, J.A.; Puertollano, R.; Li, Y.; Chalasani, N.; et al. Dynamic MTORC1-TFEB feedback signaling regulates hepatic autophagy, steatosis and liver injury in long-term nutrient oversupply. Autophagy 2018, 14, 1779–1795. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.; Cao, J.; Otto, N.M.; Kim, D. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef]
- Sir, D.; Ou, J.J. Autophagy in Viral Replication and Pathogenesis. Mol. Cells 2010, 29, 1–7. [Google Scholar] [CrossRef]
- Tian, Y.; Sir, D.; Kuo, C.-F.; Ann, D.K.; Ou, J.-H.J. Autophagy required for hepatitis B virus replication in transgenic mice. J. Virol. 2011, 85, 13453–13456. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, T.; Jiang, D.; Cuconati, A.; Xiao, G.-H.; Block, T.M.; Guo, J.-T. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway. J. Virol. 2007, 81, 10072–10080. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Deng, W.; Pang, J.; Kemper, T.; Hu, J.; Yin, J.; Zhang, J.; Lu, M. The microRNA-99 family modulates hepatitis B virus replication by promoting IGF-1R/PI3K/Akt/mTOR/ULK1 signaling-induced autophagy. Cell. Microbiol. 2017, 19, e12709. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wu, D.; Wu, F.; Meng, Y.; Zhang, J.; Wang, X.; Jia, L. Non-alcoholic Fatty Liver Disease Induced by Perinatal Exposure to Bisphenol a Is Associated with Activated mTOR and TLR4/NF-κB Signaling Pathways in Offspring Rats. Front. Endocrinol. 2019, 10, 620. [Google Scholar] [CrossRef]
- Noureldein, M.; Eid, A. Gut microbiota and mTOR signaling: Insight on a new pathophysiological interaction. Microb. Pathog. 2018, 118, 98–104. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Yen, C.; Izzo, J.G.; Lee, D.; Guha, S.; Wei, Y.; Wu, T.; Chen, C.; Kuo, H.; Hsu, J.; Sun, H.; et al. Bile Acid Exposure Up-regulates Tuberous Sclerosis Complex 1/Mammalian Target of Rapamycin Pathway in Barrett’s-Associated Esophageal Adenocarcinoma. Cancer Res. 2008, 68, 2632–2640. [Google Scholar] [CrossRef]
- González-Rubio, S.; López-Sánchez, L.; Muñoz-Castañeda, J.; Linares, C.I.; Aguilar-Melero, P.; Rodríguez-Perálvarez, M.; Sánchez-Sánchez, R.; Fernández-Álvarez, A.; Casado, M.; Montero-Álvarez, J.L.; et al. GCDCA down-regulates gene expression by increasing Sp1 binding to the NOS-3 promoter in an oxidative stress dependent manner. Biochem. Pharmacol. 2015, 96, 39–51. [Google Scholar] [CrossRef]
- Gao, L.; Lv, G.; Li, R.; Liu, W.; Zong, C.; Ye, F.; Li, X.; Yang, X.; Jiang, J.; Hou, X.; et al. Glycochenodeoxycholate promotes hepatocellular carcinoma invasion and migration by AMPK/mTOR dependent autophagy activation. Cancer Lett. 2019, 454, 215–223. [Google Scholar] [CrossRef]
- Yamada, S.; Takashina, Y.; Watanabe, M.; Nagamine, R.; Saito, Y.; Kamada, N.; Saito, H. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget 2018, 9, 9925–9939. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.; Wang, Y.; Stahl, A.; Bassilian, S.; Lee, W.P.; Kim, Y.; Sherwin, R.; Devaskar, S.; Lesche, R.; Magnuson, M.A.; et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Suzuki, A.; Kataoka, E.; Sasaki, T.; Hamada, K.; Sasaki, J.; Mizuno, K.; Hasegawa, G.; Kishimoto, H.; Iizuka, M.; et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Investig. 2004, 113, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Tanaka, Y.; Tateishi, K.; Yamamoto, K.; Yamamoto, S.; Mohri, D.; Isomura, Y.; Seto, M.; Nakagawa, H.; Asaoka, Y.; et al. Altered composition of fatty acids exacerbates hepatotumorigenesis during activation of the phosphatidylinositol 3-kinase pathway. J. Hepatol. 2011, 55, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Guri, Y.; Colombi, M.; Dazert, E.; Hindupur, S.K.; Roszik, J.; Moes, S.; Jenoe, P.; Heim, M.H.; Riezman, I.; Riezman, H.; et al. mTORC2 Promotes Tumorigenesis via Lipid Synthesis. Cancer Cell 2017, 32, 807–823.e12. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Weng, J.; Zhang, Y.; Liang, K.; Fu, G.; Li, Y.; Bai, X.; Gao, Y. mTOR direct crosstalk with STAT5 promotes de novo lipid synthesis and induces hepatocellular carcinoma. Cell Death Dis. 2019, 10, 619. [Google Scholar] [CrossRef]
- Menon, S.; Yecies, J.L.; Zhang, H.H.; Howell, J.J.; Nicholatos, J.; Harputlugil, E.; Bronson, R.T.; Kwiatkowski, D.J.; Manning, B.D. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci. Signal. 2012, 5, ra24. [Google Scholar] [CrossRef]
- Bhat, M.; Sonenberg, N.; Gores, G.J. The mTOR pathway in hepatic malignancies. Hepatology 2013, 58, 810–818. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 1041–1047. [Google Scholar] [CrossRef]
- Villanueva, A.; Chiang, D.Y.; Newell, P.; Peix, J.; Thung, S.; Alsinet, C.; Tovar, V.; Roayaie, S.; Minguez, B.; Sole, M.; et al. Pivotal Role of mTOR Signaling in Hepatocellular Carcinoma. Gastroenterology 2008, 135, 1972–1983. [Google Scholar] [CrossRef]
- Matter, M.S.; Decaens, T.; Andersen, J.B.; Thorgeirsson, S.S. Targeting the mTOR pathway in hepatocellular carcinoma: Current state and future trends. J. Hepatol. 2014, 60, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Kannangai, R.; Adegbola, O.; Wang, J.; Su, G.; Torbenson, M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin. Cancer Res. 2004, 10, 8421–8425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, Y.; Li, J.; Wang, Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med. Oncol. 2010, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Ferrín, G.; Rodríguez-Perálvarez, M.; González-Rubio, S.; Sánchez-Frías, M.; Amado, V.; Pozo, J.C.; Poyato, A.; Ciria, R.; Ayllón, M.D.; et al. mTOR Expression in Liver Transplant Candidates with Hepatocellular Carcinoma: Impact on Histological Features and Tumour Recurrence. Int. J. Mol. Sci. 2019, 20, 336. [Google Scholar] [CrossRef]
- Varshney, A.; Panda, J.J.; Singh, A.K.; Yadav, N.; Bihari, C.; Biswas, S.; Sarin, S.K.; Chauhan, V.S. Targeted delivery of microRNA-199a-3p using self-assembled dipeptide nanoparticles efficiently reduces hepatocellular carcinoma in mice. Hepatology 2018, 67, 1392–1407. [Google Scholar] [CrossRef]
- Villa-Cuesta, E.; Boylan, J.M.; Tatar, M.; Gruppuso, P.A. Resveratrol Inhibits Protein Translation in Hepatic Cells. PLoS ONE 2011, 6, e29513. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Chen, L.; Kong, D.; Zhang, X.; Lu, C.; Lu, Y.; Zheng, S. Curcumin attenuates angiogenesis in liver fibrosis and inhibits angiogenic properties of hepatic stellate cells. J. Cell Mol. Med. 2014, 18, 1392–1406. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Dyson, J.K.; Alexander, G.J.M.; Chapman, M.H.; Collier, J.; Hübscher, S.; Patanwala, I.; Pereira, S.P.; Thain, C.; Thorburn, D.; et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018, 67, 1568–1594. [Google Scholar] [CrossRef]
- Arisawa, S.; Ishida, K.; Kameyama, N.; Ueyama, J.; Hattori, A.; Tatsumi, Y.; Hayashi, H.; Yano, M.; Hayashi, K.; Katano, Y.; et al. Ursodeoxycholic acid induces glutathione synthesis through activation of PI3K/Akt pathway in HepG2 cells. Biochem. Pharmacol. 2009, 77, 858–866. [Google Scholar] [CrossRef]
- Hu, J.; Hong, W.; Yao, K.; Zhu, X.; Chen, Z.; Ye, L. Ursodeoxycholic acid ameliorates hepatic lipid metabolism in LO2 cells by regulating the AKT/mTOR/SREBP-1 signaling pathway. World J. Gastroenterol. 2019, 25, 1492–1501. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Q.; Lin, T.; Zeng, Z.; Wang, G.; Zeng, D.; Ding, R.; Sun, C.; Zhang, X.; Chen, H. U12, a UDCA Derivative, Acts as an Anti-Hepatoma Drug Lead and Inhibits the mTOR/S6K1 and Cyclin/CDK Complex Pathways. PLoS ONE 2014, 9, e113479. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Blomenkamp, K.S.; Fickert, P.; Trauner, M.; Teckman, J.H. NorUDCA promotes degradation of α1-antitrypsin mutant Z protein by inducing autophagy through AMPK/ULK1 pathway. PLoS ONE 2018, 13, e0200897. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N.; Baker, H.; Vézina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. 1975, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.E.; Mercer, C.A.; Carnevalli, L.S.; Park, J.; Andersen, J.B.; Conner, E.A.; Tanaka, K.; Matsutani, T.; Iwanami, A.; Aronow, B.J.; et al. mTOR Inhibitors Synergize on Regression, Reversal of Gene Expression, and Autophagy in Hepatocellular Carcinoma. Sci. Transl. Med. 2012, 4, 139ra84. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A.I. Autophagy Protects against CYP2E1/Chronic Ethanol-Induced Hepatotoxicity. Biomolecules 2015, 5, 2659–2674. [Google Scholar] [CrossRef]
- Chao, X.; Wang, S.; Zhao, K.; Li, Y.; Williams, J.A.; Li, T.; Chavan, H.; Krishnamurthy, P.; He, X.C.; Li, L.; et al. Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology 2018, 155, 865–879. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues From Patients With Wilson Disease and From ATP7B-Deficient Animals, Protects Hepatocytes From Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189. [Google Scholar] [CrossRef]
- Allaire, M.; Rautou, P.; Codogno, P.; Lotersztajn, S. Autophagy in liver diseases: Time for translation? J. Hepatol. 2019, 70, 985–998. [Google Scholar] [CrossRef]
- Kunz, J.; Hall, M.N. Cyclosporin A, FK506 and rapamycin: More than just immunosuppression. Trends Biochem. Sci. 1993, 18, 334–338. [Google Scholar] [CrossRef]
- Aghdasi, B.; Ye, K.; Resnick, A.; Huang, A.; Ha, H.C.; Guo, X.; Dawson, T.M.; Dawson, V.L.; Snyder, S.H. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc. Natl. Acad. Sci. USA 2001, 98, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.J.; Abraham, R.T.; Yu, K. Mammalian target of rapamycin: Discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin. Oncol. 2009, 36, S3–S17. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.P.; Sehgal, S.N.; Vezina, C. Activity of rapamycin (AY-22,989) against transplanted tumors. J. Antibiot. 1984, 37, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J.; Rojo, F.; Salmena, L.; Alimonti, A.; Egia, A.; Sasaki, A.T.; Thomas, G.; Kozma, S.C.; et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-P.; Valenzuela, N.M.; Ziegler, M.E.; Rozengurt, E.; Reed, E.F. Everolimus inhibits anti-HLA I antibody-mediated endothelial cell signaling, migration and proliferation more potently than sirolimus. Am. J. Transplant. 2014, 14, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Soria, J. Targeted Therapies in Oncology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-84214-546-3. [Google Scholar]
- Martelli, A.M.; Buontempo, F.; McCubrey, J.A. Drug discovery targeting the mTOR pathway. Clin. Sci. 2018, 132, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, H.; Liu, T.; Wu, J.; Zhu, J.; Shen, X. Targeting the mTOR regulatory network in hepatocellular carcinoma: Are we making headway? BBA-Rev. Cancer 2019, 1871, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Rodrik-Outmezguine, V.S.; Chandarlapaty, S.; Pagano, N.C.; Poulikakos, P.I.; Scaltriti, M.; Moskatel, E.; Baselga, J.; Guichard, S.; Rosen, N. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011, 1, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [CrossRef]
- Liu, G.; Kuang, S.; Cao, R.; Wang, J.; Peng, Q.; Sun, C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019, 33, 10089–10103. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Manning, B.D. The TSC1-TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK and Raptor: Matching Cell Growth to Energy Supply. Mol. Cell 2008, 30, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Rudalska, R.; Dauch, D.; Longerich, T.; McJunkin, K.; Wuestefeld, T.; Kang, T.; Hohmeyer, A.; Pesic, M.; Leibold, J.; von Thun, A.; et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat. Med. 2014, 20, 1138–1146. [Google Scholar] [CrossRef]
- Llerena, S.; García-Díaz, N.; Curiel-Olmo, S.; Doblas, A.A.; García-Blanco, A.; Pisonero, H.; Varela, M.; Santibáñez, M.; Almaraz, C.; Cereceda, L.; et al. Applied diagnostics in liver cancer. Efficient combinations of sorafenib with targeted inhibitors blocking AKT/mTOR. Oncotarget 2018, 9, 30869–30882. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Hu, J.; Song, X.; Utpatel, K.; Zhang, Y.; Wang, P.; Lu, X.; Zhang, J.; Xu, M.; Su, T.; et al. Combined Treatment with MEK and mTOR Inhibitors is Effective in In Vitro and In Vivo Models of Hepatocellular Carcinoma. Cancers 2019, 11, 930. [Google Scholar] [CrossRef]
- Sieben, C.J.; Sturmlechner, I.; van de Sluis, B.; van Deursen, J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018, 28, 723–737. [Google Scholar] [CrossRef]
- Wang, C.; Vegna, S.; Jin, H.; Benedict, B.; Lieftink, C.; Ramirez, C.; de Oliveira, R.L.; Morris, B.; Gadiot, J.; Wang, W.; et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 2019, 574, 268–272. [Google Scholar] [CrossRef]
- Chresta, C.M.; Davies, B.R.; Hickson, I.; Harding, T.; Cosulich, S.; Critchlow, S.E.; Vincent, J.P.; Ellston, R.; Jones, D.; Sini, P.; et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010, 70, 288–298. [Google Scholar] [CrossRef]
- Finn, R.S.; Poon, R.; Yau, T.; Klümpen, H.; Chen, L.; Kang, Y.; Kim, T.; Gomez-Martin, C.; Rodriguez-Lope, C.; Kunz, T.; et al. Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J. Hepatol. 2013, 59, 1271–1277. [Google Scholar] [CrossRef]
- Koeberle, D.; Dufour, J.; Demeter, G.; Li, Q.; Ribi, K.; Samaras, P.; Saletti, P.; Roth, A.D.; Horber, D.; Buehlmann, M.; et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): A randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann. Oncol. 2016, 27, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.; Lim, H.Y.; Poon, R.T.P.; Blanc, J.; Vogel, A.; Chen, C.; et al. Effect of Everolimus on Survival in Advanced Hepatocellular Carcinoma After Failure of Sorafenib: The EVOLVE-1 Randomized Clinical Trial. JAMA 2014, 312, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Pomfret, E.A. Killing Two Birds with One Stone: Antineoplastic Immunosuppression. Transplantation 2016, 100, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Wiesner, R.H.; Trotter, J.F.; Klintmalm, G.; Katz, E.; Maller, E.; Roberts, J.; Kneteman, N.; Teperman, L.; Fung, J.J.; et al. De novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: The 2000-2003 phase II prospective randomized trial. Am. J. Transplant. 2014, 14, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Guerrero-Misas, M.; Thorburn, D.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Maintenance immunosuppression for adults undergoing liver transplantation: A network meta-analysis. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; Jonas, S.; Sudan, D.; Fung, J.; Fischer, L.; et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: A randomized controlled trial. Am. J. Transplant. 2012, 12, 3008–3020. [Google Scholar] [CrossRef]
- Sterneck, M.; Kaiser, G.M.; Heyne, N.; Richter, N.; Rauchfuss, F.; Pascher, A.; Schemmer, P.; Fischer, L.; Klein, C.G.; Nadalin, S.; et al. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomized trial in liver transplantation. Am. J. Transplant. 2014, 14, 701–710. [Google Scholar] [CrossRef]
- Cholongitas, E.; Mamou, C.; Rodríguez-Castro, K.I.; Burra, P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: A systematic review. Transpl. Int. 2014, 27, 1039–1049. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Guerrero, M.; Barrera, L.; Ferrín, G.; Álamo, J.M.; Ayllón, M.D.; Artacho, G.S.; Montero, J.L.; Briceño, J.; Bernal, C.; et al. Impact of Early Initiated Everolimus on the Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Transplantation 2018, 102, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Geissler, E.K.; Schnitzbauer, A.A.; Zülke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.-W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients with Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Amado, V.; de la Mata, M. Letter: Mechanistic target of rapamycin inhibitors as adjuvant therapy for patients with hepatocellular carcinoma undergoing liver transplantation. Aliment. Pharmacol. Ther. 2019, 50, 478–479. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).