Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration
Abstract
:1. Introduction
2. Results
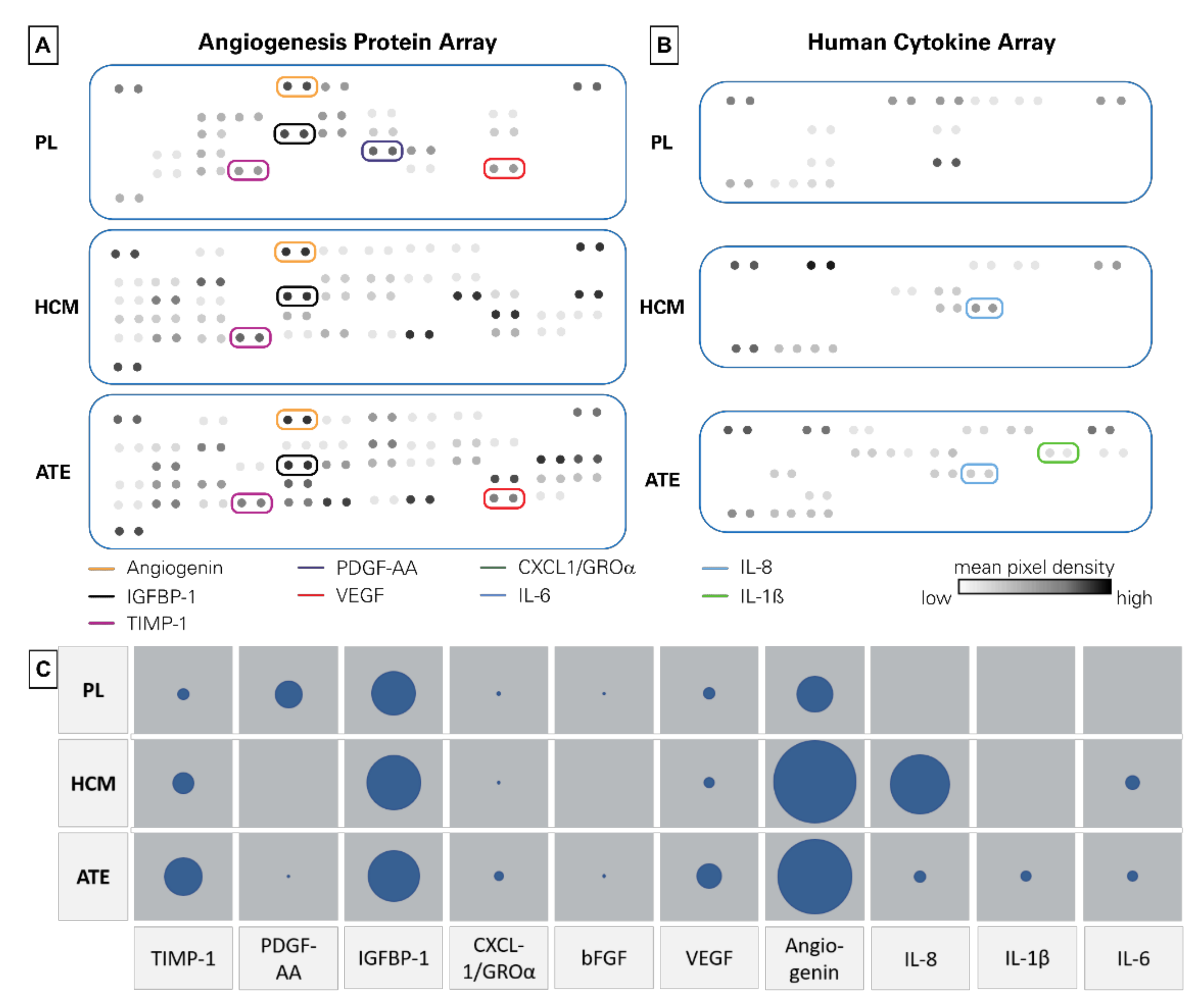
2.1. Protein Quantification, Angiogenesis Protein and Cytokine Array
2.2. Chemoattractive Potential, Proliferation and Specific ALP Activity
2.3. Angiogenic Potential of PL, HCM and ATE
3. Discussion
4. Materials and Methods
4.1. Generation of Growth Factor Mixtures
4.1.1. Preparation of Platelet lysates (PL)
4.1.2. Generation of Hypoxia Conditioned Medium (HCM)
4.1.3. Preparation of Adipose Tissue Extracts (ATE)
4.2. Biochemical Characterization of Growth Factor Mixtures
4.2.1. Protein Quantification Assay
4.2.2. Angiogenesis and Cytokine Array
4.2.3. Enzyme-Linked Immunosorbent Assay
4.3. Cell Culture Experiments to Investigate the Effects of Growth Factor Mixtures
4.3.1. Cells
4.3.2. Chemotaxis Assay
4.3.3. Osteogenic Differentiation of BM-MSC
4.3.4. Analysis of LDH and ALP Activity
4.3.5. In vitro Angiogenesis Assay
4.3.6. CD31-Staining and Analysis of Angiogenic Structures
4.3.7. Nitrate Measurement
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAP | Ascorbic acid-2-phosphate |
| ATE | Adipose tissue extract |
| AT-MSC | Adipose tissue-derived stromal cells |
| bFGF | Basic fibroblast growth factor |
| BM-MSC | Bone-marrow-derived mesenchymal stromal cells |
| CD | Cluster of differentiation |
| Ctrl | Positive control |
| Dex | Dexamethasone |
| EGF | Epidermal growth factor |
| ELISA | Enzyme-linked immunosorbent assays |
| FCS | Fetal calf serum |
| GP | Glycerophosphate |
| HCM | Hypoxia-conditioned medium |
| HMGB1 | High-mobility group protein B1 |
| hTERT-MSC | Human telomerase immortalized bone-marrow-derived mesenchymal stem cells |
| IGF-1 | Insulin-like growth factors-1 |
| IGFBP-1 | Insulin-like growth factor-binding protein 1 |
| IL | Interleukin |
| MSC | Mesenchymal stromal cells |
| NO | Nitric oxide |
| OS | Osteogenic supplements |
| PDGF-BB | Platelet-derived growth factor |
| PL | Platelet lysates |
| PRP | Platelet-rich plasma |
| TGF-ß | Transforming growth factor β |
| TIMP-1 | Tissue inhibitor of metalloproteinases-1 |
| TNFα | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Colterjohn, N.R.; Bednar, D.A. Procurement of bone graft from the iliac crest. An operative approach with decreased morbidity. J. Bone Joint Surg. Am. 1997, 79, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Toolan, B.C. Current concepts review: Orthobiologics. Foot Ankle Int. 2006, 27, 561–566. [Google Scholar] [CrossRef] [PubMed]
- De Long, W.G.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Joint Surg. Am. 2007, 89, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Hoyer, B.; Springer, A.; Mrozik, B.; Hanke, T.; Cherif, C.; Pompe, W.; Gelinsky, M. Novel Textile Scaffolds Generated by Flock Technology for Tissue Engineering of Bone and Cartilage. Mater. Basel 2012, 5, 540–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentsch, C.; Rentsch, B.; Scharnweber, D.; Zwipp, H.; Rammelt, S. Knochenersatz: Transplantate und Ersatzmaterialien–ein Update. Der. Unfallchirurg 2012, 115, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Schnabelrauch, M.; Scharnweber, D.; Schiller, J. Sulfated glycosaminoglycans as promising artificial extracellular matrix components to improve the regeneration of tissues. Curr. Med. Chem. 2013, 20, 2501–2523. [Google Scholar] [CrossRef]
- Bierbaum, S.; Hintze, V.; Scharnweber, D. Functionalization of biomaterial surfaces using artificial extracellular matrices. Biomatter 2012, 2, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Yin, G.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y.; Pu, X. Localized delivery of growth factors for angiogenesis and bone formation in tissue engineering. Int. Immunopharmacol. 2013, 16, 214–223. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Doberenz, F.; Kilian, D.; Vater, C.; Korn, P.; Lauer, G.; Lode, A.; Gelinsky, M. Bioprinting of mineralized constructs utilizing multichannel plotting of a self-setting calcium phosphate cement and a cell-laden bioink. Biofabrication 2018, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Inman, E.; Spaethe, R.; Helgerson, S. Fibrin-based biomaterials to deliver human growth factors. Thromb. Haemost. 2003, 89, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kilian, O.; Flesch, I.; Wenisch, S.; Taborski, B.; Jork, A.; Schnettler, R.; Jonuleit, T. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur. J. Med. Res. 2004, 9, 337–344. [Google Scholar] [PubMed]
- Park, S.-Y.; Kim, K.-H.; Shin, S.-Y.; Koo, K.-T.; Lee, Y.-M.; Seol, Y.-J. Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model. Tissue Eng. Part A 2013, 19, 2495–2505. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jang, S.-J.; Baek, H.-R.; Lee, K.M.; Chang, B.-S.; Lee, C.-K. Synergistic induction of early stage of bone formation by combination of recombinant human bone morphogenetic protein-2 and epidermal growth factor: Synergistic bone formation by combination of rhBMP-2 and EGF. J. Tissue Eng. Regen Med. 2015, 9, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors 2014, 40, 459–481. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Kang, D.G.; Hsu, W.K.; Lehman, R.A. Complications Associated With Bone Morphogenetic Protein in the Lumbar Spine. Orthopedics 2017, 40, e229–e237. [Google Scholar] [CrossRef] [Green Version]
- López, J.F.; Sarkanen, J.-R.; Huttala, O.; Kaartinen, I.S.; Kuokkanen, H.O.; Ylikomi, T. Adipose tissue extract shows potential for wound healing: In vitro proliferation and migration of cell types contributing to wound healing in the presence of adipose tissue preparation and platelet rich plasma. Cytotechnology 2018, 70, 1193–1204. [Google Scholar] [CrossRef]
- Malhotra, A.; Pelletier, M.; Oliver, R.; Christou, C.; Walsh, W.R. Platelet-rich plasma and bone defect healing. Tissue Eng. Part A 2014, 20, 2614–2633. [Google Scholar] [CrossRef]
- Yu, T.; Pan, H.; Hu, Y.; Tao, H.; Wang, K.; Zhang, C. Autologous platelet-rich plasma induces bone formation of tissue-engineered bone with bone marrow mesenchymal stem cells on beta-tricalcium phosphate ceramics. J. Orthop. Surg. Res. 2017, 12, 178. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, K.A.; Donath, K.; Rupprecht, S.; Falk, S.; Zimmermann, R.; Felszeghy, E.; Wiltfang, J. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 2004, 25, 5387–5393. [Google Scholar] [CrossRef]
- Kitoh, H.; Kitakoji, T.; Tsuchiya, H.; Katoh, M.; Ishiguro, N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone 2007, 40, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, A.; Knaak, S.; Gelinsky, M.; Arnhold, S.; Rösen-Wolff, A. Hypoxia-conditioned media allows species-specific attraction of bone marrow stromal cells without need for recombinant proteins. BMC Vet. Res. 2014, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE 2014, 9, e96161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummitzsch, L.; Zitta, K.; Bein, B.; Steinfath, M.; Albrecht, M. Culture media from hypoxia conditioned endothelial cells protect human intestinal cells from hypoxia/reoxygenation injury. Exp. Cell Res. 2014, 322, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Huttala, O.; Sarkanen, J.-R.; Kaartinen, I.; Kuokkanen, H.; Ylikomi, T. Cytokine-Rich Adipose Tissue Extract Production from Water-Assisted Lipoaspirate: Methodology for Clinical Use. BioRes. Open Access 2016, 5, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Guerre-Millo, M. Adipose tissue and adipokines: For better or worse. Diabetes Metab. 2004, 30, 13–19. [Google Scholar] [CrossRef]
- Muoio, D.M.; Newgard, C.B. Obesity-Related Derangements in Metabolic Regulation. Annu. Rev. Biochem. 2006, 75, 367–401. [Google Scholar] [CrossRef]
- Sarkanen, J.-R.; Kaila, V.; Mannerström, B.; Räty, S.; Kuokkanen, H.; Miettinen, S.; Ylikomi, T. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng. Part A 2012, 18, 17–25. [Google Scholar] [CrossRef]
- Sarkanen, J.-R.; Ruusuvuori, P.; Kuokkanen, H.; Paavonen, T.; Ylikomi, T. Bioactive Acellular Implant Induces Angiogenesis and Adipogenesis and Sustained Soft Tissue Restoration In Vivo. J. Tissue Eng. A 2012, 18, 2568–2580. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar] [PubMed]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Rajaram, S.; Baylink, D.J.; Mohan, S. Insulin-like growth factor-binding proteins in serum and other biological fluids: Regulation and functions. Endocr. Rev. 1997, 18, 801–831. [Google Scholar] [PubMed] [Green Version]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6, 33123. [Google Scholar] [CrossRef] [Green Version]
- Bhat, K.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. GROα overexpression drives cell migration and invasion in triple negative breast cancer cells. Oncol. Rep. 2017, 38, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef]
- Ng, Y.; Krilleke, D.; Shima, D. VEGF function in vascular pathogenesis. Exp. Cell Res. 2006, 312, 527–537. [Google Scholar] [CrossRef]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000–2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK5974/ (accessed on 18 February 2020).
- Pavlov, N.; Frendo, J.-L.; Guibourdenche, J.; Degrelle, S.A.; Evain-Brion, D.; Badet, J. Angiogenin Expression during Early Human Placental Development; Association with Blood Vessel Formation. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Bandyopadhyay, C.; Bottero, V.; Veettil, M.V.; Wilson, L.; Pins, M.R.; Johnson, K.E.; Warshall, C.; Chandran, B. Angiogenin interacts with the plasminogen activation system at the cell surface of breast cancer cells to regulate plasmin formation and cell migration. Mol. Oncol. 2014, 8, 483–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Rajarathnam, K.; Sykes, B.D.; Kay, C.M.; Dewald, B.; Geiser, T.; Baggiolini, M.; Clark-Lewis, I. Neutrophil activation by monomeric interleukin-8. Science 1994, 264, 90–92. [Google Scholar] [CrossRef]
- Simsa-Maziel, S.; Monsonego-Ornan, E. Interleukin-1β Promotes Proliferation and Inhibits Differentiation of Chondrocytes through a Mechanism Involving Down-Regulation of FGFR-3 and p21. Endocrinology 2012, 153, 2296–2310. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Jiang, C.-Y.; Fujita, T.; Luo, S.-W.; Kumamoto, E. Enhancement by Interleukin-1β of AMPA and NMDA Receptor-Mediated Currents in Adult Rat Spinal Superficial Dorsal Horn Neurons. Mol. Pain 2013, 9, 1744–8069. [Google Scholar] [CrossRef] [Green Version]
- Gabryšová, L.; Howes, A.; Saraiva, M.; O’Garra, A. The regulation of IL-10 expression. Curr. Top. Microbiol. Immunol. 2014, 380, 157–190. [Google Scholar]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Bowler, D.; Dym, H. Bone Morphogenic Protein. Dental Clinics of North. America 2015, 59, 493–503. [Google Scholar] [CrossRef]
- Flouzat-Lachaniette, C.-H.; Ghazanfari, A.; Bouthors, C.; Poignard, A.; Hernigou, P.; Allain, J. Bone union rate with recombinant human bone morphogenic protein-2 versus autologous iliac bone in PEEK cages for anterior lumbar interbody fusion. Int. Orthop. 2014, 38, 2001–2007. [Google Scholar] [CrossRef]
- Young, A.; Mirarchi, A. Soft Tissue Swelling Associated with the Use of Recombinant Human Bone Morphogenetic Protein-2 in Long Bone Non-unions. J. Orthop Case Rep. 2015, 5, 18–21. [Google Scholar]
- Papanna, M.C.; Saldanha, K.A.; Kurian, B.; Fernandes, J.A.; Jones, S. The use of recombinant morphogenic protein-2(rhBMP-2) in children undergoing revision surgery for persistent non-union. Strategies Trauma Limb Reconstr. 2016, 11, 53–58. [Google Scholar]
- Vas, W.J.; Shah, M.; Al Hosni, R.; Owen, H.C.; Roberts, S.J. Biomimetic strategies for fracture repair: Engineering the cell microenvironment for directed tissue formation. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Schive, S.W.; Mirlashari, M.R.; Hasvold, G.; Wang, M.; Josefsen, D.; Gullestad, H.P.; Korsgren, O.; Foss, A.; Kvalheim, G.; Scholz, H. Human Adipose-Derived Mesenchymal Stem Cells Respond to Short-Term Hypoxia by Secreting Factors Beneficial for Human Islets In Vitro and Potentiate Antidiabetic Effect In Vivo. Cell Med. 2017, 9, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Antebi, B.; Rodriguez, L.A.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef]
- Hersant, B.; Sid-Ahmed, M.; Braud, L.; Jourdan, M.; Baba-Amer, Y.; Meningaud, J.-P.; Rodriguez, A.-M. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Kakudo, N.; Morimoto, N.; Kushida, S.; Ogawa, T.; Kusumoto, K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med. Mol. Morphol. 2014, 47, 83–89. [Google Scholar] [CrossRef]
- Mammoto, T.; Jiang, A.; Jiang, E.; Mammoto, A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc. Res. 2013, 89, 15–24. [Google Scholar] [CrossRef]
- Bolte, J.; Vater, C.; Culla, A.C.; Ahlfeld, T.; Nowotny, J.; Kasten, P.; Disch, A.C.; Goodman, S.B.; Gelinsky, M.; Stiehler, M.; et al. Two-step stem cell therapy improves bone regeneration compared to concentrated bone marrow therapy. J. Orthop. Res. 2019, 37, 1318–1328. [Google Scholar] [CrossRef]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Quade, M.; Knaack, S.; Weber, D.; König, U.; Paul, B.; Simon, P.; Rösen-Wolff, A.; Schwartz-Albiez, R.; Gelinsky, M.; Lode, A. Heparin modification of a biomimetic bone matrix modulates osteogenic and angiogenic cell response in vitro. Eur. Cell Mater. 2017, 33, 105–120. [Google Scholar] [CrossRef]



| Mean ± SD | |
|---|---|
| PL | 11.60 ± 0.15 mg/mL |
| HCM | 0.015 ± 0.006 mg/mL |
| ATE | 4.05 ± 0.08 mg/mL |
| PL (pg/mL) | HCM (pg/mL) | ATE (pg/mL) | Function (Selected) | |
|---|---|---|---|---|
| TIMP-1 | 498 | 1992 | 924 | angiogenesis suppression [33,34] |
| PDGF | 8233 | 0 | 60 | blood vessel formation, proliferation [35] |
| IGFBP-1 | 2905 | 3026 | 1417 | cell migration and metabolism [36] |
| CXCL1 (GROα) | 1970 | 1469 | 2721 | activates neutrophils and basophils; increases cell migration [37,38] |
| bFGF | 1721 | 708 | 1071 | cell migration, activated during wound healing [39] |
| VEGF | 605 | 398 | stimulates the formation of blood vessels [40,41,42] | |
| Angiogenin | 159 | 1447 | 890 | cell migration, proliferation and formation of tubular structures [43,44] |
| IL-8 | 22 | 1105 | 2112 | innate immune response, regulated angiogenesis [45,46] |
| IL-1β | 30 | 3 | 124 | inflammatory response, cellular activities–proliferation and differentiation [47,48] |
| IL-10 | 135 | 15 | 201 | immunoregulation and inflammation [49] |
| IL-6 | 0 | 804 | 784 | pro- and anti-inflammatory properties [50,51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bretschneider, H.; Quade, M.; Lode, A.; Gelinsky, M.; Rammelt, S.; Zwingenberger, S.; Schaser, K.-D.; Vater, C. Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 1412. https://doi.org/10.3390/ijms21041412
Bretschneider H, Quade M, Lode A, Gelinsky M, Rammelt S, Zwingenberger S, Schaser K-D, Vater C. Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration. International Journal of Molecular Sciences. 2020; 21(4):1412. https://doi.org/10.3390/ijms21041412
Chicago/Turabian StyleBretschneider, Henriette, Mandy Quade, Anja Lode, Michael Gelinsky, Stefan Rammelt, Stefan Zwingenberger, Klaus-Dieter Schaser, and Corina Vater. 2020. "Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration" International Journal of Molecular Sciences 21, no. 4: 1412. https://doi.org/10.3390/ijms21041412





