Blood Metabolite Signatures of Metabolic Syndrome in Two Cross-Cultural Older Adult Cohorts
Abstract
1. Introduction
2. Results
2.1. Participants
2.2. Metabolite Concentrations: BLSA versus TMCS Differences
2.3. Associations with Metabolic Risk Factors
3. Discussion
4. Methods and Materials
4.1. Participants
4.2. Blood Samples
4.3. Other Outcomes
4.4. Metabolites
4.5. Metabolic Syndrome Risk Factors
4.6. Additional Demographic Variables and Covariates
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef]
- Birnbaum, H.G.; Mattson, M.E.; Kashima, S.; Williamson, T.E. Prevalence rates and costs of metabolic syndrome and associated risk factors using employees’ integrated laboratory data and health care claims. J. Occup. Environ. Med. 2011, 53, 27–33. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J. Exceptional Human Longevity. Mayo Clin. Proc. 2019, 94, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Foley, D.; White, L.; Burchfiel, C.M.; Curb, J.D.; Petrovitch, H.; Ross, G.W.; Havlik, R.J.; Launer, L.J. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2255–2260. [Google Scholar] [CrossRef]
- Ueshima, H.; Okayama, A.; Saitoh, S.; Nakagawa, H.; Rodriguez, B.; Sakata, K.; Okuda, N.; Choudhury, S.R.; Curb, J.D.; Group, I.R. Differences in cardiovascular disease risk factors between Japanese in Japan and Japanese-Americans in Hawaii: The INTERLIPID study. J. Hum. Hypertension 2003, 17, 631–639. [Google Scholar] [CrossRef]
- Yeom, J.; Kim, J.K.; Crimmins, E.M. Factors Associated with Body Mass Index (BMI) Among Older Adults: A Comparison Study of the U.S., Japan, and Korea. Hanguk Nonyonhak 2009, 29, 1479–1500. [Google Scholar]
- Obika, M.; Trence, D.L. Comparison of type 2 diabetes care in the United States and Japan. Endocr. Pract. 2010, 16, 707–711. [Google Scholar] [CrossRef]
- Fujiyoshi, A.; Miura, K.; Ohkubo, T.; Kadowaki, T.; Kadowaki, S.; Zaid, M.; Hisamatsu, T.; Sekikawa, A.; Budoff, M.J.; Liu, K.; et al. Cross-sectional comparison of coronary artery calcium scores between Caucasian men in the United States and Japanese men in Japan: The multi-ethnic study of atherosclerosis and the Shiga epidemiological study of subclinical atherosclerosis. Am. J. Epidemiol. 2014, 180, 590–598. [Google Scholar] [CrossRef]
- Comstock, G.W.; Suzuki, T.; Stone, R.W.; Crumrine, J.L.; Johnson, D.H.; Sakai, Y.; Matsuya, T.; Sasaki, S. Cardiovascular risk factors in American and Japanese executives. Telecom Health Research Group. J. R. Soc. Med. 1985, 78, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Namekata, T.; Moore, D.; Knopp, R.; Marcovina, S.; Perrin, E.; Hughes, D.; Suzuki, K.; Mori, M.; Sempos, C.; Hatano, S.; et al. Cholesterol levels among Japanese Americans and other populations: Seattle Nikkei Health Study. J. Atheroscler. Thromb. 1996, 3, 105–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imazu, M.; Sumida, K.; Yamabe, T.; Yamamoto, H.; Ueda, H.; Hattori, Y.; Miyauchi, A.; Hara, H.; Yamakido, M. A comparison of the prevalence and risk factors of high blood pressure among Japanese living in Japan, Hawaii, and Los Angeles. Public Health Rep. 1996, 111 (Suppl. 2), 59–61. [Google Scholar] [PubMed]
- Saito, Y.; Davarian, S.; Takahashi, A.; Schneider, E.; Crimmins, E.M. Diagnosis and Control of Hypertension in the Elderly Populations of Japan and the United States. Int. J. Popul. Stud. 2015, 1, 19–28. [Google Scholar] [CrossRef]
- Shiwa, M.; Yoneda, M.; Nakanishi, S.; Oki, K.; Yamane, K.; Kohno, N. Japanese lifestyle during childhood prevents the future development of obesity among Japanese-Americans. PLoS ONE 2015, 10, e0120804. [Google Scholar] [CrossRef]
- van Valkengoed, I.G.M.; Argmann, C.; Ghauharali-van der Vlugt, K.; Aerts, J.; Brewster, L.M.; Peters, R.J.G.; Vaz, F.M.; Houtkooper, R.H. Ethnic differences in metabolite signatures and type 2 diabetes: A nested case-control analysis among people of South Asian, African and European origin. Nutr. Diabetes 2017, 7, 300. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Wurtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef]
- Patel, M.J.; Batch, B.C.; Svetkey, L.P.; Bain, J.R.; Turer, C.B.; Haynes, C.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Shah, S.H. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. OMICS 2013, 17, 627–635. [Google Scholar] [CrossRef]
- Liver Function Tests. Available online: https://www.mayoclinic.org/tests-procedures/liver-function-tests/about/pac-20394595 (accessed on 1 December 2019).
- Senauer, B.; Gemma, M. Why Is the Obesity Rate So Low in Japan and High in the U.S.? Some Possible Economic Explanations; Minnesota, U.O., Ed.; The Food Industry Center: Saint Paul, MN, USA, 2006; p. 26. [Google Scholar]
- Akhlaghi, M. Dietary Approaches to Stop Hypertension (DASH): Potential mechanisms of action against risk factors of the metabolic syndrome. Nutr. Res. Rev. 2019, 1–18. [Google Scholar] [CrossRef]
- Joyce, B.T.; Wu, D.; Hou, L.; Dai, Q.; Castaneda, S.F.; Gallo, L.C.; Talavera, G.A.; Sotres-Alvarez, D.; Van Horn, L.; Beasley, J.M.; et al. DASH diet and prevalent metabolic syndrome in the Hispanic Community Health Study/Study of Latinos. Prev. Med. Rep. 2019, 15, 100950. [Google Scholar] [CrossRef]
- Ghorabi, S.; Salari-Moghaddam, A.; Daneshzad, E.; Sadeghi, O.; Azadbakht, L.; Djafarian, K. Association between the DASH diet and metabolic syndrome components in Iranian adults. Diabetes Metab. Syndr. 2019, 13, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.L.; Hagedorn, A.; Yeom, J.; Saito, Y.; Yokoyama, E.; Crimmins, E.M. A tale of two countries—The United States and Japan: Are differences in health due to differences in overweight? J. Epidemiol. 2008, 18, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.; Byrne, C.D. Bidirectional Relationships and Disconnects between NAFLD and Features of the Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Ren, J.; Pulakat, L.; Whaley-Connell, A.; Sowers, J.R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. (Berlin) 2010, 88, 993–1001. [Google Scholar] [CrossRef]
- Supale, S.; Li, N.; Brun, T.; Maechler, P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol. Metab. 2012, 23, 477–487. [Google Scholar] [CrossRef]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Kedenko, L.; Paulweber, B.; Stadlmayr, A.; Huber-Schonauer, U.; Niederseer, D.; Stickel, F.; Auer, S.; et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non-alcoholic Fatty Liver. Am. J. Gastroenterol. 2017, 112, 102–110. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guenard, F.; Garneau, V.; Cormier, H.; Barbier, O.; Perusse, L.; Vohl, M.C. Association between Metabolite Profiles, Metabolic Syndrome and Obesity Status. Nutrients 2016, 8, 324. [Google Scholar] [CrossRef]
- Lo, C.J.; Tang, H.Y.; Huang, C.Y.; Lin, C.M.; Ho, H.Y.; Shiao, M.S.; Cheng, M.L. Metabolic Signature Differentiated Diabetes Mellitus from Lipid Disorder in Elderly Taiwanese. J. Clin. Med. 2018, 8, 13. [Google Scholar] [CrossRef]
- Yang, S.J.; Kwak, S.Y.; Jo, G.; Song, T.J.; Shin, M.J. Serum metabolite profile associated with incident type 2 diabetes in Koreans: Findings from the Korean Genome and Epidemiology Study. Sci. Rep. 2018, 8, 8207. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; De Schrijver, E.; Verhoeven, G.; Swinnen, J.V. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005, 65, 6719–6725. [Google Scholar] [CrossRef] [PubMed]
- Cowart, L.A. Sphingolipids and Metabolic Disease; Cowart, L.A., Ed.; Springer: New York, NY, USA, 2011; Volume 721, p. 152. [Google Scholar]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Bullo, M.; Ruiz-Canela, M.; Dennis, C.; Deik, A.; Wang, D.; Guasch-Ferre, M.; Yu, E.; Razquin, C.; Corella, D.; et al. Plasma metabolites predict both insulin resistance and incident type 2 diabetes: A metabolomics approach within the Prevencion con Dieta Mediterranea (PREDIMED) study. Am. J. Clin. Nutr. 2019, 109, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Arnold, M.; Kastenmuller, G.; Chang, R.; Baillie, R.A.; Han, X.; Thambisetty, M.; Tenenbaum, J.D.; Suhre, K.; Thompson, J.W.; et al. Metabolic network failures in Alzheimer’s disease: A biochemical road map. Alzheimers Dement. 2017, 13, 965–984. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Misialek, J.R.; Boerwinkle, E.; Gottesman, R.F.; Sharrett, A.R.; Mosley, T.H.; Coresh, J.; Wruck, L.M.; Knopman, D.S.; Alonso, A. Prospective associations of plasma phospholipids and mild cognitive impairment/dementia among African Americans in the ARIC Neurocognitive Study. Alzheimers Dement. (Amsterdam) 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Mathews, A.T.; Famodu, O.A.; Olfert, M.D.; Murray, P.J.; Cuff, C.F.; Downes, M.T.; Haughey, N.J.; Colby, S.E.; Chantler, P.D.; Olfert, I.M.; et al. Efficacy of nutritional interventions to lower circulating ceramides in young adults: FRUVEDomic pilot study. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Knebel, B.; Strassburger, K.; Szendroedi, J.; Kotzka, J.; Scheer, M.; Nowotny, B.; Mussig, K.; Lehr, S.; Pacini, G.; Finner, H.; et al. Specific Metabolic Profiles and Their Relationship to Insulin Resistance in Recent-Onset Type 1 and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2130–2140. [Google Scholar] [CrossRef]
- Kamaura, M.; Nishijima, K.; Takahashi, M.; Ando, T.; Mizushima, S.; Tochikubo, O. Lifestyle modification in metabolic syndrome and associated changes in plasma amino acid profiles. Circ. J. 2010, 74, 2434–2440. [Google Scholar] [CrossRef]
- Nakamura, H.; Jinzu, H.; Nagao, K.; Noguchi, Y.; Shimba, N.; Miyano, H.; Watanabe, T.; Iseki, K. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 2014, 4, e133. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Han, Q.; Liu, Y.; Sun, C.; Gang, X.; Wang, G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef] [PubMed]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. (London) 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Harada, S.; Kurihara, A.; Fukai, K.; Kuwabara, K.; Sugiyama, D.; Takeuchi, A.; Okamura, T.; Akiyama, M.; Nishiwaki, Y.; et al. Profiling of plasma metabolites in postmenopausal women with metabolic syndrome. Menopause 2016, 23, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvado, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O’Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J. Clin. Investig. 2013, 123, 4309–4317. [Google Scholar] [CrossRef]
- Ferrucci, L. The Baltimore Longitudinal Study of Aging (BLSA): A 50-year-long journey and plans for the future. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1416–1419. [Google Scholar] [CrossRef]
- Resnick, S.M.; Pham, D.L.; Kraut, M.A.; Zonderman, A.B.; Davatzikos, C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J. Neurosci. 2003, 23, 3295–3301. [Google Scholar] [CrossRef]
- Harada, S.; Takebayashi, T.; Kurihara, A.; Akiyama, M.; Suzuki, A.; Hatakeyama, Y.; Sugiyama, D.; Kuwabara, K.; Takeuchi, A.; Okamura, T.; et al. Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Environ. Health Prev. Med. 2016, 21, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Casanova, R.; Varma, S.; Simpson, B.; Kim, M.; An, Y.; Saldana, S.; Riveros, C.; Moscato, P.; Griswold, M.; Sonntag, D.; et al. Blood metabolite markers of preclinical Alzheimer’s disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement. 2016, 12, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C., Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch. Intern. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.R.; Carey, W.D. Liver Test Interpretation—Approach to the Patient with Liver Disease: A Guide to Commonly Used Liver Tests. Available online: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/ (accessed on 1 December 2019).
- Cortez-Pinto, H.; Camilo, M.E.; Baptista, A.; De Oliveira, A.G.; De Moura, M.C. Non-alcoholic fatty liver: Another feature of the metabolic syndrome? Clin. Nutr. 1999, 18, 353–358. [Google Scholar] [CrossRef]
- Souza, M.R.; Diniz Mde, F.; Medeiros-Filho, J.E.; Araujo, M.S. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq. Gastroenterol. 2012, 49, 89–96. [Google Scholar] [CrossRef]
- Verbrugge, L.M.; Gruber-Baldini, A.L.; Fozard, J.L. Age differences and age changes in activities: Baltimore Longitudinal Study of Aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 1996, 51, S30–S41. [Google Scholar] [CrossRef]
- Fujii, H.; Yamamoto, S.; Takeda-Imai, F.; Inoue, M.; Tsugane, S.; Kadowaki, T.; Noda, M. Validity and applicability of a simple questionnaire for the estimation of total and domain-specific physical activity. Diabetol. Int. 2011, 2, 47–54. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
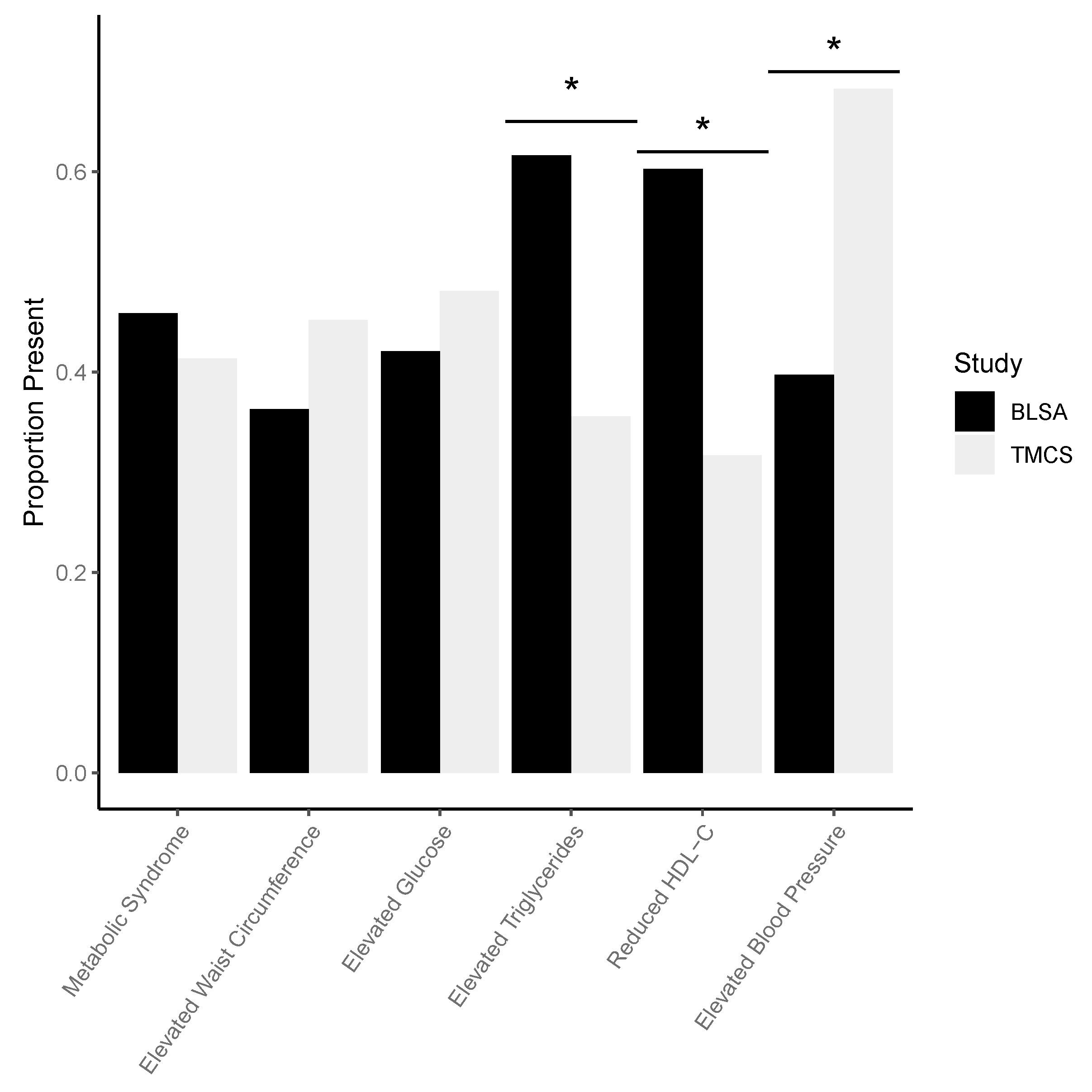

| Demographic Variable | BLSA (n = 146) | TMCS (n = 104) | p Value |
|---|---|---|---|
| Female, n (%) | 77 (52.74) | 66 (63.46) | 0.0913 |
| White, n (%) | 112 (76.71) | - | - |
| Storage time (years), Mean (SD) | 4.36 (2.33) | - | - |
| Age (years), Mean (SD) | 76.08 (8.54) | 69.67 (2.57) | <0.0001 |
| Never smoke, n (%) | 74 (50.68) | 74 (71.15) | 0.0012 |
| DASH (no Mg), Mean (SD) | 2.62 (0.78) | 2.80 (1.21) | 0.1459 |
| Drinker, n (%) | 128 (88.28) | 41 (39.42) | * |
| Physical Activity (METs/week), Mean (SD) | 77.77 (66.01) | 17.77 (15.32) | * |
| Metabolic syndrome | |||
| Metabolic syndrome, n (%) | 67 (45.89) | 43 (41.35) | 0.4756 |
| Waist circumference | |||
| Elevated waist circumference, n (%) | 53 (36.3) | 47 (45.19) | 0.1573 |
| Waist circumference (in), Mean (SD) | 35.82 (4.65) | 32.43 (3.37) | <0.0001 |
| Fasting glucose | |||
| Elevated fasting glucose, n (%) | 61 (42.07) | 50 (48.08) | 0.3469 |
| Fasting glucose (mg/dL), Mean (SD) | 101.04 (20.68) | 102.3 (15.03) | 0.5794 |
| Diabetes drug use, n (%) | 14 (9.72) | 9 (8.65) | 0.7747 |
| Triglycerides | |||
| Elevated triglyceride level, n (%) | 90 (61.64) | 37 (35.58) | <0.0001 |
| Triglyceride level (mg/dL), Mean (SD) | 97.88 (42.15) | 102.4 (60.05) | 0.5092 |
| LMT use, n (%) | 82 (56.94) | 29 (27.88) | <0.0001 |
| HDL cholesterol | |||
| Reduced HDL cholesterol, n (%) | 88 (60.27) | 33 (31.73) | <0.0001 |
| HDL cholesterol (mg/dL), Mean (SD) | 61.36 (15.12) | 68.97 (23.76) | 0.0046 |
| LMT drug use, n (%) | 82 (56.94) | 29 (27.88) | <.0001 |
| Blood Pressure | |||
| Elevated Blood Pressure, n (%) | 58 (39.73) | 71 (68.27) | <0.0001 |
| SBP (mm Hg), Mean (SD) | 114.32 (12.88) | 130.87 (16.95) | <0.0001 |
| DBP (mm Hg), Mean (SD) | 64.32 (9.06) | 74.83 (10.28) | <0.0001 |
| Hypertension drug use, n (%) | 44 (30.56) | 46 (44.23) | 0.0271 |
| Liver Enzymes | |||
| Aspartate aminotransferase (AST) (U/L), Mean (SD) | 27.11 (10.25) | 20.57 (11.17) | 0.0059 |
| Alanine aminotransferase (ALT) (U/L), Mean (SD) | 21.73 (8.88) | 24.59 (7.37) | <0.0001 |
| Metabolite | Category | BLSA | TMCS | Direction of Assoc. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio Estimate | Lower 95% CI | Upper 95% CI | p Value | FDR-Adjusted p Value | Odds Ratio Estimate | Lower 95% CI | Upper 95% CI | p Value | FDR-Adjusted p Value | |||
| Met S | ||||||||||||
| H1 * | Hexoses | 3.4 | 1.863 | 6.207 | <0.0001 | <0.0001 | 1.918 | 1.121 | 3.28 | 0.0174 | 0.01743 | + |
| PC ae C34:2 * | Phosphatidylcholines-acyl-alkyl | 0.246 | 0.129 | 0.468 | <0.0001 | 0.00014 | 0.24 | 0.108 | 0.533 | <0.0001 | 0.01429 | - |
| PC ae C34:3 * | Phosphatidylcholines-acyl-alkyl | 0.249 | 0.128 | 0.484 | <0.0001 | 0.00019 | 0.285 | 0.136 | 0.598 | <0.0001 | 0.01429 | - |
| PC ae C36:3 * | Phosphatidylcholines-acyl-alkyl | 0.221 | 0.112 | 0.436 | <0.0001 | 0.00012 | 0.326 | 0.163 | 0.654 | 0.0016 | 0.01429 | - |
| PC ae C38:2 * | Phosphatidylcholines-acyl-alkyl | 0.218 | 0.114 | 0.417 | <0.0001 | <0.0001 | 0.298 | 0.142 | 0.623 | 0.0013 | 0.01429 | - |
| SM C16:0 * | Sphingomyelins | 0.339 | 0.191 | 0.602 | 0.0002 | 0.00299 | 0.369 | 0.196 | 0.697 | 0.0021 | 0.02956 | - |
| Metabolite | Category | BLSA | TMCS | Direction of Assoc. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio Estimate | Lower 95% CI | Upper 95% CI | p Value | FDR-Adjusted p Value | Odds Ratio Estimate | Lower 95% CI | Upper 95% CI | p Value | FDR-Adjusted p Value | |||
| Glucose | ||||||||||||
| H1* | Hexoses | 24.297 | 7.99 | 73.892 | <0.0001 | <0.0001 | 70.821 | 12.211 | 410.741 | <0.0001 | <0.0001 | + |
| HDL | ||||||||||||
| PC ae C32:1 * | Phosphatidylcholines-acyl-alkyl | 0.271 | 0.142 | 0.515 | <0.0001 | 0.00125 | 0.405 | 0.208 | 0.788 | 0.0078 | 0.04004 | - |
| PC ae C34:2 * | Phosphatidylcholines-acyl-alkyl | 0.417 | 0.241 | 0.721 | 0.0018 | 0.01105 | 0.286 | 0.129 | 0.637 | 0.0022 | 0.03163 | - |
| PC ae C34:3 * | Phosphatidylcholines-acyl-alkyl | 0.455 | 0.263 | 0.785 | 0.0047 | 0.01369 | 0.253 | 0.109 | 0.586 | 0.0013 | 0.03163 | - |
| PC ae C36:3 * | Phosphatidylcholines-acyl-alkyl | 0.363 | 0.2 | 0.657 | 0.0008 | 0.00609 | 0.331 | 0.157 | 0.695 | 0.0035 | 0.03163 | - |
| PC ae C38:2 * | Phosphatidylcholines-acyl-alkyl | 0.285 | 0.156 | 0.52 | <0.0001 | 0.00125 | 0.309 | 0.143 | 0.67 | 0.0029 | 0.03163 | - |
| PC ae C40:1 * | Phosphatidylcholines-acyl-alkyl | 0.474 | 0.283 | 0.794 | 0.0046 | 0.01349 | 0.388 | 0.197 | 0.765 | 0.0063 | 0.03755 | - |
| PC ae C42:3 * | Phosphatidylcholines-acyl-alkyl | 0.433 | 0.243 | 0.773 | 0.0046 | 0.01349 | 0.385 | 0.195 | 0.761 | 0.006 | 0.03755 | - |
| PC ae C44:4 * | Phosphatidylcholines-acyl-alkyl | 0.561 | 0.357 | 0.88 | 0.0119 | 0.02359 | 0.402 | 0.2 | 0.807 | 0.0104 | 0.04673 | - |
| SM C16:0 * | Sphingomyelins | 0.407 | 0.236 | 0.703 | 0.0013 | 0.01741 | 0.293 | 0.144 | 0.594 | <0.0001 | 0.00934 | - |
| SM C16:1 * | Sphingomyelins | 0.448 | 0.245 | 0.816 | 0.0087 | 0.02928 | 0.419 | 0.218 | 0.805 | 0.0091 | 0.04231 | - |
| SM C24:1* | Sphingomyelins | 0.42 | 0.237 | 0.745 | 0.003 | 0.02039 | 0.376 | 0.194 | 0.73 | 0.0039 | 0.02695 | - |
| Triglycerides | ||||||||||||
| PC ae C34:2 * | Phosphatidylcholines-acyl-alkyl | 0.437 | 0.252 | 0.758 | 0.0032 | 0.01378 | 0.3 | 0.141 | 0.641 | 0.0019 | 0.03356 | - |
| PC ae C34:3 | Phosphatidylcholines-acyl-alkyl | 0.535 | 0.313 | 0.914 | 0.0221 | 0.04221 | 0.294 | 0.138 | 0.63 | 0.0016 | 0.03356 | - |
| PC ae C38:2 * | Phosphatidylcholines-acyl-alkyl | 0.301 | 0.165 | 0.55 | <0.0001 | 0.00343 | 0.344 | 0.168 | 0.708 | 0.0037 | 0.04498 | - |
| SM C16:0 | Sphingomyelins | 0.446 | 0.26 | 0.766 | 0.0034 | 0.04703 | 0.328 | 0.168 | 0.638 | 0.001 | 0.00722 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajan, U.V.; Varma, V.R.; Huang, C.-W.; An, Y.; Tanaka, T.; Ferrucci, L.; Takebayashi, T.; Harada, S.; Iida, M.; Legido-Quigley, C.; et al. Blood Metabolite Signatures of Metabolic Syndrome in Two Cross-Cultural Older Adult Cohorts. Int. J. Mol. Sci. 2020, 21, 1324. https://doi.org/10.3390/ijms21041324
Mahajan UV, Varma VR, Huang C-W, An Y, Tanaka T, Ferrucci L, Takebayashi T, Harada S, Iida M, Legido-Quigley C, et al. Blood Metabolite Signatures of Metabolic Syndrome in Two Cross-Cultural Older Adult Cohorts. International Journal of Molecular Sciences. 2020; 21(4):1324. https://doi.org/10.3390/ijms21041324
Chicago/Turabian StyleMahajan, Uma V., Vijay R. Varma, Chiung-Wei Huang, Yang An, Toshiko Tanaka, Luigi Ferrucci, Toru Takebayashi, Sei Harada, Miho Iida, Cristina Legido-Quigley, and et al. 2020. "Blood Metabolite Signatures of Metabolic Syndrome in Two Cross-Cultural Older Adult Cohorts" International Journal of Molecular Sciences 21, no. 4: 1324. https://doi.org/10.3390/ijms21041324
APA StyleMahajan, U. V., Varma, V. R., Huang, C.-W., An, Y., Tanaka, T., Ferrucci, L., Takebayashi, T., Harada, S., Iida, M., Legido-Quigley, C., & Thambisetty, M. (2020). Blood Metabolite Signatures of Metabolic Syndrome in Two Cross-Cultural Older Adult Cohorts. International Journal of Molecular Sciences, 21(4), 1324. https://doi.org/10.3390/ijms21041324





