Characterization of a Unique Bordetella bronchiseptica vB_BbrP_BB8 Bacteriophage and Its Application as an Antibacterial Agent
Abstract
1. Introduction
2. Results
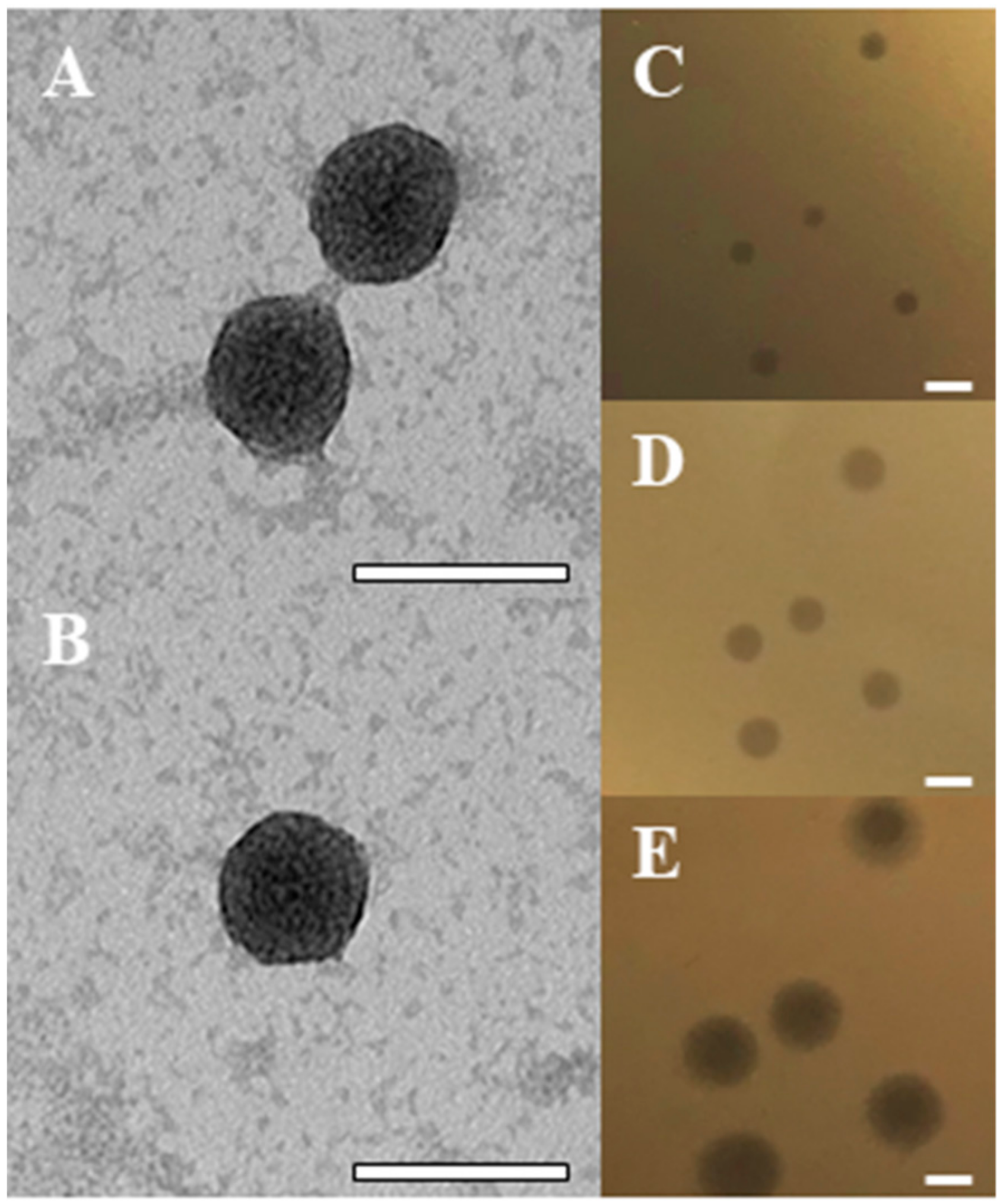
2.1. Isolation of the BB8 Phage
2.2. Phage BB8 Resistance to Physico-Chemical Factors
2.3. Adsorption, One-Step Growth and Collapse Assays
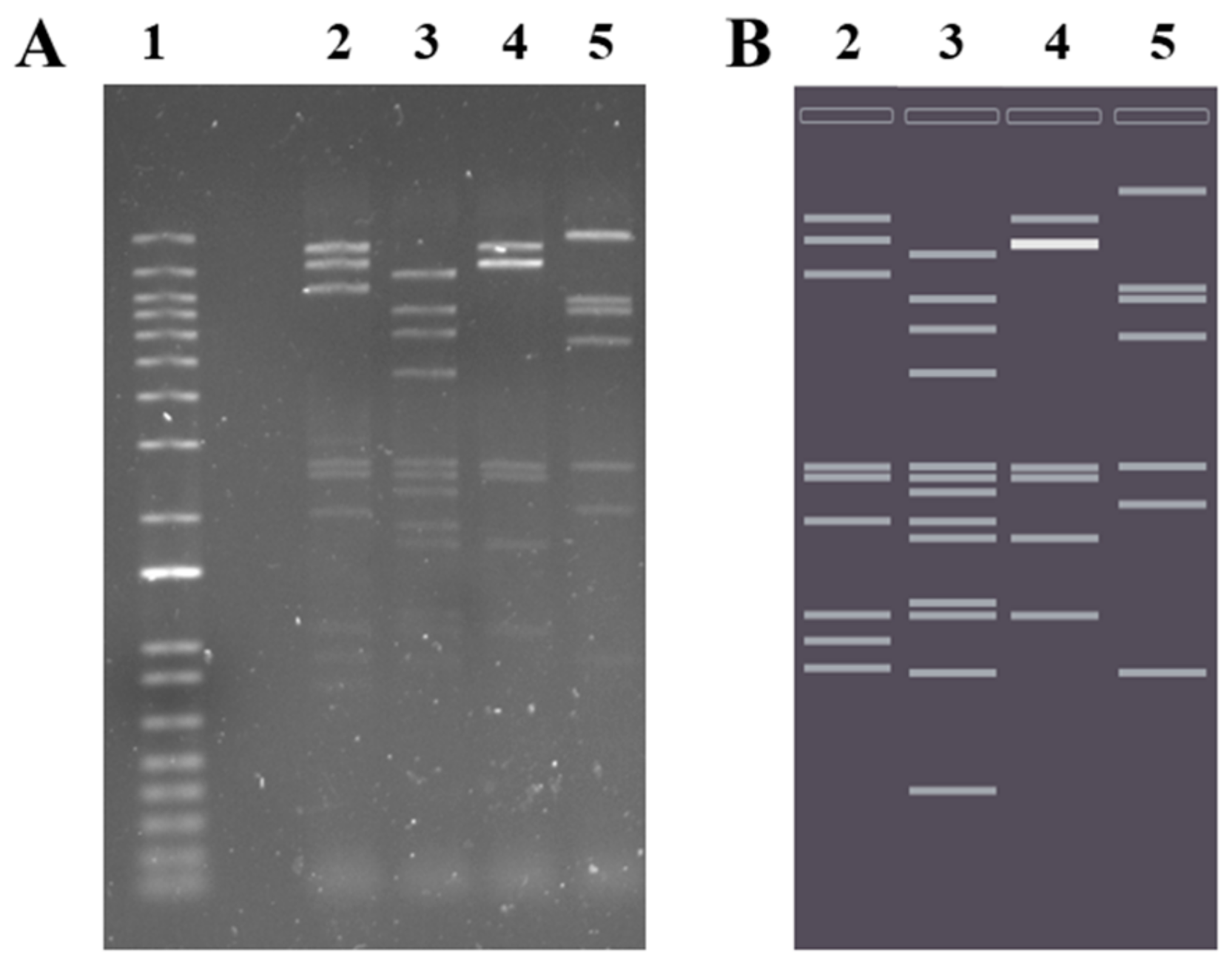
2.4. Phage Genome Exploration
2.5. Applicability of BB8 Phage as an Antibacterial Agent
3. Discussion
4. Materials and Methods
4.1. Sample Origin
4.2. Bacterial Host and Culture Conditions
4.3. Phage Isolation
4.4. Phage Propagation and Purification
4.5. Thermal and pH Stability of the Phage
4.6. Phage Resistance to UV Light
4.7. Transmission Electron Microscopy (TEM)
4.8. Adsorption Kinetics
4.9. One-Step Growth Curve
4.10. Bacterial Culture Collapse
4.11. Biofilm Formation and Assessment of Phage Lytic Activity in Biofilm
4.12. Scanning Electron Microscopy (SEM)
4.13. In Vivo Assessment of Phage Activity in B. bronchiseptica-Infected Galleria mellonella Larvae
4.14. Phage DNA Extraction and Restriction Enzyme Digestion
4.15. Whole Genome Sequencing (WGS)
4.16. Bioinformatics
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BHI | Brain heart infusion |
| CFU | Colony forming unit |
| CRAB | Carbapenem resistant Acinetobacter baumannii |
| HSP | High scoring pair |
| IC | Infection center |
| LB | Lysogeny Broth |
| MALDI | Matrix-assisted laser desorption/ionization |
| MOI | Multiplicity of infection |
| NCBI | National Center for Biotechnology Information |
| PCR | Polymerase chain reaction |
| PFU | Plaque forming units |
| RNAP | RNA Polymerase |
| RT | Room temperature |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TOF | Time-of-flight |
References
- Cattelan, N.; Dubey, P.; Arnal, L.; Yantorno, O.M.; Deora, R. Bordetella biofilms: A lifestyle leading to persistent infections. Pathog. Dis. 2016, 74, ftv108. [Google Scholar] [CrossRef]
- Datz, C. Bordetella Infections in Dogs and Cats: Pathogenesis, Clinical Signs, and Diagnosis. Compend. Contin. Educ. Pract. Vet. N. Am. Ed. 2003, 25, 896–901. [Google Scholar]
- Dawson, S.; Jones, D.; McCracken, C.M.; Gaskell, R.M.; Hart, C.A.; Gaskell, C.J. Bordetella bronchiseptica infection in cats following contact with infected dogs. Vet. Rec. 2000, 146, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B. Emerging and Re-emerging zoonoses of dogs and cats. Animals 2014, 4, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Ner, Z.; Ross, L.A.; Horn, M.V.; Keens, T.G.; MacLaughlin, E.F.; Starnes, V.A.; Woo, M.S. Bordetella bronchiseptica infection in pediatric lung transplant recipients. Pediatr. Transplant. 2003, 7, 413–417. [Google Scholar] [CrossRef]
- Wernli, D.; Emonet, S.; Schrenzel, J.; Harbarth, S. Evaluation of eight cases of confirmed Bordetella bronchiseptica infection and colonization over a 15-year period. Clin. Microbiol. Infect. 2011, 17, 201–203. [Google Scholar] [CrossRef]
- Monti, M.; Diano, D.; Allegrini, F.; Delmonte, A.; Fausti, V.; Cravero, P.; Marcantognini, G.; Frassineti, G.L. Bordetella bronchiseptica pneumonia in a patient with lung cancer; a case report of a rare infection. BMC Infect. Dis. 2017, 17, 644. [Google Scholar] [CrossRef]
- Trainor, E.A.; Nicholson, T.L.; Merkel, T.J. Bordetella pertussis transmission. Pathog. Dis. 2015, 73, ftv068. [Google Scholar] [CrossRef]
- Egberink, H.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Bordetella Bronchiseptica Infection in Cats: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 610–614. [Google Scholar] [CrossRef]
- Brockmeier, S.L.; Register, K.B.; Magyar, T.; Lax, A.J.; Pullinger, G.D.; Kunkle, R.A. Role of the dermonecrotic toxin of Bordetella bronchiseptica in the pathogenesis of respiratory disease in swine. Infect. Immun. 2002, 70, 481–490. [Google Scholar] [CrossRef]
- Kureljušić, B.; Weissenbacher-Lang, C.; Nedorost, N.; Stixenberger, D.; Weissenböck, H. Association between Pneumocystis spp. and co-infections with Bordetella bronchiseptica, Mycoplasma hyopneumoniae and Pasteurella multocida in Austrian pigs with pneumonia. Vet. J. 2016, 207, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Stepniewska, K.; Markowska-Daniel, I. Occurrence of genes encoding virulence factors in Bordetella bronchiseptica strains isolated from infected and healthy pigs. Bull. Vet. Inst. Pulawy 2012, 56, 483–487. [Google Scholar] [CrossRef][Green Version]
- Chanter, N.; Magyar, T.; Rutter, J.M. Interactions between Bordetella bronchiseptica and toxigenic Pasteurella multocida in atrophic rhinitis of pigs. Res. Vet. Sci. 1989, 47, 48–53. [Google Scholar] [CrossRef]
- Ellis, J.A. How well do vaccines for Bordetella bronchiseptica work in dogs? A critical review of the literature 1977–2014. Vet. J. 2015, 204, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, K.; Schwarz, S. Antimicrobial Resistance in Bordetella bronchiseptica. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Gueirard, P.; Weber, C.; Le Coustumier, A.; Guiso, N. Human Bordetella bronchiseptica infection related to contact with infected animals: Persistence of bacteria in host. J. Clin. Microbiol. 1995, 33, 2002–2006. [Google Scholar] [CrossRef]
- Cisek, A.A.; Dąbrowska, I.; Gregorczyk, K.P.; Wyżewski, Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years After the Discovery of Bacteriophages. Curr. Microbiol. 2017, 74, 277–283. [Google Scholar] [CrossRef]
- Petrovic, A.; Kostanjsek, R.; Rakhely, G.; Knezevic, P. The First Siphoviridae Family Bacteriophages Infecting Bordetella bronchiseptica Isolated from Environment. Microb. Ecol. 2017, 73, 368–377. [Google Scholar] [CrossRef]
- Liu, M.; Deora, R.; Doulatov, S.R.; Gingery, M.; Eiserling, F.A.; Preston, A.; Maskell, D.J.; Simons, R.W.; Cotter, P.A.; Parkhill, J.; et al. Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 2002, 295, 2091–2094. [Google Scholar] [CrossRef]
- Joo, J.; Gunny, M.; Cases, M.; Hudson, P.; Albert, R.; Harvill, E. Bacteriophage-mediated competition in Bordetella bacteria. Proc. R. Soc. B Biol. Sci. 2006, 273, 1843–1848. [Google Scholar] [CrossRef]
- Rauch, H.C.; Pickett, M.J. Bordetella bronchiseptica bacteriophage. Can. J. Microbiol. 1961, 7, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Holzmayer, T.A.; Karataev, G.I.; Rozinov, M.N.; Moskvina, I.L.; Shumakov, Y.L.; Motin, V.L.; Mebel, S.M.; Gershanovich, V.N.; Lapaeva, I.A. Bacteriophages of Bordetella sp.: Features of lysogeny and conversion. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1988, 269, 147–155. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Sun, E.; Song, J.; Wu, B. Characterisation of a newly detected bacteriophage infecting Bordetella bronchiseptica in swine. Arch. Virol. 2019, 164, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Twest, R.; Kropinski, A.M. Bacteriophage Enrichment from Water and Soil. In Bacteriophages. Methods in Molecular Biology™; Clokie, M.R., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 15–21. [Google Scholar]
- Adriaenssens, E.M.; Rodney Brister, J. How to Name and Classify Your Phage: An Informal Guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, U.; Liu, M.; Tomida, S.; Park, J.; Souda, P.; Whitelegge, J.; Li, H.; Harvill, E.T.; Parkhill, J.; Miller, J.F. Phenotypic and genomic analysis of hypervirulent human-associated Bordetella bronchiseptica. BMC Microbiol. 2012, 12, 167. [Google Scholar] [CrossRef]
- Speakman, A.J.; Dawson, S.; Corkill, J.E.; Binns, S.H.; Hart, C.A.; Gaskell, R.M. Antibiotic susceptibility of canine Bordetella bronchiseptica isolates. Vet. Microbiol. 2000, 71, 193–200. [Google Scholar] [CrossRef]
- Kropinski, A.M. Bacteriophage research—What we have learnt and what still needs to be addressed. Res. Microbiol. 2018, 169, 481–487. [Google Scholar] [CrossRef]
- Hansen, M.F.; Svenningsen, S.L.; Røder, H.L.; Middelboe, M.; Burmølle, M. Big Impact of the Tiny: Bacteriophage–Bacteria Interactions in Biofilms. Trends Microbiol. 2019, 27, 739–752. [Google Scholar] [CrossRef]
- Brennan, M.; Thomas, D.Y.; Whiteway, M.; Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002, 34, 153–157. [Google Scholar] [CrossRef]
- Maguire, R.; Duggan, O.; Kavanagh, K. Evaluation of Galleria mellonella larvae as an in vivo model for assessing the relative toxicity of food preservative agents. Cell Biol. Toxicol. 2016, 32, 209–216. [Google Scholar] [CrossRef]
- Seed, K.D.; Dennis, J.J. Experimental bacteriophage therapy increases survival of Galleria mellonella larvae infected with clinically relevant strains of the Burkholderia cepacia complex. Antimicrob. Agents Chemother. 2009, 53, 2205–2208. [Google Scholar] [CrossRef] [PubMed]
- Abbasifar, R.; Kropinski, A.M.; Sabour, P.M.; Chambers, J.R.; MacKinnon, J.; Malig, T.; Griffiths, M.W. Efficiency of bacteriophage therapy against Cronobacter sakazakii in Galleria mellonella (greater wax moth) larvae. Arch. Virol. 2014, 159, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Beeton, M.L.L.; Alves, D.R.R.; Enright, M.C.C.; Jenkins, A.T.A.T.A. Assessing phage therapy against Pseudomonas aeruginosa using a Galleria mellonella infection model. Int. J. Antimicrob. Agents 2015, 46, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Park, J.H.; Yong, D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Tsai, C.J.Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and phage-derived proteins—Application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant. Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, P.; Soni, P.; Gupta, B. Ralstonia picketti neonatal sepsis: A case report. BMC Res. Notes 2017, 10, 28. [Google Scholar] [CrossRef]
- Golec, P.; Karczewska-Golec, J.; Loś, M.; Wegrzyn, G. Bacteriophage T4 can produce progeny virions in extremely slowly growing Escherichia coli host: Comparison of a mathematical model with the experimental data. FEMS Microbiol. Lett. 2014, 351, 156–161. [Google Scholar] [CrossRef]
- Golec, P.; Karczewska-Golec, J.; Voigt, B.; Albrecht, D.; Schweder, T.; Hecker, M.; Wȩgrzyn, G.; Łoś, M. Proteomic profiles and kinetics of development of bacteriophage T4 and its ri and riii mutants in slowly growing Escherichia coli. J. Gen. Virol. 2013, 94, 896–905. [Google Scholar] [CrossRef]
- Latimer, J.; Forbes, S.; McBain, A.J. Attenuated virulence and biofilm formation in Staphylococcus aureus following sublethal exposure to triclosan. Antimicrob. Agents Chemother. 2012, 56, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.E.; Vuorela, P.M.; Fallarero, A. Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. J. Antibiot. (Tokyo) 2012, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, A.; Bacal, P.; Wasiluk, A.; Trybunko, A.; Adamczyk-Poplawska, M. The dam replacing gene product enhances Neisseria gonorrhoeae FA1090 viability and biofilm formation. Front. Microbiol. 2014, 5, 712. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Zarnowiec, P.; Kaca, W.; Danis-Wlodarczyk, K.; Augustyniak, D.; Drevinek, P.; de Soyza, A.; McClean, S.; Drulis-Kawa, Z. In vitro and in vivo antibacterial activity of environmental bacteriophages against Pseudomonas aeruginosa strains from cystic fibrosis patients. Appl. Microbiol. Biotechnol. 2015, 99, 6021–6033. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.; Berriman, M.; Tivey, A.; Patel, C.; Böhme, U.; Barrell, B.G.; Parkhill, J.; Rajandream, M.A. Artemis and ACT: Viewing, annotating and comparing sequences stored in a relational database. Bioinformatics 2008, 24, 2672–2676. [Google Scholar] [CrossRef]
- Mcnair, K.; Zhou, C.; Dinsdale, E.A.; Souza, B.; Edwards, R.A.; Hancock, J. PHANOTATE: A novel approach to gene identification in phage genomes. Bioinformatics 2019, 35, 4537–4542. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33, 244. [Google Scholar] [CrossRef]
- Darzentas, N. Circoletto: Visualizing sequence similarity with Circos. Bioinformatics 2010, 26, 2620–2621. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, M.; Grygorcewicz, B.; Karczewska-Golec, J.; Decewicz, P.; Pankowski, J.A.; Országh-Szturo, H.; Bącal, P.; Dołęgowska, B.; Golec, P. Characterization of a Unique Bordetella bronchiseptica vB_BbrP_BB8 Bacteriophage and Its Application as an Antibacterial Agent. Int. J. Mol. Sci. 2020, 21, 1403. https://doi.org/10.3390/ijms21041403
Szymczak M, Grygorcewicz B, Karczewska-Golec J, Decewicz P, Pankowski JA, Országh-Szturo H, Bącal P, Dołęgowska B, Golec P. Characterization of a Unique Bordetella bronchiseptica vB_BbrP_BB8 Bacteriophage and Its Application as an Antibacterial Agent. International Journal of Molecular Sciences. 2020; 21(4):1403. https://doi.org/10.3390/ijms21041403
Chicago/Turabian StyleSzymczak, Mateusz, Bartłomiej Grygorcewicz, Joanna Karczewska-Golec, Przemysław Decewicz, Jarosław Adam Pankowski, Hanna Országh-Szturo, Paweł Bącal, Barbara Dołęgowska, and Piotr Golec. 2020. "Characterization of a Unique Bordetella bronchiseptica vB_BbrP_BB8 Bacteriophage and Its Application as an Antibacterial Agent" International Journal of Molecular Sciences 21, no. 4: 1403. https://doi.org/10.3390/ijms21041403
APA StyleSzymczak, M., Grygorcewicz, B., Karczewska-Golec, J., Decewicz, P., Pankowski, J. A., Országh-Szturo, H., Bącal, P., Dołęgowska, B., & Golec, P. (2020). Characterization of a Unique Bordetella bronchiseptica vB_BbrP_BB8 Bacteriophage and Its Application as an Antibacterial Agent. International Journal of Molecular Sciences, 21(4), 1403. https://doi.org/10.3390/ijms21041403





