Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology
Abstract
1. Introduction
2. Etiology and Pathogenesis of Endometritis
2.1. Infectious Endometritis
2.2. Non-Infectious Endometritis
2.3. Innate Immune Response to Endometritis
3. Diagnosis
4. Treatment
4.1. Ecbolics
4.2. Antibiotics
4.3. Uterine Lavage and Treatment for Biofilm
4.4. Immunomodulatory Agents
4.5. Lactoferrin
4.6. Platelet-Rich Plasma
4.7. Stem Cells
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Antigen-presenting cells |
| BAFF | B cell-activating factor |
| C1q | Complement component 1 |
| C3a | Complement 3a |
| C3b | Complement 3b |
| C4a | Complement 4a |
| C4b | Complement 4b |
| C5a | Complement 5a |
| CD14 CD40 | Cluster of differentiation 14 Cluster of differentiation 40 |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| CXCL8 | Chemokine ligand 8 |
| EC | Epithelial cells |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid-2-amino-2-hydroxymethyl-propane-1,3-diol |
| FoxP3 | Forkhead box protein P3 |
| GnRH | Gonadotropin-releasing hormone |
| IFN | Interferon |
| IFNα | Interferon type I α |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL1 | Interleukin1 |
| IL1RN | Interleukin 1 receptor antagonist |
| IL10 | Interleukin 10 |
| IL13 | Interleukin 13 |
| IL17 | Interleukin 17 |
| IL1α | Interleukin 1alpha |
| IL1β | Interleukin 1Beta |
| IL4 | Interleukin 4 |
| IL6 | Interleukin 6 |
| LH | Luteinizing hormone |
| LPS LRS | Lipopolysaccharides Lactated Ringer’s Solution |
| MAC | Membrane attack complex |
| MCWE | Mycobacterium phlei cell wall extract |
| MMP-3 | Metalloproteinase-3 |
| MMPs | Matrix metalloproteinases |
| MSCs | Mesenchymal stem cells |
| MyD88 | Myeloid differentiation primary response 88 |
| NETs | Neutrophil extracellular traps |
| NF-κB | Nuclear factor kappa-B |
| NK | Natural killer cells |
| NLR | NOD-like receptors |
| NO | Nitric oxide |
| NOD | Nucleotide-binding and oligomerization domain |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PABA PBIE | Para-aminobenzoic acid Persistent breeding-induced endometritis |
| PBPs PGF2α | Penicillin-binding proteins Prostaglandin 2α |
| PMNs | Polymorphonuclear neutrophils |
| PRP | Platelet-rich plasma |
| PRRs | Pattern recognition receptors |
| SAA | Serum amyloid A |
| TIMPs | Tissue inhibitors of MMPs |
| TLR2 | Toll-like receptors type 2 |
| TLR4 | Toll-like receptors type 4 |
| TLRs | Toll-like receptors |
| TNFα | Tumor necrosis factor-α |
| TRAF6 | Receptor-associated factor 6 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| WBC | White blood cells |
References
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [PubMed]
- Troedsson, M.H.T. Uterine clearance and resistance to persistent endometritis in the mare. Theriogenology 1999, 52, 461–471. [Google Scholar] [CrossRef]
- Leblanc, M.M.; Neuwirth, L.; Asbury, A.C.; Tran, T.; Mauragis, D.; Klapstein, E. Scintigraphic measurement of uterine clearance in normal mares and mares with recurrent endometritis. Equine Vet. J. 1994, 26, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T.; Desvousges, A.; Macpherson, M.L.; Pozor, M.P. Persistent breeding-induced endometritis. Pferdeheilkunde 1994, 24, 56–60. [Google Scholar] [CrossRef]
- Scoggin, C.F. Not just a number: Effect of age on fertility, pregnancy and offspring vigour in thoroughbred brood-mares. Reprod. Fertil. Dev. 2015, 27, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Squires, E.L.; Troedsson, M.H.T. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef]
- Allen, W.; Pycock, J. Cyclical accumulation of uterine fluid in mares with lowered resistance to endometritis. Vet. Rec. 1988, 122, 489–490. [Google Scholar] [CrossRef]
- Zent, W.W.; Troedsson, M.H.T.; Xue, J.-L. Postbreeding uterine fluid accumulation in a normal population of Thoroughbred mares: A field study. In Proceedings of the 40th Annual Convention of the American Association of Equine Practitioners, Baltimore, MD, USA, 6 December 1998; Volume 44, pp. 64–65. [Google Scholar]
- Troedsson, M.H.; Liu, I.K.; Crabo, B. Sperm transport and survival in the mare. Theriogenology 1998, 49, 905–915. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N.; Carlson, C.S.; Troedsson, M.H.T. Nitric oxide levels and nitric oxide synthase expression in uterine samples from mares susceptible and resistant to persistent breeding-induced endometritis. Am. J. Reprod. Immunol. 2005, 53, 230–237. [Google Scholar] [CrossRef]
- Katila, T. Onset and duration of uterine inflammatory response of mares after insemination with fresh semen. Biol. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Therapeutic considerations for mating-induced endometritis. Pferdeheilkunde 1997, 13, 516–520. [Google Scholar] [CrossRef]
- Troedsson, M.H.; Liu, I.K. Uterine clearance of non-antigenic markers (51Cr) in response to a bacterial challenge in mares potentially susceptible and resistant to chronic uterine infections. J. Reprod. Fertil. Suppl. 1991, 44, 283–288. [Google Scholar] [PubMed]
- Carnevale, E.M.; Ramirez, R.J.; Squires, E.L.; Alvarenga, M.A.; Vanderwall, D.K.; McCue, P.M. Factors affecting pregnancy rates and early embryonic death after equine embryo transfer. Theriogenology 2000, 54, 965–979. [Google Scholar] [CrossRef]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The use of dexamethasone administered to mares at breeding time in the modulation of persistent mating induced endometritis. Theriogenology 2008, 70, 1093–1100. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial inflammatory markers of the early immune response in mares susceptible or resistant to persistent breeding-induced endometritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef]
- Freeman, D.A.; Weber, J.A.; Geary, R.T.; Woods, G.L. Time of embryo transport through the mare oviduct. Theriogenology 1991, 36, 823–830. [Google Scholar] [CrossRef]
- Robertson, S.A.; Chin, P.Y.; Femia, J.G.; Brown, H.M. Embryotoxic cytokines—Potential roles in embryo loss and fetal programming. J. Reprod. Immunol. 2018, 125, 80–88. [Google Scholar] [CrossRef]
- Squires, E.L. Embryo transfer challenges and perspectives. Rev. Bras. Reprod. Anim. 2013, 37, 105–107. [Google Scholar]
- Canisso, I.F.; Stewart, J.; Coutinho da Silva, M.A. Endometritis: Managing persistent post-breeding endometritis. Vet. Clin. N. Am. Equine Pract. 2016, 32, 465–480. [Google Scholar] [CrossRef]
- Scoggin, C.F. Endometritis: Nontraditional therapies. Vet. Clin. N. Am. Equine Pract. 2016, 32, 499–511. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Breeding-induced endometritis in mares. Vet. Clin. N. Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.K.M.; Troedsson, M.H.T. The diagnosis and treatment of endometritis in the mare: Yesterday and today. Theriogenology 2008, 70, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T. Mating-induced endometritis: Physiology or pathology? Vet. J. 2014, 199, 9–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Troedsson, M.H.T.; Woodward, E.M. Our current understanding of the pathophysiology of equine endometritis with an emphasis on breeding-induced endometritis. Reprod. Biol. 2016, 16, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Evaluation of diagnostic methods in equine endometritis. Reprod. Biol. 2016, 16, 189–196. [Google Scholar] [CrossRef]
- Woodward, E.M.; Troedsson, M.H.T. Inflammatory mechanisms of endometritis. Equine Vet. J. 2015, 47, 384–389. [Google Scholar] [CrossRef]
- Marth, C.D.; Firestone, S.M.; Hanlon, D.; Glenton, L.Y.; Browning, G.F.; Young, N.D.; Krekeler, N. Innate immune genes in persistent mating-induced endometritis in horses. Reprod. Fertil. Dev. 2018, 30, 533–545. [Google Scholar] [CrossRef]
- Marth, C.D.; Young, N.D.; Glenton, L.Y.; Noden, D.M.; Browning, G.F.; Krekeler, N. Deep sequencing of the uterine immune response to bacteria during the equine oestrous cycle. BMC Genom. 2015, 16, 1–19. [Google Scholar] [CrossRef]
- Nash, D.M.; Sheldon, I.M.; Herath, S.; Lane, E.A. Markers of the uterine innate immune response of the mare. Anim. Reprod. Sci. 2010, 119, 31–39. [Google Scholar] [CrossRef]
- Fumuso, E.A.; Aguilar, J.; Giguère, S.; Rivulgo, M.; Wade, J.; Rogan, D. Immune parameters in mares resistant and susceptible to persistent post-breeding endometritis: Effects of immunomodulation. Vet. Immunol. Immunopathol. 2007, 118, 30–39. [Google Scholar] [CrossRef]
- Fumuso, E.; Giguère, S.; Wade, J.; Rogan, D.; Videla-Dorna, I.; Bowden, R.A. Endometrial IL-1beta, IL-6 and TNF-alpha, mRNA expression in mares resistant or susceptible to post-breeding endometritis. Effects of estrous cycle, artificial insemination and immunomodulation. Vet. Immunol. Immunopathol. 2003, 96, 31–41. [Google Scholar] [CrossRef]
- Christoffersen, M.; Troedsson, M.H.T.; Woodward, E.M.; Lehn-Jensen, H.; Bojesen, A.M.; Squires, E.L.; Petersen, M.R. Effect of immunomodulatory therapy on the endometrial inflammatory response to induced infectious endometritis in susceptible mares. Theriogenology 2012, 78, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.M.; Christoffersen, M.; Horohov, D.; Squires, E.L.; Troedsson, M.H.T. The effect of treatment with immune modulators on endometrial cytokine expression in mares susceptible to persistent breeding-induced endometritis. Equine Vet. J. 2015, 47, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.; Christoffersen, M.; Campos, J.; Horohov, D.; Scoggin, K.; Squires, E.; Troedsson, M. An investigation of uterine nitric oxide production in mares susceptible and resistant to persistent breeding-induced endometritis and the effects of immunomodulation. Reprod. Domest. Anim. 2013, 48, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T.; Crabo, B.G.; Ibrahim, N.; Scott, M.; Ing, M. Mating-induced endometritis: Mechanisms, clinical importance and consequences. Proc. 40th Am. Assoc. Equine Pract. 1994, 41, 11–12. [Google Scholar]
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leukocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Trotter, G.W.; McKinnon, A.O. Surgery for abnormal vulvar and perineal conformation in the mare. Vet. Clin. N. Am. Equine Pract. 1988, 4, 389–405. [Google Scholar] [CrossRef]
- Christoffersen, M.; Söderlind, M.; Rudefalk, S.R.; Pedersen, H.G.; Allen, J.; Krekeler, N. Risk factors associated with uterine fluid after breeding caused by Streptococcus zooepidemicus. Theriogenology 2015, 84, 1283–1290. [Google Scholar] [CrossRef]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships between uterine culture, cytology and pregnancy rates in a Thoroughbred practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Wingfield Digby, N.J.; Ricketts, S.W. Results of concurrent bacteriological and cytological examinations of the endometrium of mares in routine stud farm practice 1978–1981. J. Reprod. Fertil. Suppl. 1982, 32, 181–185. [Google Scholar]
- Leblanc, M.; Causey, R. Clinical and subclinical endometritis in the mare: Both threats to fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- Overbeck, W.; Witte, T.S.; Heuwieser, W. Comparison of three diagnostic methods to identify subclinical endometritis in mares. Theriogenology 2011, 75, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, S.W.; Mackintosh, M.E. Role of anaerobic bacteria in equine endometritis. J. Reprod. Fertil. Suppl. 1987, 35, 343–351. [Google Scholar] [PubMed]
- Collins, S. A study of the incidence of cervical and uterine infection in Thoroughbred mares in Ireland. Vet. Rec. 1964, 66, 673–676. [Google Scholar]
- Bain, A.M. The role of infection in infertility in the thoroughbred mare. Vet. Rec. 1966, 78, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Neuberg, K.P.; Failing, K.; Wehrend, A. Cytological diagnosis of endometritis in the mare: Investigations of sampling techniques and relation to bacteriological results. Anim. Reprod. Sci. 2012, 132, 178–186. [Google Scholar] [CrossRef]
- LeBlanc, M.M.; Magsig, J.; Stromberg, A.J. Use of a low-volume uterine flush for diagnosing endometritis in chronically infertile mares. Theriogenology 2007, 68, 403–412. [Google Scholar] [CrossRef]
- Beltaire, K.A.; Cheong, S.H.; Coutinho da Silva, M.A. Retrospective study on equine uterine fungal isolates and antifungal susceptibility patterns (1999–2011). Equine Vet. J. Suppl. 2012, 84–87. [Google Scholar] [CrossRef]
- Frontoso, R.; De Carlo, E.; Pasolini, M.P.; van der Meulen, K.; Pagnini, U.; Iovane, G.; De Martino, L. Retrospective study of bacterial isolates and their antimicrobial susceptibilities in equine uteri during fertility problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef]
- Albihn, A.; Båverud, V.; Magnusson, U. Uterine microbiology and antimicrobial susceptibility in isolated bacteria from mares with fertility problems. Acta Vet. Scand. 2003, 44, 121–129. [Google Scholar] [CrossRef]
- Petersen, M.R.; Skive, B.; Christoffersen, M.; Lu, K.; Nielsen, J.M.; Troedsson, M.H.T.; Bojesen, A.M. Activation of persistent Streptococcus equi subspecies zooepidemicus in mares with subclinical endometritis. Vet. Microbiol. 2015, 179, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Coutinho da Silva, M.A.; Alvarenga, M.A. Fungal endometritis. In Equine Reproduction; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 2643–2651. [Google Scholar]
- Dascanio, J.J.; Schweizer, C.; Ley, W.B. Equine fungal endometritis. Equine Vet. Educ. 2010, 13, 324–329. [Google Scholar] [CrossRef]
- Hinrichs, K.; Spensley, M.S.; McDonough, P.L. Evaluation of progesterone treatment to create a model for equine endometritis. Equine Vet. J. 1992, 24, 457–461. [Google Scholar] [CrossRef]
- Stout, T.A.E. Fungal endometritis in the mare. Pferdeheilkunde 2008, 24, 83–87. [Google Scholar] [CrossRef]
- Ferris, R.A. Current understanding of bacterial biofilms and latent infections: A clinical perspective. Rev. Bras. Reprod. Anim. 2017, 41, 74–80. [Google Scholar]
- Beehan, D.P.; Wolfsdorf, K.; Elam, J.; Krekeler, N.; Paccamonti, D.; Lyle, S.K. The evaluation of biofilm-forming potential of escherichia coli collected from the equine female reproductive tract. J. Equine Vet. Sci. 2015, 35, 935–939. [Google Scholar] [CrossRef]
- Ferris, R.A. Bacterial endometritis: A focus on biofilms. Clin. Theriogenol. 2014, 6, 315–319. [Google Scholar]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Ann. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Shah, D.; Zhang, Z.; Khodursky, A.; Kaldalu, N.; Kurg, K.; Lewis, K. Persisters: A distinct physiological state of E. coli. BMC Microbiol. 2006, 6, 53. [Google Scholar] [CrossRef]
- Jensen, E.T.; Kharazmi, A.; Lam, K.; Costerton, J.W.; Høiby, N. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun. 1990, 58, 2383–2385. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Brown, M.R.W.; Allison, D.G.; Gilbert, P. Resistance of bacterial biofilms to antibiotics a growth-rate related effect? J. Antimicrob. Chemother. 1988, 22, 777–780. [Google Scholar] [CrossRef]
- Anwar, H.; Strap, J.L.; Costerton, W. Establishment of aging biofilms: Possible mechanism of bacterial resistance to antimicrobial therapy. Antimicrob. Agents Chemother. 1992, 36, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The First 25 Years of a Universal Bacterial Second Messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Hickman, J.W.; Tifrea, D.F.; Harwood, C.S. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. USA 2005, 102, 14422–14427. [Google Scholar] [CrossRef]
- Merighi, M.; Lee, V.T.; Hyodo, M.; Hayakawa, Y.; Lory, S. The second messenger bis-(3’-5’)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 876–895. [Google Scholar] [CrossRef]
- Hay, I.D.; Remminghorst, U.; Rehm, B.H.A. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2009, 75, 1110–1120. [Google Scholar] [CrossRef]
- Kuchma, S.L.; Connolly, J.P.; O’Toole, G.A. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 1441–1454. [Google Scholar] [CrossRef]
- Ma, L.; Jackson, K.D.; Landry, R.M.; Parsek, M.R.; Wozniak, D.J. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 2006, 188, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Kundukad, B.; Seviour, T.; Van der Maarel, J.R.C.; Yang, L.; Rice, S.A.; Doyle, P.; Kjelleberg, S. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. mBio 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.; Vallet-Gely, I.; Soscia, C.; Genin, S.; Filloux, A. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 2005, 151, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.A.; Wittstock, S.M.; McCue, P.M.; Borlee, B.R. Evaluation of biofilms in gram-negative bacteria isolated from the equine uterus. J. Equine Vet. Sci. 2014, 34, 121. [Google Scholar] [CrossRef]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Loncar, K.D.; Hennet, M.L.; Borlee, B.R. In vitro efficacy of nonantibiotic treatments on biofilm disruption of gram-negative pathogens and an in vivo model of infectious endometritis utilizing isolates from the equine uterus. J. Clin. Microbiol. 2016, 54, 631–639. [Google Scholar] [CrossRef]
- Morse, D.J.; Wilson, M.J.; Wei, X.; Lewis, M.A.O.; Bradshaw, D.J.; Murdoch, C.; Williams, D.W. Denture-associated biofilm infection in three-dimensional oral mucosal tissue models. J. Med. Microbiol. 2018, 67, 364–375. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral biofilms: Development, control, and analysis. High Throughput 2018, 7, E24. [Google Scholar]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Glapa, K.E.; Martin, K.H.; Mangalea, M.R.; Hennet, M.L.; Wolfe, L.M.; Broeckling, C.D.; Borlee, B.R. Model of chronic equine endometritis involving a Pseudomonas aeruginosa biofilm. Infect. Immun. 2017, 85, e00332-17. [Google Scholar] [CrossRef]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota—New player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van de Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D. Review: Maternal health and the placental microbiome. Placenta 2017, 54, 30–37. [Google Scholar] [CrossRef]
- Heil, B.A.; Thompson, S.K.; Kearns, T.A.; Davolli, G.M.; King, G.; Sones, J.L. Metagenetic characterization of the resident equine uterine microbiome using multiple techniques. J. Equine Vet. Sci. 2018, 66, 111. [Google Scholar] [CrossRef]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.; Love, B.; DeSilva, U.; Rezabek, G.; Carrington, S.; Holyoak, G.; Carroll, B.; Gragg, D. Detectable differences in the endometrial microbiome between normal and susceptible mares using metagenomic profiling and conventional bacterial culture. In Proceedings of the Society of Theriogenology, Milwaukee, WI, USA, 11 August 2011. [Google Scholar]
- Subramaniam, A.; Ptacek, T.; Lobashevsky, E.; Cliver, S.; Lefkowitz, E.; Morrow, C.; Biggio, J.; Edwards, R. Midtrimester Cervicovaginal Microbiota: Identification of Microbial Variations Associated with Puerperal Infection at Term. Am. J. Perinatol. 2016, 33, 1165–1175. [Google Scholar] [PubMed]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near-neutral pH. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Ball, B.A.; Cray, C.; Williams, N.M.; Scoggin, K.E.; Davolli, G.M.; Squires, E.L.; Troedsson, M.H. Serum amyloid A and haptoglobin concentrations are increased in plasma of mares with ascending placentitis in the absence of changes in peripheral leukocyte counts or fibrinogen concentration. Am. J. Reprod. Immunol. 2014, 72, 376–385. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Loset, K.; Alghamdi, A.M.; Dahms, B.; Crabo, B.G. Interaction between equine semen and the endometrium: The inflammatory response to semen. Anim. Reprod. Sci. 2001, 68, 273–278. [Google Scholar] [CrossRef]
- Lieberman, J. The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat. Rev. Immunol. 2003, 3, 361–370. [Google Scholar] [CrossRef]
- Muraille, E.; Goriely, S. The nonspecific face of adaptive immunity. Curr. Opin. Immunol. 2017, 48, 38–43. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Ann. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 1998, 10, 351–353. [Google Scholar] [CrossRef]
- Moldenhauer, L.M.; Diener, K.R.; Thring, D.M.; Brown, M.P.; Hayball, J.D.; Robertson, S.A. Crosspresentation of male seminal fl uid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 2009, 182, 8080–8093. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Robertson, S.A. Seminal fluid signaling in the female reproductive tract: Implications for reproductive outcome and offspring health. Adv. Exp. Med. Biol. 2015, 868, 127–158. [Google Scholar] [PubMed]
- Jørgensen, N.; Persson, G.; Hviid, T.V.F. The tolerogenic function of regulatory T cells in pregnancy and cancer. Front. Immunol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell 2000, 101, 455–458. [Google Scholar] [CrossRef]
- Shevach, E.M. CD4+ CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002, 2, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25 + CD4 + regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hori, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Khattri, R.; Cox, T.; Yasayko, S.A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. J. Immunol. 2003, 198, 993–998. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Loux, S.L.; Scoggin, K.E.; Adams, A.A.; Troedsson, M.H.T.; Ball, B.A. Alterations in T cell-related transcripts at the feto-maternal interface throughout equine gestation. Placenta 2019, 89, 78–87. [Google Scholar] [CrossRef]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.-C.; Heuze-Vourc’h, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 induces FoxP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Kumar, V. The complement system, toll-like receptors and inflammasomes in host defense: Three musketeers’ one target. Int. Rev. Immunol. 2019, 38, 131–156. [Google Scholar] [CrossRef]
- Hakansson, A.; Albihn, A.; Magnusson, U. The contribution of complement to opsonic activity in the uterine secretions of mares free of endometritis. Theriogenology 1993, 39, 601–609. [Google Scholar] [CrossRef]
- Grossman, T.R.; Hettrick, L.A.; Johnson, R.B.; Hung, G.; Peralta, R.; Watt, A.; Henry, S.P.; Adamson, P.; Monia, B.P.; McCaleb, M.L. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology 2016, 221, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.M.; Daha, M.R.; Austen, K.F.; Fearon, D.T. Control of the amplification convertase of complement by the plasma protein β1H. Proc. Natl. Acad. Sci. USA. 1976, 73, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.H.; Carlos, J.R.; Ruddy, S. Interaction of β1H globulin with cell-bound C3b: Quantitative analysis of binding and influence of alternative pathway components on binding. J. Exp. Med. 1978, 147, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Pangburn, M.K.; Schreiber, R.D.; Müller-Eberhard, H.J. Human complement C3b inactivator: Isolation, characterization, and demonstration of an absolute requirement for the serum protein β1H for cleavage of C3b and C4b in solution. J. Exp. Med. 1977, 146, 257–270. [Google Scholar] [CrossRef]
- Ahearn, J.M.; Fearon, D.T. Structure and Function of the Complement Receptors, CR1 (CD35) and CR2 (CD21). Adv. Immunol. 1989, 46, 183–219. [Google Scholar]
- Krych-Goldberg, M.; Atkinson, J.P. Structure-function relationships of complement receptor type 1. Immunol. Rev. 2001, 180, 112–122. [Google Scholar] [CrossRef]
- Nonaka, M. Evolution of the complement system. Subcell. Biochem. 2014, 80, 31–43. [Google Scholar]
- Walport, M.J. Complement. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
- Meri, S. Self-nonself discrimination by the complement system. FEBS Lett. 2016, 590, 2418–2434. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D.; Stokes, C.R.; Bourne, F.J. Influence of arachidonic acid metabolites in vitro and in uterine washings on migration of equine neutrophils under agarose. Res. Vet. Sci. 1987, 43, 203–207. [Google Scholar] [CrossRef]
- Pycock, J.F.; Allen, W.E. Pre-chemotactic and chemotactic properties of uterine fluid from mares with experimentally induced endometritis. Vet. Rec. 1988, 123, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Pycock, J.F.; Allen, W.E. Inflammatory components in uterine fluid from mares with experimentally induced bacterial endometritis. Equine Vet. J. 1990, 22, 422–425. [Google Scholar] [CrossRef]
- Watson, E.D.; Stokes, C.R.; Bourne, F.J. Cellular and humoral defence mechanisms in mares susceptible and resistant to persistent endometritis. Vet. Immunol. Immunopathol. 1987, 16, 107–121. [Google Scholar] [CrossRef]
- Asbury, A.C.; Gorman, N.T.; Foster, G.W. Uterine defense mechanisms in the mare: Serum opsonins affecting phagocytosis of Streptococcus zooepidemicus by equine neutrophils. Theriogenology 1984, 21, 375–385. [Google Scholar] [CrossRef]
- Watson, E.D. Opsonins in uterine washings influencing in vitro activity of equine neutrophils. Equine Vet. J. 1988, 20, 435–437. [Google Scholar] [CrossRef]
- Asbury, A.C.; Halliwell, R.E.; Foster, G.W.; Longino, S.J. Immunoglobulins in uterine secretions of mares with differing resistance to endometritis. Theriogenology 1980, 14, 299–308. [Google Scholar] [CrossRef]
- Mitchell, G.; Liu, I.K.; Perryman, L.E.; Stabenfeldt, G.H.; Hughes, J.P. Preferential production and secretion of immunoglobulins by the equine endometrium—A mucosal immune system. J. Reprod. Fertil. Suppl. 1982, 32, 161–168. [Google Scholar]
- Troedsson, M.H.T.; Liu, I.K.M.; Thurmond, M. Immunoglobulin (IgG and IgA) and complement (C3) concentrations in uterine secretion following an intrauterine challenge of Streptococcus Zooepidemicus in mares susceptible to versus resistant to chronic uterine infection1. Biol. Reprod. 1993, 49, 502–506. [Google Scholar] [CrossRef]
- Widders, P.R.; Stokes, C.R.; David, J.S.; Bourne, F.J. Quantitation of the immunoglobulins in reproductive tract secretions of the mare. Res. Vet. Sci. 1984, 37, 324–330. [Google Scholar] [CrossRef]
- Williamson, P.; Dunning, A.; O’Connor, J.; Penhale, W.J. Immunoglobulin levels, protein concentrations and alkaline phosphatase activity in uterine flushings from mares with endometritis. Theriogenology 1983, 19, 441–448. [Google Scholar] [CrossRef]
- Dell’Aqua, J.A., Jr.; Papa, F.O.; Lopes, M.D.; Alvarenga, M.A.; Macedo, L.P.; Melo, C.M. Modulation of acute uterine inflammatory response after artificial insemination with equine frozen semen. Anim. Reprod. Sci. 2006, 94, 270–273. [Google Scholar]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef]
- Kitaya, K.; Yamada, H. Pathophysiological Roles of Chemokines in Human Reproduction: An Overview. Am. J. Reprod. Immunol. 2011, 65, 449–459. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Sentman, C.L.; Pioli, P.A.; Shen, L. Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol. Rev. 2005, 206, 306–335. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Gerberick, G.F.; Saito, F.H.; Ledger, W.J.; Witkin, S.S. Innate Immunity in the Lower Female Mucosal Tract. J. Steroids Horm. Sci. 2011, 2, 2. [Google Scholar] [CrossRef]
- An, H.; Yu, Y.; Zhang, M.; Xu, H.; Qi, R.; Yan, X.; Liu, S.; Wang, W.; Guo, Z.; Guo, J.; et al. Involvement of ERK, p38 and NF-κB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology 2002, 106, 38–45. [Google Scholar] [CrossRef]
- Chen, C.; Zibiao, H.; Ming, Z.; Shiyi, C.; Ruixia, L.; Jie, W.; SongJia, L. Expression pattern of Toll-like receptors (TLRs) in different organs and effects of lipopolysaccharide on the expression of TLR 2 and 4 in reproductive organs of female rabbit. Dev. Comp. Immunol. 2014, 46, 341–348. [Google Scholar] [CrossRef]
- Silva, E.; Leitão, S.; Henriques, S.; Kowalewski, M.P.; Hoffmann, B.; Ferreira-Dias, G.; Lopes da Costa, L.; Mateus, L. Gene transcription of TLR2, TLR4, LPS ligands and prostaglandin synthesis enzymes are up-regulated in canine uteri with cystic endometrial hyperplasia-pyometra complex. J. Reprod. Immunol. 2010, 84, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Swangchan-Uthai, T.; Lavender, C.R.M.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.L.; Healey, G.D.; Sheldon, I.M. Immunity and inflammation in the uterus. Reprod. Domest. Anim. 2012, 47, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Adib-Conquy, M.; Cavaillon, J.M. Natural killer (NK) cells in antibacterial innate immunity: Angels or devils? Mol. Med. 2012, 18, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Fujita, Y.; Mihara, T.; Okazaki, T.; Shitanaka, M.; Kushino, R.; Ikeda, C.; Negishi, H.; Liu, Z.; Richards, J.S.; Shimada, M. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum. Reprod. 2011, 26, 2799–2806. [Google Scholar] [CrossRef]
- Akthar, I.; Suarez, S.; Morillo, V.A.; Sasaki, M.; Ezz, M.A.; Takahashi, K.I.; Shimada, M.; Marey, M.A.; Miyamoto, A. Sperm enter glands of preovulatory bovine endometrial explants and initiate inflammation. Reproduction 2019, 159, 181–192. [Google Scholar] [CrossRef]
- Ezz, M.A.; Marey, M.A.; Elweza, A.E.; Kawai, T.; Heppelmann, M.; Pfarrer, C.; Balboula, A.Z.; Montaser, A.; Imakawa, K.; Zaabel, S.M.; et al. TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Elweza, A.E.; Ezz, M.A.; Acosta, T.J.; Talukder, A.K.; Shimizu, T.; Hayakawa, H.; Shimada, M.; Imakawa, K.; Zaghloul, A.H.; Miyamoto, A. Aproinflammatory response of bovine endometrial epithelial cells to active sperm in vitro. Mol. Reprod. Dev. 2018, 85, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Warner, N.; Viani, K.; Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Chamaillard, M.; McDonald, C.; Nuñez, G. NOD-LRR Proteins: Role in Host-Microbial Interactions and Inflammatory Disease. Ann. Rev. Biochem. 2005, 74, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Barbé, F.; Douglas, T.; Saleh, M. Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev. 2014, 25, 681–697. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Benko, S.; Magalhaes, J.G.; Philpott, D.J.; Girardin, S.E. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 2010, 185, 1681–1691. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, L.; Xia, X.; Wang, H.Y.; Legras, X.; Hong, J.; Ji, J.; Shen, P.; Zheng, S.; Chen, Z.J.; et al. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell 2010, 141, 483–496. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Ann. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Zandi, E.; Rothwarf, D.M.; Delhase, M.; Hayakawa, M.; Karin, M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for Iκb phosphorylation and NF-κB activation. Cell 1997, 91, 243–252. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IkB kinases: Key regulators of the NF-kB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Dejardin, E.; Droin, N.M.; Delhase, M.; Haas, E.; Cao, Y.; Makris, C.; Li, Z.W.; Karin, M.; Ware, C.F.; Green, D.R. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 2002, 17, 525–535. [Google Scholar] [CrossRef]
- Bonizzi, G.; Bebien, M.; Otero, D.C.; Johnson-Vroom, K.E.; Cao, Y.; Vu, D.; Jegga, A.G.; Aronow, B.J.; Ghosh, G.; Rickert, R.C.; et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 2004, 23, 4202–4210. [Google Scholar] [CrossRef]
- Novack, D.V.; Yin, L.; Hagen-Stapleton, A.; Schreiber, R.D.; Goeddel, D.V.; Ross, F.P.; Teitelbaum, S.L. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J. Exp. Med. 2003, 198, 771–781. [Google Scholar] [CrossRef]
- Matsushima, A.; Kaisho, T.; Rennert, P.D.; Nakano, H.; Kurosawa, K.; Uchida, D.; Takeda, K.; Akira, S.; Matsumoto, M. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 2001, 193, 631–636. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Girling, J.E.; Hedger, M.P. Toll-like receptors in the gonads and reproductive tract: Emerging roles in reproductive physiology and pathology. Immunol. Cell Biol. 2007, 85, 481–489. [Google Scholar] [CrossRef]
- Chandrasekharan, N.V.; Simmons, D.L. The cyclooxygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef]
- Gohda, J.; Matsumura, T.; Inoue, J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Van de Craen, M.; Declercq, W.; Van den brande, I.; Fiers, W.; Vandenabeele, P. The proteolytic procaspase activation network: An in vitro analysis. Cell Death Differ. 1999, 6, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Hazuda, D.J.; Strickler, J.; Kueppers, F.; Simon, P.L.; Young, P.R. Processing of precursor interleukin1ß and inflammatory disease. J. Biol. Chem. 1990, 265, 6318–6322. [Google Scholar]
- Black, R.A.; Kronheim, S.R.; Cantrell, M.; Deeley, M.C.; March, C.J.; Prickett, K.S.; Wignall, J.; Conlon, P.J.; Cosman, D.; Hopp, T.P.; et al. Generation of biologically active interleukin 1b by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 1988, 263, 9437–9442. [Google Scholar]
- Ito, A.; Mukaiyama, A.; Itoh, Y.; Nagase, H.; Thogersen, I.B.; Enghild, J.J.; Sasaguri, Y.; Mori, Y. Degradation of interleukin 1beta by matrix metalloproteinases. J. Biol. Chem. 1996, 271, 14657–14660. [Google Scholar] [CrossRef]
- Boerboom, D.; Brown, K.A.; Vaillancourt, D.; Poitras, P.; Goff, A.K.; Watanabe, K.; Doré, M.; Sirois, J. Expression of key prostaglandin synthases in equine endometrium during late diestrus and early pregnancy. Biol. Reprod. 2004, 70, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Walter, I.; Budik, S.; Kolodziejek, J.; Nowotny, N.; Aurich, C. Influence of different semen extenders and seminal plasma on PMN migration and on expression of IL-1β, IL-6, TNF-α and COX-2 mRNA in the equine endometrium. Theriogenology 2008, 70, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Contran, R.S.; Kumar, V.; Collins, T.; Robbins, S.L. Robbins Pathologic Basic of Disease, 6th ed.; W.B. Saunders: Philadelphia, PA, USA, 1999. [Google Scholar]
- Fumuso, E.; Aguilar, J.; Gigu, S. Interleukin-8 (IL-8) and 10 (IL-10) mRNA transcriptions in the endometrium of normal mares and mares susceptible to persistent post-breeding endometritis. Anim. Reprod. Sci. 2006, 94, 282–285. [Google Scholar]
- Doré, M.; Sirois, J. Regulation of P-selectin expression by inflammatory mediators in canine jugular endothelial cells. Vet. Pathol. 1996, 33, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R. Innate immunity: The recognition of invaders. In Veterinary Immunology, 9th ed.; Elsevier Saunders: St. Louis, MI, USA, 2009; Volume 1, pp. 11–20. [Google Scholar]
- Troedsson, M.H.T.; Liu, I.K.M.; Ing, M.; Pascoe, J. Smooth muscle electrical activity in the oviduct, and the effect of oxytocin, prostaglandin F2α, and prostaglandin E2 on the myometrium and the oviduct of the cycling mare. Biol. Reprod. 1995, 52, 475–488. [Google Scholar] [CrossRef]
- Lögters, T.; Margraf, S.; Altrichter, J.; Cinatl, J.; Mitzner, S.; Windolf, J.; Scholz, M. The clinical value of neutrophil extracellular traps. Med. Microbiol. Immunol. 2009, 198, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Brinkmann, V. Neutrophil extracellular traps in microbial infections. In Neutrophils Ininfectious Diseases; Tacchini-Cottier, F., Zandbergen, G., Eds.; Bentham eBooks: Dubais, UAE, 2011; pp. 3–10. [Google Scholar]
- Weinrauch, Y.; Drujan, D.; Shapiro, S.D.; Weiss, J.; Zychlinsky, A. Neutrophil elastase targets virulence factors of enterobacteria. Nature 2002, 417, 91–94. [Google Scholar] [CrossRef]
- Wartha, F.; Beiter, K.; Albiger, B.; Fernebro, J.; Zychlinsky, A.; Normark, S.; Henriques-Normark, B. Capsule and d-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 2007, 9, 1162–1171. [Google Scholar] [CrossRef]
- Van der Windt, D.; Bootsma, H.J.; Burghout, P.; Van der Gaast-de Jongh, C.E.; Hermans, P.W.M.; Van der Flier, M. Nonencapsulated Streptococcus pneumoniae resists extracellular human neutrophil elastase- and cathepsin G-mediated killing. FEMS Immunol. Med. Microbiol. 2012, 66, 445–448. [Google Scholar] [CrossRef]
- Marin-Esteban, V.; Turbica, I.; Dufour, G.; Semiramoth, N.; Gleizes, A.; Gorges, R.; Beau, I.; Servin, A.L.; Lievin-Le, M.V.; Sandré, C.; et al. Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect. Immun. 2012, 80, 1891–1899. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Carneiro, C.; Alexandre-Pires, G.; Brito, P.; Pereira, C.; Nunes, T.; Galvão, A.; Leitão, A.; Vilela, C.; Ferreira-Dias, G. Neutrophil extracellular traps formation by bacteria causing endometritis in the mare. J. Reprod. Immunol. 2014, 106, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.; Woodward, E.; Bojesen, A.M.; Jacobsen, S.; Petersen, M.R.; Troedsson, M.H.; Lehn-Jensen, H. Inflammatory responses to induced infectious endometritis in mares resistant or susceptible to persistent endometritis. BMC Vet. Res. 2012, 8, 41. [Google Scholar] [CrossRef]
- Arend, W.P.; Guthridge, C.J. Biological role of interleukin 1 receptor antagonist isoforms. Ann. Rheum. Dis. 2000, 59 (Suppl. S1), i60–i64. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Dripps, D.J.; Brandhuber, B.J.; Thompson, R.C.; Eisenberg, S.P. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J. Biol. Chem. 1991, 266, 10331–10336. [Google Scholar] [PubMed]
- Cassatella, M.A.; Meda, L.; Gasperini, S.; Calzetti, F.; Bonora, S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J. Exp. Med. 1994, 179, 1695–1699. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar]
- Troedsson, M.H.T.; Liu, I.K.M.; Ing, M.; Pascoe, J.; Thurmond, M. Multiple site electromyography recordings of uterine activity following an intrauterine bacterial challenge in mares susceptible and resistant to chronic uterine infection. J. Reprod. Fertil. 1993, 99, 307–313. [Google Scholar] [CrossRef]
- Griscavage, J.M.; Wilk, S.; Ignarro, L.J. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 1996, 93, 3308–3312. [Google Scholar] [CrossRef]
- Liu, I.; Rakestraw, P.; Coit, C.; Harmon, F.; Snyder, J. An in vitro investigation of the mechanism of neuromuscular regulation in myometrial contractility. Pferdeheilkunde 1997, 13, 557. [Google Scholar]
- Khan, F.A.; Chenier, T.S.; Murrant, C.L.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Dose-dependent inhibition of uterine contractility by nitric oxide: A potential mechanism underlying persistent breeding-induced endometritis in the mare. Theriogenology 2017, 90, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Chenier, T.S.; Foster, R.A.; Hewson, J.; Scholtz, E.L. Endometrial nitric oxide synthase activity in mares susceptible or resistant to persistent breeding-induced endometritis and the effect of a specific iNOS inhibitor in vitro. Reprod. Domest. Anim. 2018, 53, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Hamer, J.M.; Gason, L.M.; Graham, C.S.; Asbury, A.C.; Irvine, C.H. Clearance of bacteria and non-antigenic markers following intra-uterine inoculation into maiden mares: Effect of steroid hormone environment. Theriogenology 1986, 26, 37–50. [Google Scholar] [CrossRef]
- Bowdish, D.M.E.; Davidson, D.J.; Hancock, R.E.W. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 2006, 306, 27–66. [Google Scholar] [PubMed]
- Oddsdóttir, C.; Riley, S.C.; Leask, R.; Edwards, D.R. Activities of matrix metalloproteinases-9 and -2 in uterine fluid during induced equine endometritis. Pferdeheilkunde 2008, 24, 70–73. [Google Scholar]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases. An overview. Mol. Cell. Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef]
- Curry, T.E.; Osteen, K.G. The matrix metalloproteinase system: Changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev. 2003, 24, 428–465. [Google Scholar] [CrossRef]
- Rebordão, M.; Galvão, A.; Szóstek, A.; Amaral, A.; Mateus, L.; Skarzynski, D.; Ferreira-Dias, G. Physiopathologic mechanisms involved in mare endometrosis. Reprod. Domest. Anim. 2014, 49, 82–87. [Google Scholar] [CrossRef]
- Alpoim-Moreira, J.; Fernandes, C.; Rebordão, M.R.; Amaral, A.; Pinto-Bravo, P.; Bliebernicht, M.; Skarzynski, D.J.; Ferreira-Dias, G. Collagens and DNA methyltransferases in mare endometrosis. Reprod. Domest. Anim. 2019, 54, 46–52. [Google Scholar] [CrossRef]
- Hoffmann, C.; Ellenberger, C.; Mattos, R.C.; Aupperle, H.; Dhein, S.; Stief, B.; Schoon, H.-A. The equine endometrosis: New insights into the pathogenesis. Anim. Reprod. Sci. 2009, 111, 261–278. [Google Scholar] [CrossRef]
- Rawdanowicz, T.J.; Hampton, A.L.; Nagase, H.; Woolley, D.E.; Salamonsen, L.A. Matrix metalloproteinase production by cultured human endometrial stromal cells: Identification of interstitial collagenase, gelatinase-A, gelatinase-B, and stromelysin-1 and their differential regulation by interleukin-1 alpha and tumor necrosis factor-alpha. J. Clin. Endocrinol. Metab. 1994, 79, 530–536. [Google Scholar]
- Singer, C.F.; Marbaix, E.; Lemoine, P.; Courtoy, P.J.; Eeckhout, Y. Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur. J. Biochem. 1999, 259, 40–45. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Nowak, R.A. Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer. Am. J. Reprod. Immunol. 2006, 56, 201–214. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Lukasik, K.; Szóstek-Mioduchowska, A.; Baclawska, A.; Rebordão, M.R.; Aguiar-Silva, J.; Pinto-Bravo, P.; Skarzynski, D.J.; Ferreira-Dias, G. Elastase inhibition affects collagen transcription and prostaglandin secretion in mare endometrium during the estrous cycle. Reprod. Domest. Anim. 2018, 53, 66–69. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.Z.; Lukasik, K.; Skarzynski, D.J.; Okuda, K. Effect of transforming growth factor -β1 on α-smooth muscle actin and collagen expression in equine endometrial fibroblasts. Theriogenology 2019, 124, 9–17. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the interleukin system in equine endometrium during the course of endometrosis. Biol. Reprod. 2013, 89, 79. [Google Scholar] [CrossRef]
- Vesey, D.A.; Cheung, C.; Cuttle, L.; Endre, Z.; Gobe, G.; Johnson, D.W. Interleukin-1 β stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-β-dependent mechanism. J. Lab. Clin. Med. 2002, 140, 342–350. [Google Scholar] [CrossRef]
- Xiao, H.; Ji, A.M.; Li, Z.L.; Song, X.D.; Su, D.; Chen, A.H. Interleukin-1β inhibits collagen synthesis and promotes its decomposition in cultured cardiac fibroblasts. Sheng Li Xue Bao 2008, 60, 355–361. [Google Scholar]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Linde, A.; Ross, C.R.; Davis, E.G.; Dib, L.; Blecha, F.; Melgarejo, T. Innate immunity and host defense peptides in veterinary medicine. J. Vet. Intern. Med. 2008, 22, 247–265. [Google Scholar] [CrossRef]
- Nevalainen, T.J.; Graham, G.G.; Scott, K.F. Antibacterial actions of secreted phospholipases A2. Review. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 1–9. [Google Scholar] [CrossRef]
- Tomee, J.F.C.; Koëter, G.H.; Hiemstra, P.S.; Kauffman, H.F. Secretory leukoprotease inhibitor: A native antimicrobial protein presenting a new therapeutic option? Thorax 1998, 53, 114–116. [Google Scholar] [CrossRef]
- Couto, M.A.; Harwig, S.S.; Lehrer, R.I. Selective inhibition of microbial serine proteases by eNAP-2, an antimicrobial peptide from equine neutrophils. Infect. Immun. 1993, 61, 2991–2994. [Google Scholar] [CrossRef]
- Couto, M.A.; Harwig, S.S.; Cullor, J.S.; Hughes, J.P.; Lehrer, R.I. eNAP-2, a novel cysteine-rich bactericidal peptide from equine leukocytes. Infect. Immun. 1992, 60, 5042–5047. [Google Scholar] [CrossRef]
- Kolm, G.; Klein, D.; Knapp, E.; Watanabe, K.; Walter, I. Lactoferrin expression in the horse endometrium: Relevance in persisting mating-induced endometritis. Vet. Immunol. Immunopathol. 2006, 114, 159–167. [Google Scholar] [CrossRef]
- Hultén, C.; Grönlund, U.; Hirvonen, J.; Tulamo, R.-M.; Suominen, M.M.; Marhaug, G.; Forsberg, M. Dynamics in serum of the inflammatory markers serum amyloid A (SAA), haptoglobin, fibrinogen and α2-globulins during induced noninfectious arthritis in the horse. Equine Vet. J. 2010, 34, 699–704. [Google Scholar] [CrossRef]
- Hultén, C.; Sandgren, B.; Skiöldebrand, E.; Klingeborn, B.; Marhaug, G.; Forsberg, M. The acute phase protein serum amyloid A (SAA) as an inflammatory marker in equine influenza virus infection. Acta Vet. Scand. 1999, 40, 323–333. [Google Scholar]
- Nunokawa, Y.; Fujinaga, T.; Taira, T.; Okumura, M.; Yamashita, K.; Tsunoda, N.; Hagio, M. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J. Vet. Med. Sci. 1993, 55, 1011–1016. [Google Scholar] [CrossRef]
- Tuppits, U.; Orro, T.; Einarsson, S.; Kask, K.; Kavak, A. Influence of the uterine inflammatory response after insemination with frozen-thawed semen on serum concentrations of acute phase proteins in mares. Anim. Reprod. Sci. 2014, 146, 182–186. [Google Scholar] [CrossRef]
- Berg, L.C.; Thomsen, P.D.; Andersen, P.H.; Jensen, H.E.; Jacobsen, S. Serum amyloid A is expressed in histologically normal tissues from horses and cattle. Vet. Immunol. Immunopathol. 2011, 144, 155–159. [Google Scholar] [CrossRef]
- Mette, C.; Camilla Dooleweerdt, B.; Stine, J.; Anders Miki, B.; Morten Roenn, P.; Henrik, L.-J.; Bojesen, A.M.; Anders Miki, B.; Petersen, M.R.; Morten Roenn, P.; et al. Evaluation of the systemic acute phase response and endometrial gene expression of serum amyloid A and pro- and anti-inflammatory cytokines in mares with experimentally induced endometritis. Vet. Immunol. Immunopathol. 2010, 138, 95–105. [Google Scholar] [CrossRef]
- Nielsen, J.M. Endometritis in the mare: A diagnostic study comparing cultures from swab and biopsy. Theriogenology 2005, 64, 510–518. [Google Scholar] [CrossRef]
- Ferris, R.A.; Bohn, A.; McCue, P.M. Equine endometrial cytology: Collection techniques and interpretation. Equine Vet. Educ. 2015, 27, 316–322. [Google Scholar] [CrossRef]
- Bohn, A.A.; Ferris, R.A.; Mccue, P.M. Comparison of equine endometrial cytology samples collected with uterine swab, uterine brush, and low-volume lavage from healthy mares. Vet. Clin. Pathol. 2014, 43, 594–600. [Google Scholar] [CrossRef]
- Cocchia, N.; Paciello, O.; Auletta, L.; Uccello, V.; Silvestro, L.; Mallardo, K.; Paraggio, G.; Pasolini, M.P. Comparison of the cytobrush, cottonswab, and low-volume uterine flush techniques to evaluate endometrial cytology for diagnosing endometritis in chronically infertile mares. Theriogenology 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Christoffersen, M.; Brandis, L.; Samuelsson, J.; Bojesen, A.M.; Troedsson, M.H.T.; Petersen, M.R. Diagnostic double-guarded low-volume uterine lavage in mares. Theriogenology 2015, 83, 222–227. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Nielsen, F.H.; Petersen, M.R.; Dyrehospital, A. Diagnosis of equine endometritis-Microbiology, cytology and histology of endometrial biopsies and the correlation to fertility. Pferdeheilkunde 2012, 28, 8–13. [Google Scholar]
- Nielsen, J.M.; Troedsson, M.H.; Pedersen, M.R.; Bojesen, A.M.; Lehn-Jensen, H.; Zent, W.W. Diagnosis of endometritis in the mare based on bacteriological and cytological examinations of the endometrium: Comparison of results obtained by swabs and biopsies. J. Equine Vet. Sci. 2010, 30, 27–30. [Google Scholar] [CrossRef]
- Ball, B.A.; Shin, S.J.; Patten, V.H.; Lein, D.H.; Woods, G.L. Use of a low-volume uterine flush for microbiologic and cytologic examination of the mare’s endometrium. Theriogenology 1988, 29, 1269–1283. [Google Scholar] [CrossRef]
- Ferris, R.A. Endometritis: Diagnostic tools for infectious endometritis. Vet. Clin. N. Am. Equine Pract. 2016, 32, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.A.; Dern, K.; Veir, J.K.; Hawley, J.R.; Lappin, M.R.; McCue, P.M. Development of a broad-range quantitative polymerase chain reaction assay to detect and identify fungal dna in equine endometrial samples. Am. J. Vet. Res. 2013, 74, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M. Prognostic value of endometrial biopsy of the mare. J. Reprod. Fertil. Suppl. 1975, 23, 347–348. [Google Scholar]
- Doig, P.A.; McKnight, J.D.; Miller, R.B. The use of endometrial biopsy in the infertile mare. Can. Vet. J. 1981, 22, 72–76. [Google Scholar]
- Ricketts, S.W.; Alonso, S. Assessment of the breeding prognosis of mares using paired endometrial biopsy techniques. Equine Vet. J. 1991, 23, 185–188. [Google Scholar] [CrossRef]
- Kenney, R.M.; Doig, P.A. Equine endometrial biopsy. In Current Therapy in Theriogenology; Morrow, D.A., Ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1986; pp. 723–729. [Google Scholar]
- Cadario, M.E.; Thatcher, M.J.D.; Leblanc, M.M. Relationship between prostaglandin and uterine clearance of radiocolloid in the mare. Biol. Reprod. 1995, 52, 495–500. [Google Scholar] [CrossRef]
- Rasch, K.; Schoon, H.A.; Sieme, H.; Klug, E. Histomorphological endometrial status and influence of oxytocin on the uterine drainage and pregnancy rate in mares. Equine Vet. J. 1996, 28, 455–460. [Google Scholar] [CrossRef]
- Allen, W.E. Investigations into the use of exogenous oxytocin for promoting uterine drainage in mares susceptible to endometritis. Vet. Rec. 1991, 128, 593–594. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Varner, D.D.; Blanchard, T.L. The effect of uterine lavage performed four hours post insemination on pregnancy rate in mares. Theriogenology 1991, 35, 1111–1119. [Google Scholar] [CrossRef]
- Cadario, M.E.; Thatcher, W.W.; Klapstein, E.; Merrit, A.M.; Archbald, L.F.; Thatcher, M.J.; LeBlanc, M.M. Dynamics of prostaglandin secretion, intrauterine fluid and uterine clearance in reproductively normal mares and mares with delayed uterine clearance. Theriogenology 1999, 52, 1181–1192. [Google Scholar] [CrossRef]
- Madill, S.; Troedsson, M.H.T.; Santschi, E.M.; Malone, E.D. Dose-response effect of intramuscular oxytocin treatment on myometrial contraction of reproductively normal mares during estrus. Theriogenology 2002, 58, 479–481. [Google Scholar]
- Campbell, M.L.H.; England, G.C.W. A comparison of the ecbolic efficacy of intravenous and intrauterine oxytocin treatments. Theriogenology 2002, 58, 473–477. [Google Scholar]
- Brendemuehl, J.P. Effect of oxytocin and PGF2α on luteal formation, function and pregnancy rates in mares. Theriogenology 2002, 58, 623–626. [Google Scholar]
- LeBlanc, M.M. Persistent Mating-Induced Endometritis. In Current Therapy in Equine Medicine, 5th ed.; Sprayberry, K.A., Robinson, E., Eds.; Elsevier Saunders: St. Louis, MI, USA, 2003; pp. 234–237. [Google Scholar]
- Irvine, C.H.G.; Mckeough, V.-L.; Turner, J.E.; Alexander, S.L.; Taylor, T.B. Effectiveness of a two-dose regimen of prostaglandin administration in inducing luteolysis without adverse side effects in mares. Equine Vet. J. 2002, 34, 191–194. [Google Scholar] [CrossRef]
- Nie, G.J.; Johnson, K.E.; Wenzel, J.G.W.; Braden, T.D. Effect of periovulatory ecbolics on luteal function and fertility. Theriogenology 2002, 58, 461–463. [Google Scholar]
- Schramme, A.R.; Pinto, C.R.F.; Davis, J.; Whisnant, C.S.; Whitacre, M.D. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, following intravenous administration in horses. Equine Vet. J. 2008, 40, 658–661. [Google Scholar] [CrossRef]
- Steckler, D.; Naidoo, V.; Gerber, D.; Kähn, W. Ex vivo influence of carbetocin on equine myometrial muscles and comparison with oxytocin. Theriogenology 2012, 78, 502–509. [Google Scholar] [CrossRef]
- Dascanio, J.J. How and When to Treat Endometritis With Systemic or Local Antibiotics. In Proceedings of the 57th American Association of Equine Practitioners, San Antonio, TX, USA, 1 January 2011; p. 57. [Google Scholar]
- Benko, T.; Boldizar, M.; Novotny, F.; Hura, V.; Valocky, I.; Dudrikova, K.; Karamanova, M.; Petrovic, V. Incidence of bacterial pathogens in equine uterine swabs, their antibiotic resistance patterns, and selected reproductive indices in English thoroughbred mares during the foal heat cycle. Vet. Med. 2015, 60, 613–620. [Google Scholar] [CrossRef]
- Giguère, S. Antimicrobial drug action and interaction: An introduction. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 3–10. [Google Scholar]
- Vila, J.; Martí, S.; Sánchez-Céspedes, J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 2007, 59, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.S.; Han, S.; Nielsen, S.; Pearson, L.K.; Gay, J.M.; Tibary, A. Consequences of intrauterine enrofloxacin infusion on mare endometrium. J. Equine Vet. Sci. 2012, 32, 106–111. [Google Scholar] [CrossRef]
- Schnobrich, M.R.; Pearson, L.K.; Barber, B.K.; Bradecamp, E.; Tibary, A. Effects of intrauterine infusion of a water-based suspension of enrofloxacin on mare endometrium. J. Equine Vet. Sci. 2015, 35, 662–667. [Google Scholar] [CrossRef]
- Leblanc, M. The current status of antibiotic use in equine reproduction. Equine Vet. Educ. 2009, 21, 156–167. [Google Scholar] [CrossRef]
- Giguère, S. Antifungal chemotherapy. In Antimicrobial Therapy in Veterinary Medicine; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 333–355. [Google Scholar]
- Brinsko, S.P.; Rigby, S.L.; Varner, D.; Blanchard, T.L. A practical method for recognizing mares susceptible to post-breeding endometritis. AAEP Proc. 2003, 49, 363–365. [Google Scholar]
- Vanderwall, D.K.; Woods, G.L. Effect on fertility of uterine lavage performed immediately prior to insemination in mares. J. Am. Vet. Med. Assoc. 2003, 222, 1108–1110. [Google Scholar] [CrossRef]
- Hua, Q.; Joyce, A.R.; Palsson, B.Ø.; Fong, S.S. Metabolic characterization of Escherichia coli strains adapted to growth on lactate. Appl. Environ. Microbiol. 2007, 73, 4639–4647. [Google Scholar] [CrossRef]
- Eisenberg, R.C.; Dobrogosz, W.J. Gluconate metabolism in Escherichia coli. J. Bacteriol. 1967, 93, 941–949. [Google Scholar] [CrossRef]
- Knutti, B.; Pycock, J.F.; Weijden, G.C.; Küpfer, U. The influence of early postbreeding uterine lavage on pregnancy rate in mares with intrauterine fluid accumulations after breeding. Equine Vet. Educ. 2010, 12, 267–270. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Varner, D.D.; Blanchard, T.L.; Meyers, S.A. The effect of postbreeding uterine lavage on pregnancy rate in mares. Theriogenology 1990, 33, 465–475. [Google Scholar] [CrossRef]
- Fiala, S.M.; Pimentel, C.A.; Mattos, A.L.G.; Gregory, R.M.; Mattos, R.C. Effect of sperm numbers and concentration on sperm transport and uterine inflammatory response in the mare. Theriogenology 2007, 67, 556–562. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M. Effects of oxytocin, prostaglandin and phenylbutazone on uterine clearance of radiocolloid. Pferdeheilkunde 1997, 13, 483–485. [Google Scholar] [CrossRef][Green Version]
- Reilas, T.; Risco, A.M.; Kareskoski, M.; Katila, T. Effect of flunixin meglumine and oxytocin on uterine response to insemination in mares. Anim. Reprod. Sci. 2006, 94, 252–253. [Google Scholar]
- Cuervo-Arango, J. The effect of treatment with flunixin meglumine at different times relative to hCG administration on ovulation failure and luteal function in mares. Anim. Reprod. Sci. 2011, 127, 84–90. [Google Scholar] [CrossRef]
- Armstrong, D.T. Prostaglandins and follicular functions. J. Reprod. Fertil. 1981, 62, 283–291. [Google Scholar] [CrossRef]
- Donnelly, C.G.; Sones, J.L.; Dockweiler, J.C.; Norberg, L.A.; Norberg, L.E.; Cheong, S.H.; Gilbert, R.O. Effects of flunixin meglumine on postponement of ovulation in mares. Am. J. Vet. Res. 2019, 80, 306–310. [Google Scholar] [CrossRef]
- Aurich, C.; Rojer, H.; Walter, I. Treatment of estrous mares with the non-steroidal anti-inflammatory drug vedaprofen reduces the inflammatory response of the endometrium to insemination. Anim. Reprod. Sci. 2010, 121, 104–106. [Google Scholar]
- Cook, V.L.; Blikslager, A.T. The use of nonsteroidal anti-inflammatory drugs in critically ill horses. J. Vet. Emerg. Crit. Care 2015, 25, 76–88. [Google Scholar] [CrossRef]
- Friso, A.M.; Segabinazzi, L.G.T.M.; Cyrino, M.; Correal, S.B.; Freitas-Dell’Aqua, C.P.; Teoro do Carmo, M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Periovulatory administration of firocoxib did not alter ovulation rates and mitigated post-breeding inflammatory response in mares. Theriogenology 2019, 138, 24–30. [Google Scholar] [CrossRef]
- Rojer, H.; Aurich, C. Treatment of persistent mating-induced endometritis in mares with the non-steroid anti-inflammatory drug vedaprofen. Reprod. Domest. Anim. 2010, 45, 2009–2011. [Google Scholar] [CrossRef] [PubMed]
- Papa, F.O.; Dell’Aqua, J.A., Jr.; Alvarenga, M.A.; Melo-Oña, C.M.; Zahn, F.; Lopes, M.D. Use of corticosteroid therapy on the modulation of uterine inflammatory response in mares after artificial insemination with frozen semen. Pferdeheilkunde 2007, 24, 79–82. [Google Scholar] [CrossRef]
- Ruijter-Villani, M.; de Grauw, J.C.; de Stout, T.A.E. Post-breeding endometritis: Effect of dexamethasone treatment on the expression of inflammatory markers. In Proceedings of the 7th International Conference on Equine Reproductive Medicine, Leipzig, Germany, 20–21 January 2012; Leipziger blaue Hefte Band 2: Leipzig, Germany; pp. 286–288. [Google Scholar]
- Arlas, T.R.; Wolf, C.A.; Petrucci, B.P.L.; Estanislau, J.F.; Gregory, R.M.; Jobim, M.I.M.; Mattos, R.C. Proteomics of endometrial fluid after dexamethasone treatment in mares susceptible to endometritis. Theriogenology 2015, 84, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ohman, T.; Klein, C.; Doty, A.; Troedsson, M.H.T. The phagocytic function of blood-derived polymorphonuclear neutrophils after administration of dexamethasone for the modulation of post-breeding endometritis in the mare. Pferdeheilkunde 2011, 27, 267–270. [Google Scholar] [CrossRef][Green Version]
- Wolf, C.A.; Maslchitzky, E.; Gregory, R.M.; Jobim, M.I.M.; Mattos, R.C. Effect of corticotherapy on proteomics of endometrial fluid from mares susceptible to persistent postbreeding endometritis. Theriogenology 2012, 77, 1351–1359. [Google Scholar] [CrossRef]
- Ferris, R.A.; Mccue, P.M. The effects of dexamethasone and prednisolone on pituitary and ovarian function in the mare. Equine Vet. J. 2010, 42, 438–443. [Google Scholar] [CrossRef]
- McNeill-Wiest, D.R.; Thompson, D.L.; Wiest, J.J. Gonadotropin secretion in ovariectomized pony mares treated with dexamethasone or progesterone and subsequently with dihydrotestosterone. Domest. Anim. Endocrinol. 1988, 5, 149–155. [Google Scholar] [CrossRef]
- Thompson, D.L.; Garza, F.; St George, R.L.; Rabb, M.H.; Barry, B.E.; French, D.D. Relationships among LH, FSH and prolactin secretion, storage and response to secretagogue and hypothalamic GnRH content in ovariectomized pony mares administered testosterone, dihydrotestosterone, estradiol, progesterone, dexamethasone or follicular fluid. Domest. Anim. Endocrinol. 1991, 8, 189–199. [Google Scholar] [CrossRef]
- Andrews, R.V. Influence of the adrenal gland on gonadal function. Adv. Sex Horm. Res. 1977, 3, 197–215. [Google Scholar] [PubMed]
- Schreiber, J.R.; Nakamura, K.; Erickson, G.F. Rat ovary glucocorticoid receptor: Identification and characterization. Steroids 1982, 39, 569–584. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Erickson, G.F. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids 1978, 32, 639–648. [Google Scholar] [CrossRef]
- Rogan, D.; Fumuso, E.; Rodríguez, E.; Wade, J.; Sánchez Bruni, S.F. Use of a Mycobacterial cell wall extract (MCWE) in susceptible mares to clear experimentally induced endometritis with Streptococcus zooepidemicus. J. Equine Vet. Sci. 2007, 27, 112–117. [Google Scholar] [CrossRef]
- Rohrbach, B.W.; Sheerin, P.C.; Cantrell, C.K.; Matthews, P.M.; Steiner, J.V.; Dodds, L.E. Effect of adjunctive treatment with intravenously administered Propionibacterium acnes on reproductive performance in mares with persistent endometritis. J. Am. Vet. Med. Assoc. 2007, 231, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.; Copié, V. Mini-review: Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Woodward, E.M.; Scoggin, K.E.; Esteller-Vico, A.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The effect of cysteine-rich secretory protein-3 and lactoferrin on endometrial cytokine mRNA expression after breeding in the horse. J. Equine Vet. Sci. 2017, 48, 136–142. [Google Scholar] [CrossRef]
- Coutinho da Silva, M.A.; Darr, C.R.; Moraes, L.E.; Forshey, B.S. Lactoferrin modulates uterine inflammation postbreeding in the mare. J. Equine Vet. Sci. 2017, 56, 63–67. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Scoggin, K.E.; Woodward, E.M.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The effect of select seminal plasma proteins on endometrial mRNA cytokine expression in mares susceptible to persistent mating-induced endometritis. Reprod. Domest. Anim. 2017, 52, 89–96. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Scoggin, K.E.; Boakari, Y.L.; Hoppe, N.E.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The anti-inflammatory effect of exogenous lactoferrin on breeding-induced endometritis when administered post-breeding in susceptible mares. Theriogenology 2018, 114, 63–69. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Ward, L.S.; Fisher, S.T.; Wolcott, R.D.; James, G.A. In vitro susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol. Int. J. Antimicrob. Agents 2009, 33, 230–236. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Ward, L.S.; James, G.A. Anti-biofilm efficacy of a lactoferrin/xylitol wound hydrogel used in combination with silver wound dressings. Int. Wound J. 2011, 8, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.D.F.; De La Côrte, F.D.; Brass, K.E.; da Silva Azevedo, M.; Gallio, M.; Cantarelli, C.; Dau, S.L.; Cezar, A.S.; Inkelmann, M.A. Evaluation of three methods of platelet-rich plasma for treatment of equine distal limb skin wounds. J. Equine Vet. Sci. 2019, 72, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.U.; Argüelles, D.; Climent, F.; Prades, M. Autologous platelet concentrates as a treatment of horses with osteoarthritis: A preliminary pilot clinical study. J. Equine Vet. Sci. 2007, 27, 167–170. [Google Scholar] [CrossRef]
- Argüelles, D.; Carmona, J.U.; Climent, F.; Muñoz, E.; Prades, M. Autologous platelet concentrates as a treatment for musculoskeletal lesions in five horses. Vet. Rec. 2008, 162, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Georg, R.; Maria, C.; Gisela, A.; Bianca, C. Autologous conditioned plasma as therapy of tendon and ligament lesions in seven horses. J. Vet. Sci. 2010, 11, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.R. Effect of Adding Autologous Plasma to an intrauterine antibiotic therapy after breeding on pregnancy rates in mares. Biol. Reprod. 1995, 52, 539–543. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yeom, J.S.; Koh, Y.-G.; Yeo, J.-E.; Kang, K.-T.; Kang, Y.-M.; Chang, B.-S.; Lee, C.-K. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J. Orthop. Res. 2014, 32, 551–556. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Karas, V.; Della Valle, C.; Tetreault, M.W.; Mohammed, H.O.; Fortier, L.A. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am. J. Sports Med. 2014, 42, 35–41. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, W.-H.; Zao, B.; Lai, P.-L.; Lin, T.-C.; Lo, H.-Y.; Shieh, Y.-H.; Wu, C.-H.; Deng, W.-P. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials 2011, 32, 5847–5854. [Google Scholar] [CrossRef]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef]
- Mazzocca, A.D.; McCarthy, M.B.R.; Intravia, J.; Beitzel, K.; Apostolakos, J.; Cote, M.P.; Bradley, J.; Arciero, R.A. An in vitro evaluation of the anti-inflammatory effects of platelet-rich plasma, ketorolac, and methylprednisolone. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cieslik-Bielecka, A.; Bielecki, T.; Gazdzik, T.S.; Arendt, J.; Król, W.; Szczepanski, T. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfus. Apher. Sci. 2009, 41, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, C.C.; Stammers, A.H.; Woods, E.; Yen, B.R.; Klayman, M.; Gilbert, C. Use of platelet gel and its effects on infection in cardiac surgery. J. Extra Corpor. Technol. 2005, 37, 381–386. [Google Scholar] [PubMed]
- Yuan, T.; Zhang, C.; Zeng, B. Treatment of chronic femoral osteomyelitis with platelet-rich plasma (PRP): A case report. Transfus. Apher. Sci. 2008, 38, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alonso, R.; Girbau, C.; Aguirre, J.J.; Muruzabal, F.; Orive, G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin. Exp. Dermatol. 2012, 37, 652–657. [Google Scholar] [CrossRef]
- Álvarez, M.; López, C.; Giraldo, C.; Samudio, I.; Carmona, J. In vitro bactericidal activity of equine platelet concentrates, platelet poor plasma, and plasma against methicillin-resistant Staphylococcus aureus. Arch. Med. Vet. 2011, 43, 155–161. [Google Scholar] [CrossRef][Green Version]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Kròl, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances. J. Bone Joint Surg. Br. 2007, 89, 417–420. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.-L.; Wu, Y.-W.; Su, C.-Y.; Lee, L.-W. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion 2013, 53, 138–146. [Google Scholar] [CrossRef]
- Moojen, D.J.F.; Everts, P.A.M.; Schure, R.-M.; Overdevest, E.P.; van Zundert, A.; Knape, J.T.A.; Castelein, R.M.; Creemers, L.B.; Dhert, W.J.A. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J. Orthop. Res. 2008, 26, 404–410. [Google Scholar] [CrossRef]
- Reghini, M.F.S.; Ramires Neto, C.; Segabinazzi, L.G.; Castro Chaves, M.M.B.; Dell’Aqua, C.D.P.F.; Bussiere, M.C.C.; Dell’Aqua, J.A.; Papa, F.O.; Alvarenga, M.A. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology 2015, 86, 516–522. [Google Scholar] [CrossRef]
- Segabinazzi, L.G.; Friso, A.M.; Correal, S.B.; Crespilho, A.M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Uterine clinical findings, fertility rate, leucocyte migration, and COX-2 protein levels in the endometrial tissue of susceptible mares treated with platelet-rich plasma before and after AI. Theriogenology 2017, 104, 120–126. [Google Scholar] [CrossRef]
- Metcalf, E.S. The effect of Platelet-Rich Plasma (PRP) on intraluminal fluid and pregnancy rates in mares susceptible to persistent mating-induced endometritis (PMIE). J. Equine Vet. Sci. 2014, 34, 128. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Scoggin, K.; Troedsson, M.H.T. The effect of platelet-rich plasma on endometrial pro-inflammatory cytokines in susceptible mares following semen deposition. J. Equine Vet. Sci. 2012, 32, 498. [Google Scholar] [CrossRef]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.M.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Remacha, A.; Romero, A.; Vázquez, F.; Albareda, J.; Prades, M.; Ranera, B.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet. Immunol. Immunopathol. 2016, 171, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.E.; Bruscia, E.; Krause, D.S. Plasticity of bone marrow-derived stem cells. Stem Cells 2004, 22, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef]
- Alvarenga, M.A.; do Carmo, M.T.; Segabinazzi, L.G.; Guastali, M.D.; Maia, L.; Landim-Alvarenga, F.C. Feasibility and safety of endometrial injection of autologous bone marrow mesenchymal stem cells in mares. J. Equine Vet. Sci. 2016, 42, 12–18. [Google Scholar] [CrossRef]
- Mambelli, L.I.; Mattos, R.C.; Winter, G.H.Z.; Madeiro, D.S.; Morais, B.P.; Malschitzky, E.; Miglino, M.A.; Kerkis, A.; Kerkis, I. Changes in expression pattern of selected endometrial proteins following mesenchymal stem cells infusion in mares with endometrosis. PLoS ONE 2014, 9, e97889. [Google Scholar] [CrossRef]
- Ferris, R.A.; Frisbie, D.D.; McCue, P.M. Use of mesenchymal stem cells or autologous conditioned serum to modulate the inflammatory response to spermatozoa in mares. Theriogenology 2014, 82, 36–42. [Google Scholar] [CrossRef]
- Rink, B.E.; Beyer, T.; French, H.M.; Watson, E.; Aurich, C.; Donadeu, F.X. The fate of autologous endometrial mesenchymal stromal cells after application in the healthy equine uterus. Stem Cells Dev. 2018, 27, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Mambelli, L.I.; Winter, G.H.Z.; Kerkis, A.; Malschitzky, E.; Mattos, R.C.; Kerkis, I. A novel strategy of mesenchymal stem cells delivery in the uterus of mares with endometrosis. Theriogenology 2013, 79, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod. Sci. 2015, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Xu, W.R.; Qian, H.; Zhu, W.; Yan, Y.M.; Shao, Q.X.; Xu, H.X. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm. Res. 2010, 59, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef]
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef]
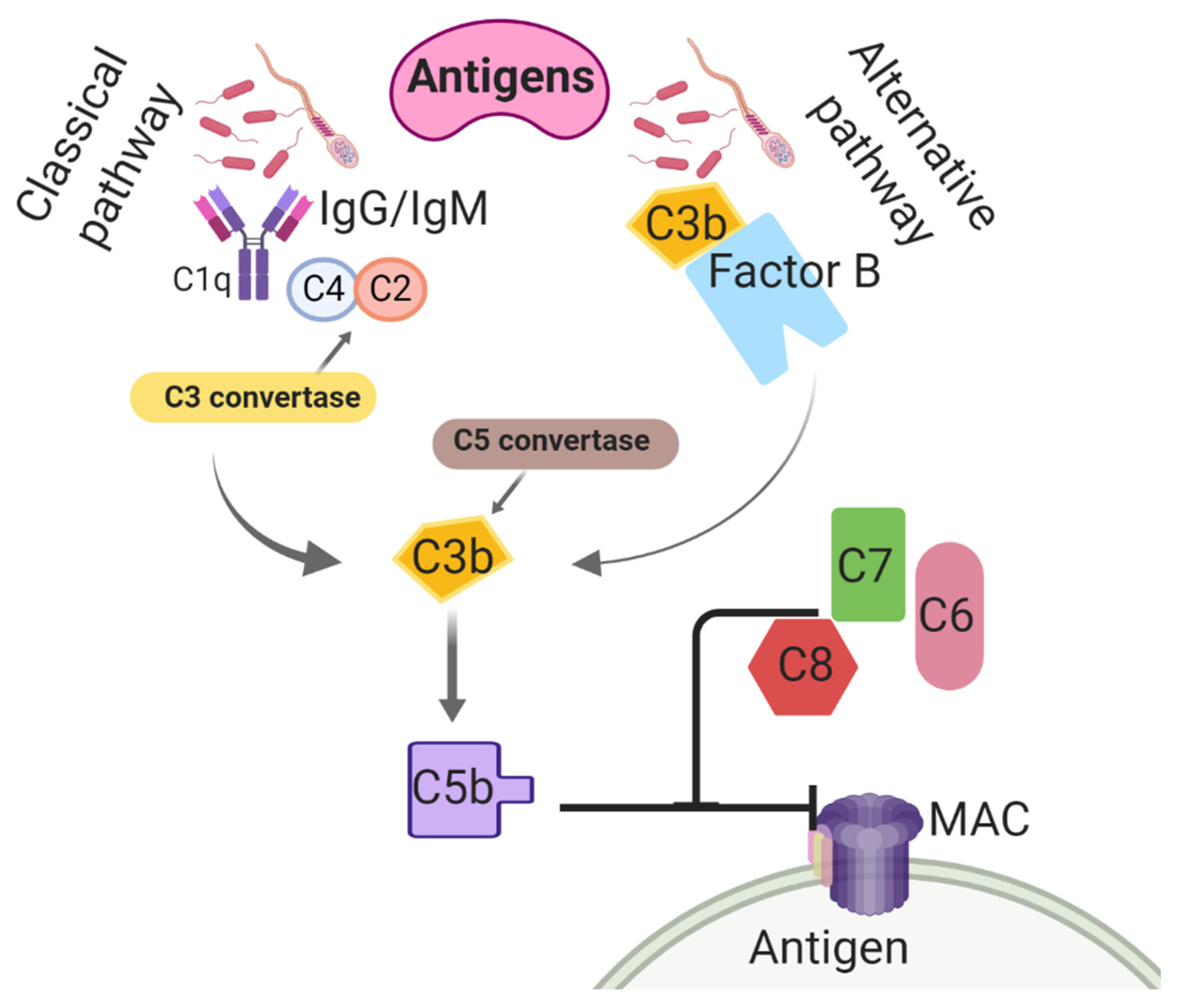



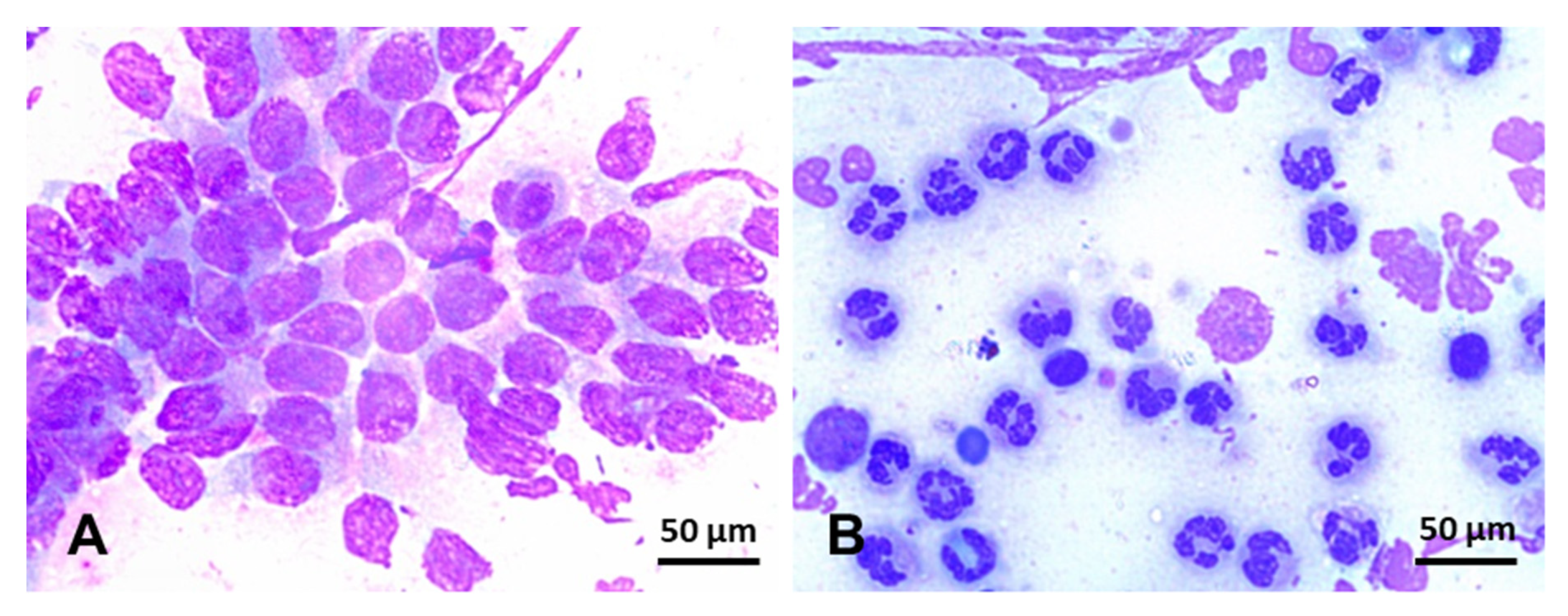

| Microorganisms | Superfamily | Features | |
|---|---|---|---|
| Bacteria | Streptococcus zooepidemicus | Lactobacillales | G+, opportunistic agent, potentially venereal. |
| Escherichia coli | Enterobacterales | G-, opportunistic, facultative anaerobic | |
| Pseudomonas aeruginosa | Pseudomonadales | G +, potentially venereal, aerobic | |
| Klebsiella pneumoniae | Enterobacterales | G-, opportunistic agent, facultatively anaerobic, potentially venereal | |
| Staphylococcus spp | Bacillales | G+, opportunistic, facultative anaerobic | |
| Taylorella equigenitalis | Burkholderiales | G-, venereal, microaerophilic, cause severe purulent endometritis | |
| Enterobacter cloacae | Enterobacterales | G-, opportunistic, facultative anaerobic | |
| Proteus spp | Enterobacterales | G-, opportunistic, anaerobic | |
| Fungus | Candida spp. | Saccharomycetales | Yeast, 58–69% of fungal endometritis |
| Aspergillus spp. | Eurotiales | Mold with septate hyphae, 25–26% of fungal endometritis | |
| Mucor spp. | Mucorales | Mold with aseptate hyphae, 5–12% of fungal endometritis |
| Technique | Approach and Applications | Limitations |
|---|---|---|
| Ultrasound | Used as a screening tool to detect the presence, amount, and appearance of IUF, which can be suggestive of endometritis. | Not all mares affected by endometritis, particularly chronic endometritis, accumulate IUF. The amount and echogenicity of fluid can be useful to direct the need for additional diagnostic techniques and therapeutic regimens. |
| Cotton-tip swab | Fast, user-friendly, and inexpensive approach to collect samples for culture and cytology. Results in combination with cytology can be used to dictate therapeutic approaches | Only a small segment of the uterus is sampled, and thus, focal infections not generating a diffuse endometrial response can be missed. In comparison with cytobrush, fewer cells are recovered, and cells are slightly compressed, making the evaluation more difficult |
| Cytobrush | Fast, user-friendly, and inexpensive approach to collect samples for culture and cytology, although it is more commonly used for cytology. | Only a small segment of the uterus is sampled, and thus, focal infections can be missed. Bacteria in biofilm may not be detected. |
| Low-volume uterine lavage | The whole surface of the uterus can be sampled for culture and cytology, and thus this technique is more utilized for the diagnosis of challenge and chronic endometritis. The recovered fluid can be centrifuged or allowed to decant before cytological evaluation. | There is a risk of contamination with commensal microorganisms of the caudal reproductive tract. It requires at least one well-trained clinician and an assistant. An excessive amount of fluid can overdilute the sample and cause a false-negative and may challenge the cytological evaluation. Mares with a pendulous uterus can have poor fluid recovery. |
| Endometrium biopsy | While this approach is primarily used for histological evaluation, endometrium biopsy is a sensitive and specific approach to diagnose endometritis in mares by histological evaluation and culture of the biopsy. Particularly useful for deep endometrium infection. Results may guide the treatment strategies employed. | It requires a biopsy, which is a minor procedure but still invasive. It also requires well-trained laboratory personnel capable of performing cultures and histological evaluations |
| Drug Class | Therapeutics | Mechanism of Action | AMR |
|---|---|---|---|
| Aminoglycosides (e.g., amikacin sulfate, gentamicin sulfate, and neomycin) | Concentration-dependent, bactericidal, broad-spectrum, G- | Irreversible inhibition of bacterial protein synthesis by binding to the 30S subunit of the bacterial ribosome | Low incidence, efflux pumps, mutation at the drug binding site |
| Cephalosporin (β-lactam, third generation) (e.g., ceftiofur sodium, and ceftiofur crystalline-free-acid) | Time-dependent, bactericidal, broad-spectrum, G- and G+ | Inhibition of cell wall synthesis by disruption of the peptidoglycan layer | Growing resistance based on PBP mutation-reduced permeability and enzymatic inactivation by β -lactamase. Most G- rods can produce β-lactamase |
| Fluoroquinolones (e.g., enrofloxacin, and ciprofloxacin) | Concentration-dependent, bactericidal, broad-spectrum, G- and some G+ | Inhibits DNA gyrase (topoisomerase II) and topoisomerase IV | Mediated by target mutations in DNA gyrase |
| Extended-spectrum penicillins (β-lactam) (e.g., Ampicillin, and Ticarcillin) | Time-dependent, bactericidal, broad-spectrum, G+ and some G- | Interference in bacterial cell membrane synthesis by inhibition of the transpeptidases and peptidoglycan enzymes | Acquired resistance in G- by plasmid- or integron-mediated |
| Penicillins (natural β-lactam) (e.g., K penicillin, Na penicillin, and G Procaine) | Time-dependent, bactericidal, broad-spectrum, G+ | Lysis of cells weakened by the loss of the peptidoglycan layer in the membrane by binding the PBPs in the outside of the bacteria wall | Mutation of PBPs that reduces bacterial permeability, and production of β-lactamase |
| Polymyxins (e.g., polymyxin B) | Concentration-dependent, bactericidal, broad-spectrum, G- (e.g., Pseudomonas spp). | Disorganize the membrane by binding LPS, disrupting the cell wall membrane, and increasing cell permeability by detergent-like action | Rare; modification of the LPS in the bacterial membrane and development of an efflux pump/potassium system |
| Sulfonamides (e.g., sulfamethoxazole) associated with pyrimidine (trimethoprim) | Time-dependent, bacteriostatic, broad-spectrum, G- and G+ (Streptococcus spp) | Interference in the biosynthesis of folic acid by competition with PABA for dihydropteroate synthetase | Mediated via a chromosomal mutation causing the hyper-production of PABA or insensitive dihydropteroate synthase |
| Nitroimidazole (e.g., metronidazole) | Concentration-dependent, G+ anaerobes |
| Drug Class | Therapeutics | Mechanism of Action | AMR |
|---|---|---|---|
| Polyenes (e.g., amphotericin B, natamycin, and nystatin) | Fungicidal or fungistatic, broad-spectrum against Candida spp, Aspergillus spp, and Mucor spp | Binding to ergosterol in the membrane to disrupt the cell wall | Rare; the only mutant fungus enhances synthetic pathways for alternative sterols that replace ergosterol in the cell membrane |
| Imidazoles (e.g., clotrimazole, ketoconazole, miconazole) | Broad-spectrum activity against Candida spp | Inhibition of ergosterol synthesis in the fungal cell membrane by inhibiting the enzyme 14-α-demethylase, ultimately increasing cellular permeability and cell leakage | Resistance is found in filamentous fungi and after prolonged therapeutic regimens |
| Triazoles (e.g., fluconazole, itraconazole) | Potent anti-Aspergillus activity | Blockage of cytochrome P450–dependent enzyme C-14-α-demethylase (necessary for the conversion of lanosterol to ergosterol) | Resistance involves a single-point mutation in the cyp51A gene, which encodes for 14-α sterol demethylase |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. https://doi.org/10.3390/ijms21041432
Canisso IF, Segabinazzi LGTM, Fedorka CE. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. International Journal of Molecular Sciences. 2020; 21(4):1432. https://doi.org/10.3390/ijms21041432
Chicago/Turabian StyleCanisso, Igor F., Lorenzo G.T.M. Segabinazzi, and Carleigh E. Fedorka. 2020. "Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology" International Journal of Molecular Sciences 21, no. 4: 1432. https://doi.org/10.3390/ijms21041432
APA StyleCanisso, I. F., Segabinazzi, L. G. T. M., & Fedorka, C. E. (2020). Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. International Journal of Molecular Sciences, 21(4), 1432. https://doi.org/10.3390/ijms21041432





