Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels
Abstract
:1. Introduction
2. Results
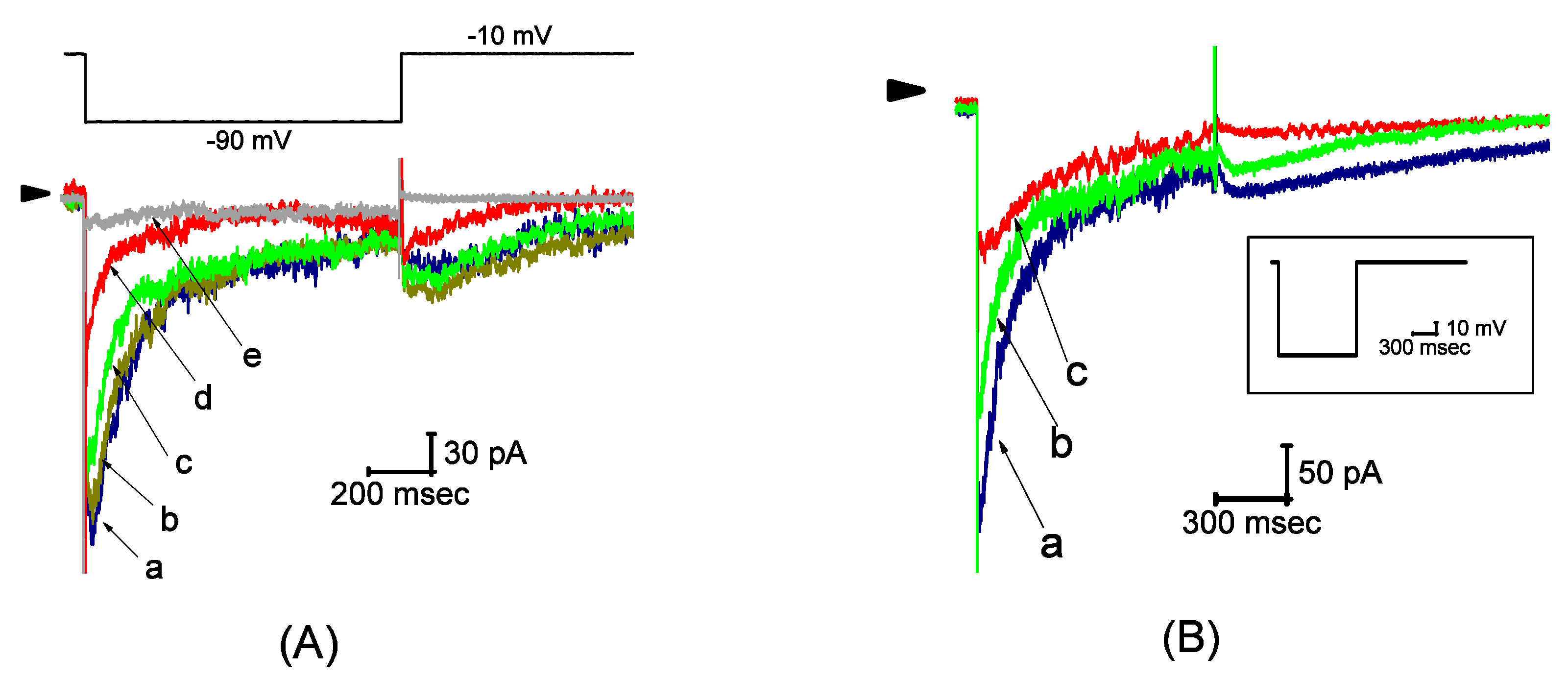
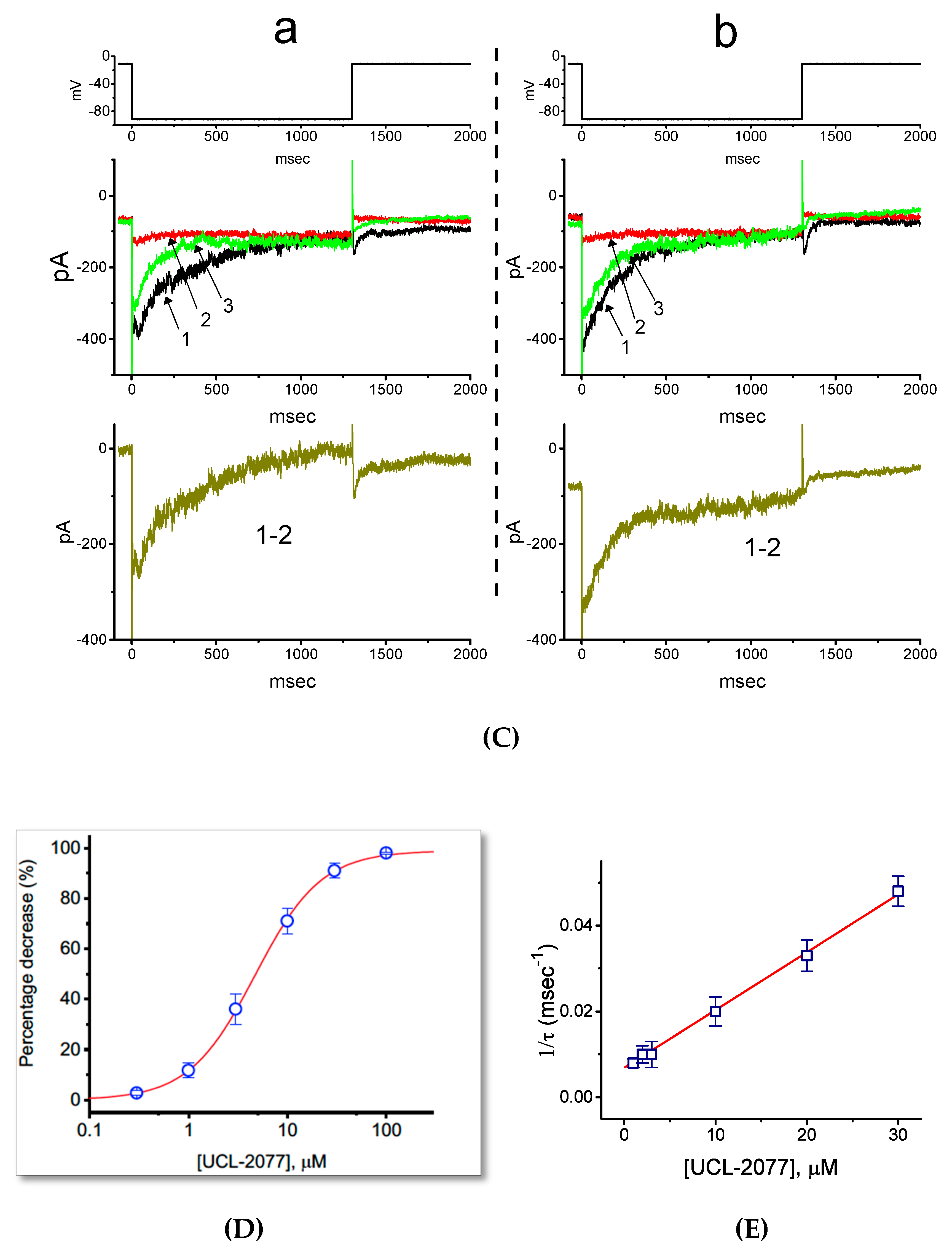
2.1. Effect of UCL-2077 on IK(erg) in Pituitary GH3 Cells
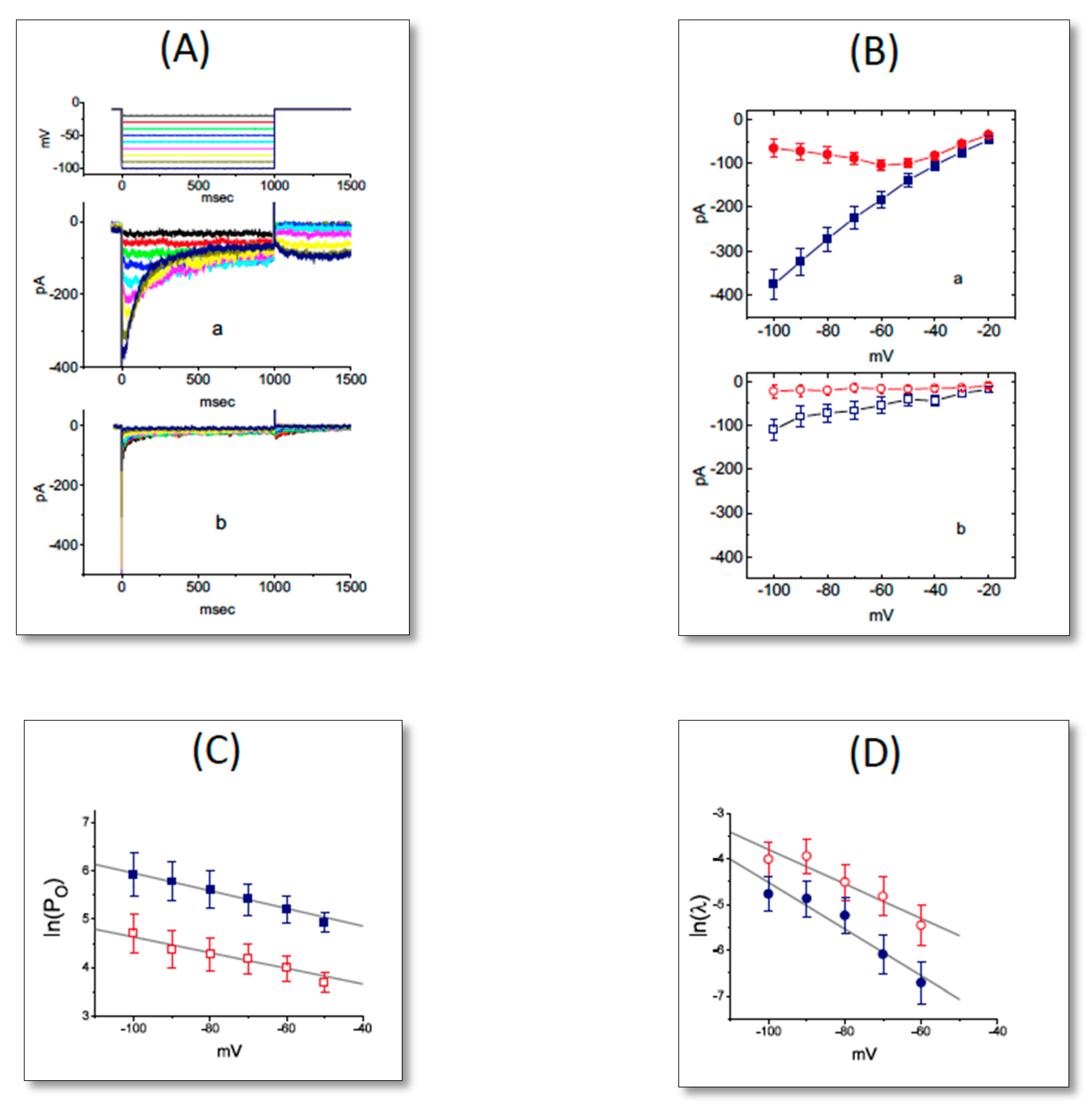
2.2. Effect of UCL-2077 on the Gating Charge of IK(erg) Activation and Deactivation
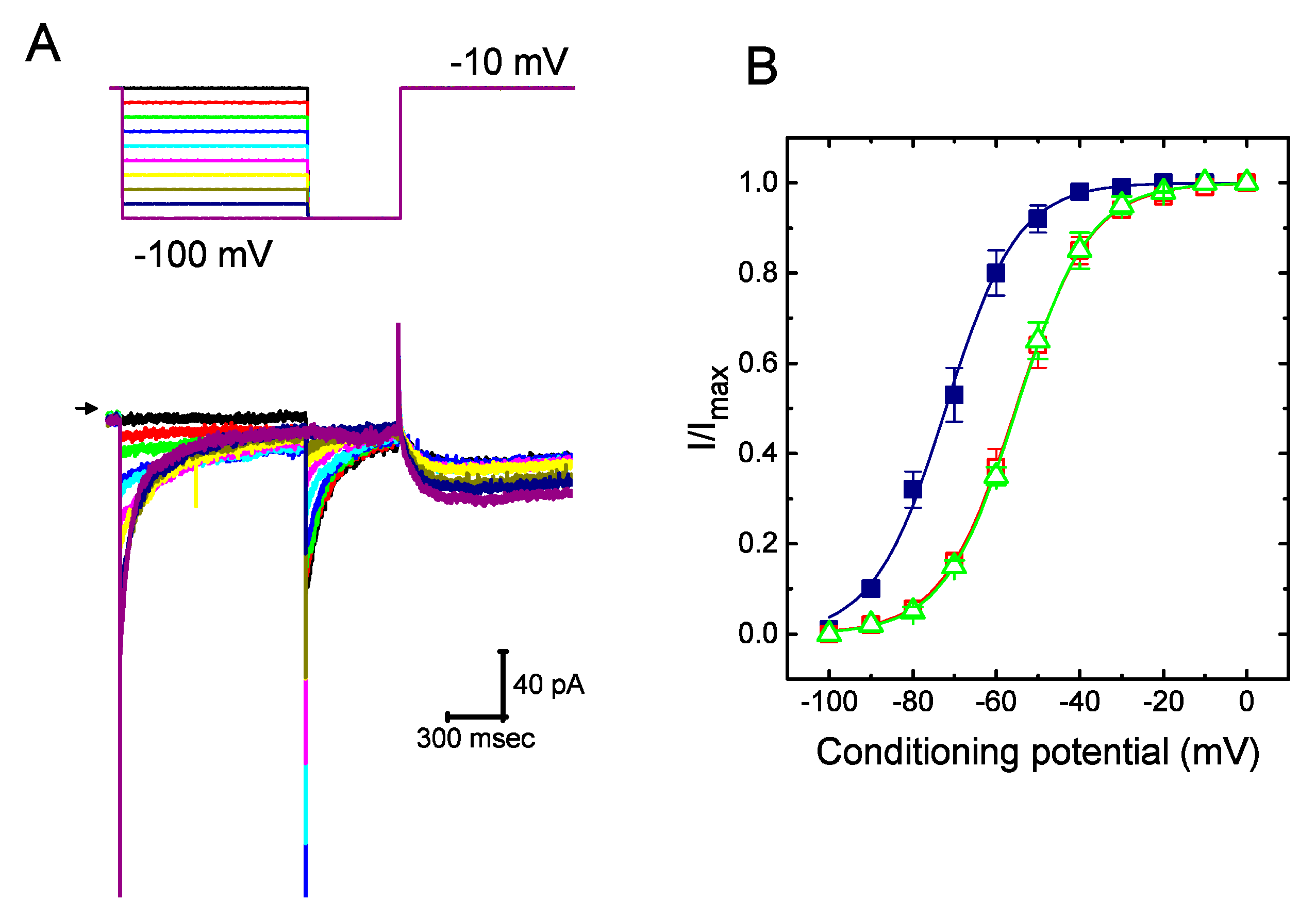
2.3. Steady-State Activation of IK(erg) Altered by the Presence of UCL-2077
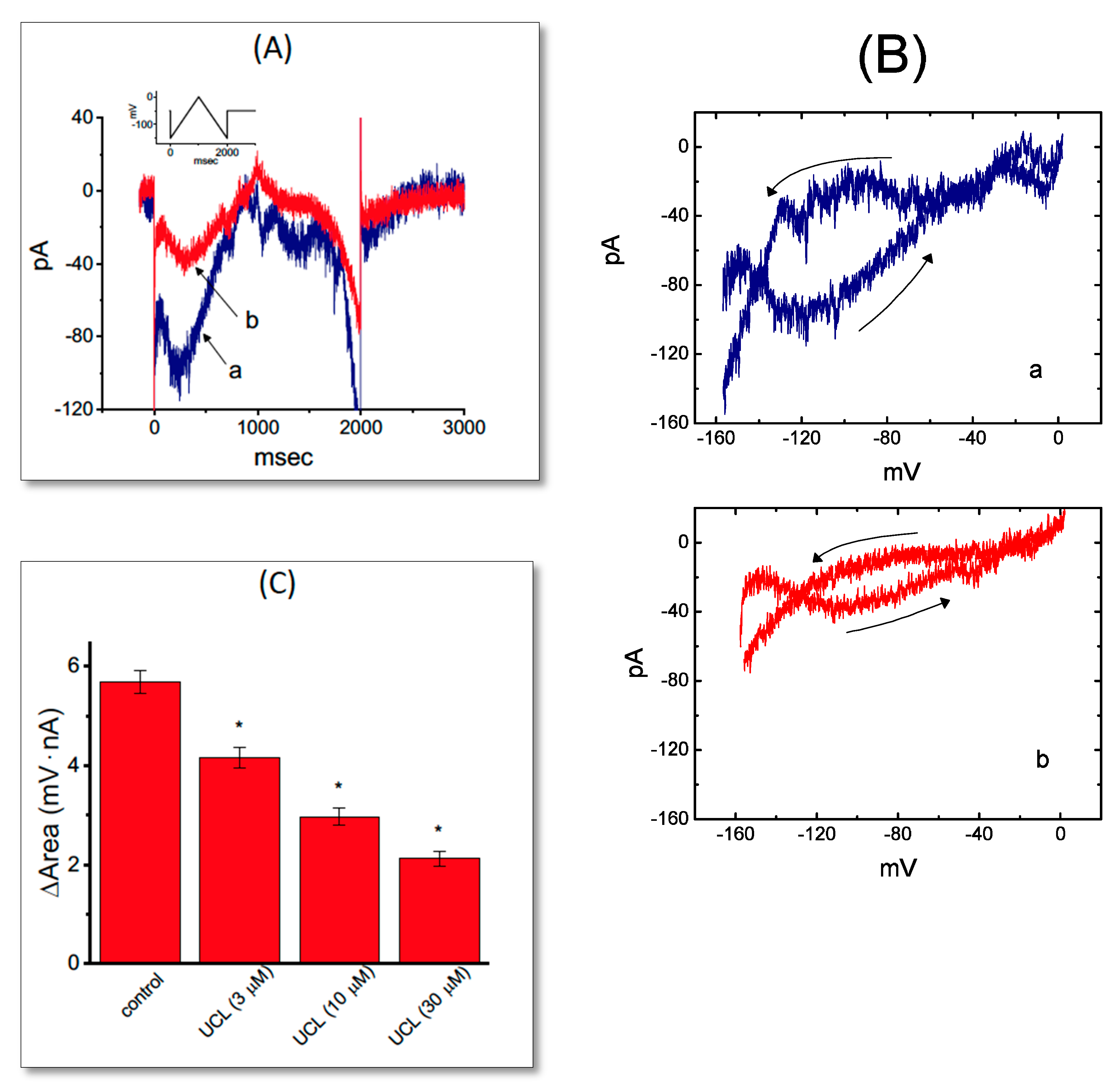
2.4. Effect of UCL-2077 on the Voltage Hysteresis Elicited in Response to Triangular Ramp Pulse
2.5. Inhibitory Effect of UCL-2077 on Delayed-Rectifier K+ Current (IK(DR))
2.6. Failure of UCL-2077 to Alter the Activity of BKCa Channels
2.7. Suppressive Effect of UCL-2077 on the Activity of IKCa Channels
2.8. Effect of UCL-2077 on the Frequency of Spontaneous Action Currents (ACs)
3. Discussion
4. Materials and Methods
4.1. Drugs and Solutions
4.2. Cell Preparations
4.3. Transfection with siRNAs
4.4. Electrophysiological Measurements
4.5. Data Recordings and Analyses
4.6. Single-Channel Analyses
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | action current |
| AP | action potential |
| BKCa channel | large-conductance Ca2+-activated K+ channel |
| Erg | ether-à-go-go-related gene |
| HH model | Hodgkin–Huxley model |
| IK(DR) | delayed rectifier K+ current |
| IK(erg) | erg-mediated K+ current |
| I-V | current versus voltage |
| IC50 | the concentration required for a 50% inhibition |
| IKCa channel | intermediate-conductance Ca2+-activated K+ channel |
| IK(DR) | delayed-rectifier K+ current |
| KD | dissociation constant |
| Kerg channel | erg-mediated K+ channel |
| Λ | rate constant |
| SEM | standard error of mean |
| siRNA | short-interfering RNA |
| TTX | tetrodotoxin |
| za | gating charge for IK(erg) activation |
| zαα | gating charge for αα transition rate embedded in modeled Kerg channel |
| zdeact | gating charge for IK(erg) deactivation |
References
- Zunszain, P.A.; Shah, M.M.; Miscony, Z.; Javadzadeh-Tabatabaie, M.; Haylett, D.G.; Ganellin, C.R. Tritylamino aromatic heterocycles and related carbinols as blockers of ca 2+-activated potassium ion channels underlying neuronal hyperpolarization. Arch Pharm 2002, 335, 159–166. [Google Scholar] [CrossRef]
- Shah, M.M.; Javadzadeh-Tabatabaie, M.; Benton, D.C.; Ganellin, C.R.; Haylett, D.G. Enhancement of hippocampal pyramidal cell excitability by the novel selective slow-afterhyperpolarization channel blocker 3-(triphenylmethylaminomethyl)pyridine (UCL2077). Mol. Pharmacol. 2006, 70, 1494–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Duan, W.; Sneyd, J.; Herbison, A.E. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J. Neurosci. 2010, 30, 6214–6224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soh, H.; Tzingounis, A.V. The specific slow afterhyperpolarization inhibitor UCL2077 is a subtype-selective blocker of the epilepsy associated KCNQ channels. Mol. Pharmacol. 2010, 78, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Lee, K.; Herbison, A.E.; Sneyd, J. A mathematical model of adult GnRH neurons in mouse brain and its bifurcation analysis. J. Theor. Biol. 2011, 276, 22–34. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Anwyl, R. Inhibition of the slow afterhyperpolarization restores the classical spike timing-dependent plasticity rule obeyed in layer 2/3 pyramidal cells of the prefrontal cortex. J. Neurophysiol. 2012, 107, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ouyang, M.; Ganellin, C.R.; Thomas, S.A. The slow afterhyperpolarization: A target of beta1-adrenergic signaling in hippocampus-dependent memory retrieval. J. Neurosci. 2013, 33, 5006–5016. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, M.; Gumenchuk, I.; Miyazaki, K.; Inoue, T.; Ross, W.N.; Leonard, C.S. Hypocretin/Orexin Peptides Alter Spike Encoding by Serotonergic Dorsal Raphe Neurons through Two Distinct Mechanisms That Increase the Late Afterhyperpolarization. J. Neurosci. 2016, 36, 10097–10115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kolaj, M.; Renaud, L.P. Ca2+-dependent and Na+-dependent K+ conductances contribute to a slow AHP in thalamic paraventricular nucleus neurons: A novel target for orexin receptors. J. Neurophysiol. 2010, 104, 2052–2062. [Google Scholar] [CrossRef] [Green Version]
- Raschi, E.; Vasina, V.; Poluzzi, E.; De Ponti, F. The hERG K+ channel: Target and antitarget strategies in drug development. Pharmacol. Res. 2008, 57, 181–195. [Google Scholar] [CrossRef]
- Hardman, R.M.; Forsythe, I.D. Ether-a-go-go-related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons. J. Physiol. 2009, 587, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Martinson, A.S.; van Rossum, D.B.; Diatta, F.H.; Layden, M.J.; Rhodes, S.A.; Martindale, M.Q.; Jegla, T. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc. Natl. Acad. Sci. USA 2014, 111, 5712–5717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.N.; Jan, C.R.; Li, H.F.; Chiang, H.T. Characterization of inhibition by risperidone of the inwardly rectifying K(+) current in pituitary GH(3) cells. Neuropsychopharmacology 2000, 23, 676–689. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, S.N. Block of erg current by linoleoylamide, a sleep-inducing agent, in pituitary GH3 cells. Eur. J. Pharmacol. 2003, 458, 37–47. [Google Scholar] [CrossRef]
- Huang, M.H.; Shen, A.Y.; Wang, T.S.; Wu, H.M.; Kang, Y.F.; Chen, C.T.; Hsu, T.I.; Chen, B.S.; Wu, S.N. Inhibitory action of methadone and its metabolites on erg-mediated K+ current in GH(3) pituitary tumor cells. Toxicology 2011, 280, 1–9. [Google Scholar] [CrossRef]
- Ji, H.; Tucker, K.R.; Putzier, I.; Huertas, M.A.; Horn, J.P.; Canavier, C.C.; Levitan, E.S.; Shepard, P.D. Functional characterization of ether-a-go-go-related gene potassium channels in midbrain dopamine neurons—Implications for a role in depolarization block. Eur. J. Neurosci. 2012, 36, 2906–2916. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.N.; Li, H.F.; Jan, C.R.; Chen, I.J.; Lo, Y.C. Selective block by glyceryl nonivamide of inwardly rectifying K+ current in rat anterior pituitary GH3 cells. Life Sci. 1998, 63, PL281–PL288. [Google Scholar] [CrossRef]
- Wu, S.N.; Yang, W.H.; Yeh, C.C.; Huang, H.C. The inhibition by di(2-ethylhexyl)-phthalate of erg-mediated K(+) current in pituitary tumor (GH(3)) cells. Arch. Toxicol. 2012, 86, 713–723. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [Green Version]
- Banderali, U.; Belke, D.; Singh, A.; Jayanthan, A.; Giles, W.R.; Narendran, A. Curcumin blocks Kv11.1 (erg) potassium current and slows proliferation in the infant acute monocytic leukemia cell line THP-1. Cell. Physiol. Biochem. 2011, 28, 1169–1180. [Google Scholar] [CrossRef]
- Mork, H.K.; Haug, T.M.; Sand, O. Contribution of different Ca-activated K channels to the first phase of the response to TRH in clonal rat anterior pituitary cells. Acta Physiol. Scand. 2005, 184, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Huang, Y.M.; Wu, S.N. The Inhibition by Oxaliplatin, a Platinum-Based Anti-Neoplastic Agent, of the Activity of Intermediate-Conductance Ca(2)(+)-Activated K(+) Channels in Human Glioma Cells. Cell. Physiol. Biochem. 2015, 37, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.S.; Wu, S.J.; Hung, T.Y.; Huang, Y.M.; Hsu, C.W.; Sze, C.I.; Hsieh, Y.J.; Huang, C.W.; Wu, S.N. Evidence for the Inhibition by Temozolomide, an Imidazotetrazine Family Alkylator, of Intermediate-Conductance Ca2+-Activated K+ Channels in Glioma Cells. Cell. Physiol. Biochem. 2016, 38, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.M.; Silvia, C.; Hirschberg, B.; Bond, C.T.; Adelman, J.P.; Maylie, J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA 1997, 94, 11651–11656. [Google Scholar] [CrossRef] [Green Version]
- Wulff, H.; Kolski-Andreaco, A.; Sankaranarayanan, A.; Sabatier, J.M.; Shakkottai, V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr. Med. Chem. 2007, 14, 1437–1457. [Google Scholar] [CrossRef]
- King, B.; Rizwan, A.P.; Asmara, H.; Heath, N.C.; Engbers, J.D.; Dykstra, S.; Bartoletti, T.M.; Hameed, S.; Zamponi, G.W.; Turner, R.W. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep. 2015, 11, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Matsuyama, H.; Baell, J.; Hunne, B.; Fowler, C.J.; Smith, J.E.; Nurgali, K.; Furness, J.B. Effects of compounds that influence IK (KCNN4) channels on afterhyperpolarizing potentials, and determination of IK channel sequence, in guinea pig enteric neurons. J. Neurophysiol. 2007, 97, 2024–2031. [Google Scholar] [CrossRef] [Green Version]
- Bouhy, D.; Ghasemlou, N.; Lively, S.; Redensek, A.; Rathore, K.I.; Schlichter, L.C.; David, S. Inhibition of the Ca(2)(+)-dependent K(+) channel, KCNN4/KCa3.1, improves tissue protection and locomotor recovery after spinal cord injury. J. Neurosci. 2011, 31, 16298–16308. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.W.; Kruskic, M.; Teves, M.; Scheidl-Yee, T.; Hameed, S.; Zamponi, G.W. Neuronal expression of the intermediate conductance calcium-activated potassium channel KCa3.1 in the mammalian central nervous system. Pflug. Arch. 2015, 467, 311–328. [Google Scholar] [CrossRef]
- Wu, S.N.; Liu, S.I.; Huang, M.H. Cilostazol, an inhibitor of type 3 phosphodiesterase, stimulates large-conductance, calcium-activated potassium channels in pituitary GH3 cells and pheochromocytoma PC12 cells. Endocrinology 2004, 145, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Mannikko, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.R.; Halvorsrud, R.; Borg-Graham, L.; Storm, J.F. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J. Physiol. 1999, 521, 135–146. [Google Scholar] [CrossRef]
- Kulke, D.; von Samson-Himmelstjerna, G.; Miltsch, S.M.; Wolstenholme, A.J.; Jex, A.R.; Gasser, R.B.; Ballesteros, C.; Geary, T.G.; Keiser, J.; Townson, S.; et al. Characterization of the Ca2+-gated and voltage-dependent K+-channel Slo-1 of nematodes and its interaction with emodepside. PLoS Negl. Trop. Dis. 2014, 8, e3401. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.N.; Chen, H.Z.; Chou, Y.H.; Huang, Y.M.; Lo, Y.C. Inhibitory actions by ibandronate sodium, a nitrogen-containing bisphosphonate, on calcium-activated potassium channels in Madin-Darby canine kidney cells. Toxicol. Rep. 2015, 2, 1182–1193. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef]
- Lo, Y.C.; Tseng, Y.T.; Liu, C.M.; Wu, B.N.; Wu, S.N. Actions of KMUP-1, a xanthine and piperazine derivative, on voltage-gated Na(+) and Ca(2+) -activated K(+) currents in GH3 pituitary tumour cells. Br. J. Pharmacol. 2015, 172, 5110–5122. [Google Scholar] [CrossRef]
- Tabarean, I.V.; Narahashi, T. Kinetics of modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by tetramethrin and deltamethrin. J. Pharmacol. Exp. Ther. 2001, 299, 988–997. [Google Scholar]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-T.; Lo, Y.-C.; Wu, S.-N. Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels. Int. J. Mol. Sci. 2020, 21, 1441. https://doi.org/10.3390/ijms21041441
Hsu H-T, Lo Y-C, Wu S-N. Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels. International Journal of Molecular Sciences. 2020; 21(4):1441. https://doi.org/10.3390/ijms21041441
Chicago/Turabian StyleHsu, Hung-Te, Yi-Ching Lo, and Sheng-Nan Wu. 2020. "Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels" International Journal of Molecular Sciences 21, no. 4: 1441. https://doi.org/10.3390/ijms21041441
APA StyleHsu, H.-T., Lo, Y.-C., & Wu, S.-N. (2020). Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels. International Journal of Molecular Sciences, 21(4), 1441. https://doi.org/10.3390/ijms21041441




