New Perspectives in Food Allergy
Abstract
:1. Introduction
2. Epidemiology
3. Clinical Presentation and Natural History
4. FA Diagnostics
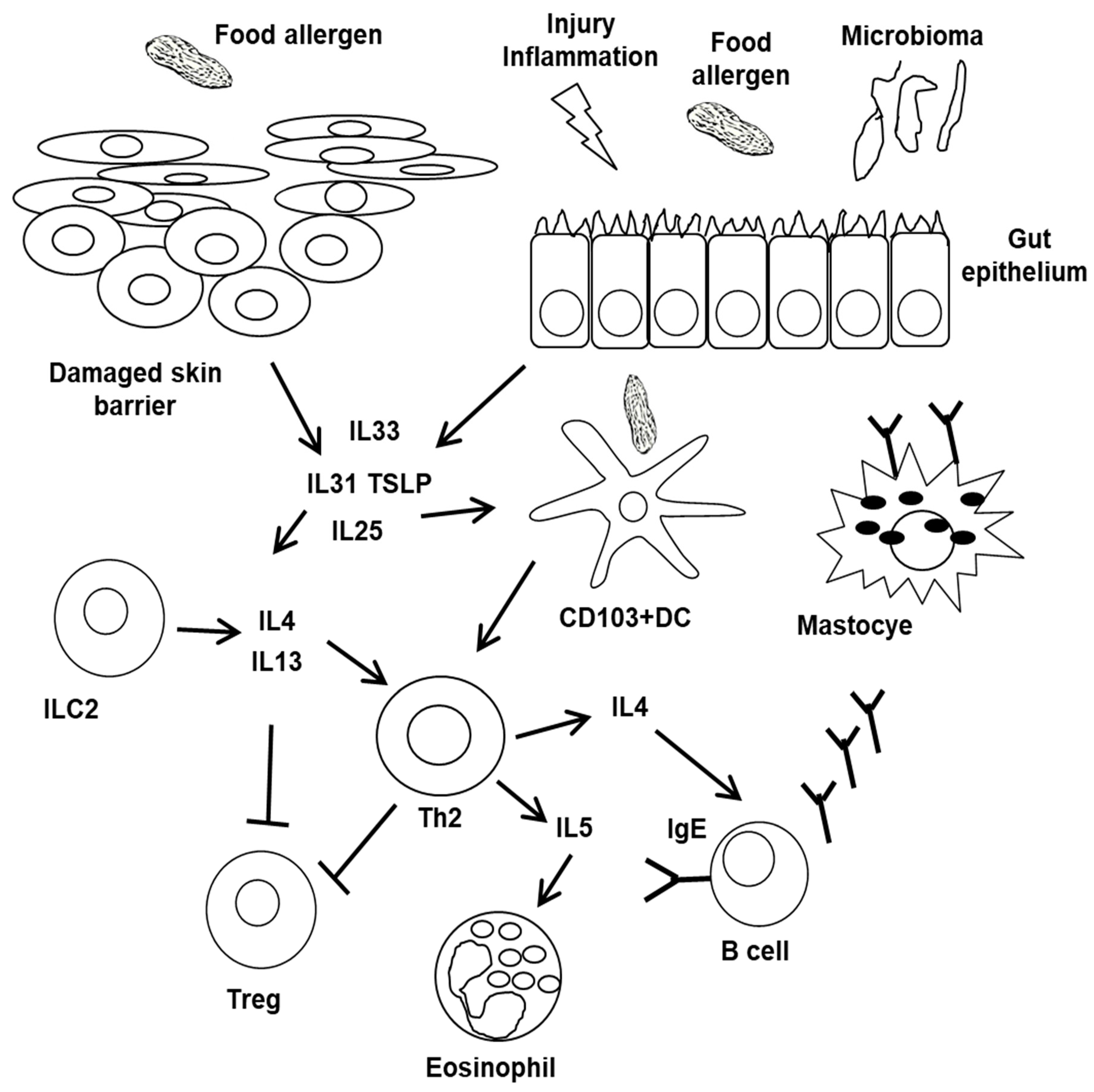
5. Pathophysiology of FA Phenotypes
6. Tolerance Disruption
7. Beyond Immune Cells
8. Current Hypotheses of Food Allergy Development: Environmental Impact and Gut Microbiota
9. Novel Treatment Strategies
9.1. Specific Immunotherapy
9.2. Pharmacological Treatments
10. Concluding Remarks
Conflicts of Interest
References
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiwegger, T.; Hung, L.; San Diego, K.E.; O’Mahony, L.; Upton, J. Recent developments and highlights in food allergy. Allergy 2019, 74, 2355–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iweala, O.I.; Choudhary, S.K.; Commins, S.P. Food Allergy. Curr. Gastroenterol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef] [Green Version]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.; Dharmage, S.C.; et al. HealthNuts Investigators. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Comberiati, P.; Costagliola, G.; Sofia D’Elios, S.; Peroni, D. Prevention of food allergy: The significance of early introduction. Medicina 2019, 55, 323. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, J.H.; Keet, C.A. Epidemiology of food allergy. Immunol. Allergy Clin. Immunol. 2018, 38, 13–25. [Google Scholar] [CrossRef]
- Sicherer, S.H. Epidemiology of food allergy. J. Allergy Clin. Immunol. 2011, 127, 594–602. [Google Scholar] [CrossRef]
- Jones, S.M.; Burks, A.W. Food Allergy. N. Engl. J. Med. 2017, 377, 1168–1176. [Google Scholar] [CrossRef]
- Berin, M.C.; Sampson, H.A. Food Allergy: An Enigmatic Epidemic. Trends Immunol. 2013, 34, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Schussler, E.; Sobel, J.; Hsu, J.; Yu, P.; Meaney-Delman, D.; Grammer, L.C.; Nowak-Węgrzyn, A. Workgroup Report by the Joint Task Force Involving American Academy of Allergy, Asthma & Immunology (AAAAI); Food Allergy, Anaphylaxis, Dermatology and Drug Allergy (FADDA) (Adverse Reactions to Foods Committee and Adverse Reactions to Drugs, Biologicals, and Latex Committee); and the Centers for Disease Control and Prevention Botulism Clinical Treatment Guidelines Work group-Allergic Reactions to Botulinum Antitoxin: A Systematic Review. Clin. Infect. Dis. 2017, 66, S65–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Allergy and aging: An old/new emerging eealth issue. Aging Dis. 2017, 8, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Di Silvestre, D.; Ginaldi, L. Sex and gender aspects for patient stratification in allergy prevention and treatment. Int. J. Mol. Sci. 2020, in press. [Google Scholar]
- De Martinis, M.; Sirufo, M.M.; Viscido, A.; Ginaldi, L. Food allergies and ageing. Int. J. Mol. Sci. 2019, 20, 5580. [Google Scholar] [CrossRef] [Green Version]
- Genuneit, J.; Seibold, A.M.; Apfelbacher, C.J.; Konstantinou, G.N.; Koplin, J.J.; La Grutta, S.; Logan, K.; Perkin, M.R.; Flohr, C. Task Force ‘Overview of Systematic Reviews in Allergy Epidemiology (OSRAE)’ of the EAACI Interest Group on Epidemiology. Overview of systematic reviews in allergy epidemiology. Allergy 2017, 72, 849–856. [Google Scholar] [CrossRef]
- De Martinis, M.; Ciccarelli, F.; Sirufo, M.M.; Ginaldi, L. An overview of environmental risk factors in systemic sclerosis. Expert Rev. Clin. Immunol. 2016, 12, 465–478. [Google Scholar] [CrossRef]
- Caraballo, L.; Zakzuk, J.; Lee, B.W.; Acevedo, N.; Soh, J.Y.; Sánchez-Borges, M.; Hossny, E.; García, E.; Rosario, N.; Ansotegui, I.; et al. Particularities of allergy in the Tropics. World Allergy Organ. J. 2016, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.L.K.; Mullins, R.J. Food allergy: Is prevalence increasing? Intern. Med. J. 2017, 47, 256–261. [Google Scholar] [CrossRef]
- Muraro, A.; Agache, I.; Clark ASheikh, A.; Roberts, G.; Akdis, C.A.; Borrego, L.M.; Higgs, J.; Hourihane, J.O.; Jorgensen, P.; Mazon, A.; et al. European Academy of Allergy and Clinical Immunology. EAACI food allergy and anaphylaxis guidelines: Managing patients with food allergy in the community. Allergy 2014, 69, 1046–1057. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Alberti, S.; Cevenini, E.; Ostan, R.; Capri, M.; Salvioli, S.; Bucci, L.; Ginaldi, L.; DeMartinis, M.; Franceschi, C.; Monti, D. Age-Dependent modifications of Type 1 and Type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech. Ageing Dev. 2006, 127, 560–566. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Viscido, A.; Ginaldi, L. Food allergy insights: A changing landscape. Arch. Immunol. Ther. Exp. 2020, in press. [Google Scholar]
- Campisi, G.; Chiappelli, M.; De Martinis, M.; Franco, V.; Ginaldi, L.; Guiglia, R.; Licastro, F.; Lio, D. Pathophysiology of age-related diseases. Immun. Ageing 2009, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Sirufo, M.M.; Suppa, M.; Ginaldi, L.; De Martinis, M. Does Allergy break bones? Osteoporosis and its connection to allergy. Int. J. Mol. Sci. 2020, 21, 712. [Google Scholar] [CrossRef] [Green Version]
- Ciccarelli, F.; De Martinis, M.; Ginaldi, L. Glucocorticoids in Patients with Rheumatic Diseases: Friends or Enemies of Bone? Curr. Med. Chem. 2015, 22, 596–603. [Google Scholar] [CrossRef]
- Ginaldi, L.; De Martinis, M. Osteoimmunology and Beyond. Curr. Med. Chem. 2016, 23, 3754–3774. [Google Scholar] [CrossRef] [Green Version]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Osteoporosis: Current and emerging therapies targeted to immunological checkpoints. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. Successful Treatment with Omalizumab in a Child with Asthma and Urticaria: A Clinical Case Report. Front. Pediatr. 2019, 7, 213. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Solar urticaria, a disease with many dark sides: Is omalizumab the right therapeutic response? Reflections from a clinical case report. Open Med. 2019, 14, 403–406. [Google Scholar] [CrossRef] [Green Version]
- Sirufo, M.M.; De Martinis, M.; Ginaldi, L. Omalizumab an effective and safe alternative therapy in severe refractory atopic dermatitis. A case report. Medicine 2018, 97, 10897. [Google Scholar] [CrossRef]
- Benedè, S.; Blázquez, A.B.; Chiang DTordesillas, L.; Berin, M.C. The rise of food allergy: Environmental factors and emerging treatments. EBioMedicine 2016, 7, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ontiveros, N.; Valdez-Meza, E.E.; Vergara-Jiménez MJCanizalez-Román, A.; Borzutzky, A.; Cabrera-Chávez, F. Parent-reported prevalence of food allergy in Mexican school children: A population-based study. Allergol. Immunopathol. 2016, 44, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Bachiloglu, R.; Ivanovic-Zuvic, D.; Álvarez JLinn, K.; Thöne, N.; de los Ángeles Paul, M.; Borzutzky, A. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergol. Immunopathol. 2014, 42, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Chávez, F.; Rodríguez-Bellegarrigue, C.I.; Figueroa-Salcido OGLopez-Gallardo, J.A.; Arámburo-Gálvez, J.G.; Vergara-Jiménez, M.J.; Castro-Acosta, M.L.; Sotelo-Cruz, N.; Gracia-Valenzuela, M.H.; Ontiveros, N. Food allergy prevalence in Salvadoran school children estimated by parent-report. Int. J. Environ. Res. Public Health 2018, 15, 2446. [Google Scholar] [CrossRef] [Green Version]
- Hossny, E.; Ebisawa, M.; El-Gamal, Y.; Arasi, S.; Dahdah, L.; El-Owaidy, R.; Galvan, C.A.; Levin, M.; Martinez, S.; Pawankar, R.; et al. Challenges of managing food allergy in the developing world. World Allergy Organ. J. 2019, 12, 100089. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [Green Version]
- Panjari, M.; Koplin, J.J.; Dharmage, S.C.; Peters, R.L.; Gurrin, L.C.; Sawyer, S.M.; McWilliam, V.; Eckert, J.K.; Vicendese, D.; Erbas, B.; et al. Nut allergy prevalence and differences between Asian-born children and Australian-born children of Asian descent: A state-wide survey of children at primary school entry in Victoria, Australia. Clin. Exp. Allergy 2016, 46, 602–609. [Google Scholar] [CrossRef]
- Shroba, J.; Rath, N.; Barnes, C. Possible role of environmental factors in the development of food allergies. Clin. Rev. Allergy Immunol. 2019, 57, 303–311. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef]
- Suaini, N.H.A.; Zhang, Y.; Vuillermin, P.J.; Allen, K.J.; Harrison, L.C. Immune modulation by vitamin D and its relevance to food allergy. Nutrients 2015, 7, 6088–6108. [Google Scholar] [CrossRef] [Green Version]
- Ciccarelli, F.; De Martinis, M.; Sirufo, M.M.; Ginaldi, L. Psoriasis Induced by Anti-Tumor Necrosis Factor Alpha Agents: A Comprehensive Review of the Literature. Acta Dermatovenerol. Croat. ADC 2016, 24, 169–174. [Google Scholar] [PubMed]
- Waserman, S.; Bégin, P.; Watson, W. IgE-mediated food allergy. Allergy Asthma Clin. Immunol. 2018, 14, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sampson, H.A. Food allergy. J. Clin. Investig. 2011, 121, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.W.; Ruiz-Garcia, M.; Patel, N.; Boyle, R.J.; Turner, P.J. Reaction phenotypes in IgE-mediated food allergy and anaphylaxis. Ann. Allergy Asthma Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Leung, D.Y. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2013. J. Allergy Clin. Immunol. 2014, 133, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Sampson, H.A. Phenotypes and endotypes of food allergy: A path to better understanding the pathogenesis and prognosis of food allergy. Ann. Allergy Asthma Immunol. 2018, 120, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, J.; Johansson, E.; Bernstein, J.A.; Chakraborty, R.; Khurana Hershey, G.K.; Rothenberg, M.E.; Mersha, T.B. Resolving the etiology of atopic disorders by genetic analysis of racial ancestry. J. Allergy Clin. Immunol. 2016, 138, 676–699. [Google Scholar] [CrossRef] [Green Version]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Cano, R.; Pascal, M.; Araujo GGoikoetxea, M.J.; Valero, A.L.; Picado, C.; Bartra, J. Mechanisms, cofactors, and augmenting factors involved in anaphylaxis. Front. Immunol. 2017, 8, 1193. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Matsuo, H.; Chinuki, Y.; Kohno, K.; Tanaka, A.; Maruyama, N.; Morita, E. Recombinant high molecular weight-glutenin subunit-specific IgE detection is useful in identifying wheat-dependent exercise-induced anaphylaxis complementary to recombinant omega-5 gliadin-specific IgE test. Clin. Exp. Allergy 2012, 42, 1293–1298. [Google Scholar] [CrossRef]
- Kuhlen, J.L., Jr.; Camargo, C.A., Jr.; Balekian, D.S.; Blumenthal, K.G.; Guyer, A.; Morris, T.; Long, A.; Banerji, A. Antibiotics are the most commonly identified cause of perioperative hypersensitivity reactions. J. Allergy Clin. Immunol. Pract. 2016, 4, 697–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platts-Mills, T.; Schuyler, A.J.; Hoyt, A.E.; Commins, S.P. Delayed anaphylaxis involving IgE to galactose-alpha-1, 3-galactose. Curr. Allergy Asthma Rep. 2015, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Jerath MRCox KErickson, L.D.; Platts-Mills, T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol. Int. 2016, 65, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, R.N.; Franco, P.F.; Rodrigues H Santos, L.C.B.; McKay, C.S.; Sanhueza, C.A.; Brito, C.R.N.; Azevedo, M.A.; Venuto, A.P.; Cowan, P.J.; Almeida, I.C.; et al. Amblyomma sculptum tick saliva: α-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int. J. Parasitol. 2016, 46, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinuki, Y.; Morita, E. Alpha-Gal-containing biologics and anaphylaxis. Allergol. Int. 2019, 68, 296–300. [Google Scholar] [CrossRef]
- Popescu, F.D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef]
- Jeebhay, M.F.; Moscato, G.; Bang, B.E.; Folletti, I.; Lipińska-Ojrzanowska, A.; Lopata, A.L.; Pala, G.; Quirce, S.; Raulf, M.; Sastre, J.; et al. Food processing and occupational respiratory allergy—An EAACI position paper. Allergy 2019, 74, 1852–1871. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef] [Green Version]
- Lukschal, A.; Wallmann, J.; Bublin, M.; Hofstetter, G.; Mothes-Luksch, N.; Breiteneder, H.; Pali-Schöll, I.; Jensen-Jarolim, E. Mimotopes for api g 5, a relevant cross-reactive allergen, in the celery-mugwort-birch-spice syndrome. Allergy Asthma Immunol. Res. 2016, 8, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K.; et al. Position paper of the EAACI: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy 2015, 70, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Chinthrajah, R.S.; Tupa, D.; Prince BTBlock, W.M.; Rosa, J.S.; Singh, A.M.; Nadeau, K. Diagnosis of Food Allergy. Pediatr. Clin. N. Am. 2015, 62, 1393–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartuzi, Z.; Kaczmarski, M.; Czerwionka-Szaflarska, M.; Małaczyńska, T.; Krogulska, A. The diagnosis and management of food allergies. Position paper of the Food Allergy Section the Polish Society of Allergology. Postepy. Dermatol. Alergol. 2017, 34, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Ansotegui, I.; Aberer WAl-Ahmad, M.; Akdis, M.; Ballmer-Weber, B.K.; Beyer, K.; Blanca, M.; Brown, S.; Bunnag, C.; Hulett, A.C.; et al. Risk and safety requirements for diagnostic and therapeutic procedures in allergology: World Allergy Organization Statement. World Allergy Organ. J. 2016, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattan, J.D.; Sicherer, S.H. Optimizing the Diagnosis of Food allergy. Immunol. Allergy Clin. N. Am. 2015, 35, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Macchia, D.; Melioli, G.; Pravettoni VNucera, E.; Piantanida, M.; Caminati, M.; Campochiaro, C.; Yacoub, M.R.; Schiavino, D.; Paganelli, R.; Di Gioacchino, M. Food Allergy Study Group (ATI) of the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Guidelines for the use and interpretation of diagnosticmethods in adult food allergy. Clin. Mol. Allergy 2015, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Sturm, E.M.; Kranzelbinder, B.; Heinemann, A.; Groselj-Strele, A.; Aberer, W.; Sturm, G.J. CD203c-based basophil activation test in allergy diagnosis: Characteristics and differences to CD63 upregulation. Cytom. B Clin. Cytom. 2010, 78, 308–318. [Google Scholar] [CrossRef]
- Santos, A.F.; Gideon Lack, G. Basophil activation test: Food challenge in a test tube or specialist research tool? Clin. Transl. Allergy 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.F.; Shreffler, W.G. Road map for the clinical application of the basophil activation test in food allergy. Clin. Exp. Allergy 2017, 47, 1115–1124. [Google Scholar] [CrossRef] [Green Version]
- Cardona, V.; Ansotegui, I.J. Component-resolved diagnosis in anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 244–249. [Google Scholar] [CrossRef]
- Arasi, S.; Mennini, M.; Valluzzi, R.; Riccardi, C.; Fiocchi, A. Precision medicine in food allergy. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 438–443. [Google Scholar] [CrossRef]
- Pomés, A.; Davies, J.M.; Gadermaier GHilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; Chan, S.K.; et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Roulias, A.; Ferreira, F.; Egger, M. Panallergens and their impact on the allergic patient. Allergy Asthma Clin. Immunol. 2010, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzucchelli, G.; Holzhauser, T.; Cirkovic Velickovic, T.; Diaz-Perales, A.; Molina, E.; Roncada, P.; Rodrigues, P.; Verhoeckx, K.; Hoffmann-Sommergruber, K. Current (food) allergenic risk assessment: Is it fit for novel foods? Status quo and identification of gaps. Mol. Nutr. Food Res. 2018, 62, 1700278. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and Cellular Mechanisms of Food Allergy and Food Tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [Green Version]
- Woodfolk, J.A.; Commins, S.P.; Schuyler, A.J.; Erwin, E.A.; Platts-Mills, T.A. Allergens, sources, particles, and molecules: Why do we make IgE responses? Allergol. Int. 2015, 64, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Price, D.; Ackland, M.L.; Suphioglu, C. Identifying Epithelial Endocytotic Mechanisms of the Peanut Allergens Ara h 1 and Ara h 2. Int. Arch. Allergy Immunol. 2017, 172, 106–115. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.E.; Schuyler, A.J.; Erwin, E.A.; Commins, S.P.; Woodfolk, J.A. IgE in the diagnosis and treatment of allergic disease. J. Allergy Clin. Immunol. 2016, 137, 1662–1670. [Google Scholar] [CrossRef] [Green Version]
- Dhanapala, P.; Withanage-Dona, D.; Tang, M.L.; Doran, T.; Suphioglu, C. Hypoallergenic variant of the major egg white allergen Gal d 1 produced by disruption of cysteine bridges. Nutrients 2017, 9, 171. [Google Scholar] [CrossRef] [Green Version]
- Hemmer, W.; Klug, C.; Swoboda, I. Update on the bird-egg syndrome and genuine poultry meat allergy. Allergo. J. Int. 2016, 25, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, C.; Ferrara, R.; Bernardi MLZennaro, D.; Tuppo, L.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Diagnosing allergic sensitizations in the third millennium: Why clinicians should know allergen molecule structures. Clin. Transl. Allergy 2017, 7, s13601–s136017. [Google Scholar] [CrossRef]
- Calamelli, E.; Liotti, L.; Beghetti, I.; Piccinno, V.; Serra, L.; Bottau, P. Component-resolved diagnosis in food allergies. Medicina 2019, 55, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmiechen, Z.C.; Weissler, K.A.; Frischmeyer-Guerrerio, P.A. Recent developments in understanding the mechanisms of food allergy. Curr. Opin. Pediatr. 2019, 31, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.K.; Chien, K.B.; Bryce, P.J. The Immunology of Food Allergy. J. Immunol. 2014, 192, 2529–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalberse, R.C.; Platts-Mills, T.A.; Rispens, T. The developmental history of IgE and IgG4 antibodies in relation to atopy, eosinophilic esophagitis and the modified TH2 response. Curr. Allergy Asthma Rep. 2016, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, H.; Zeng XGuo, L.; Sun, X.; He, S. Subsets of regulatory T cells and their roles in allergy. J. Transl. Med. 2014, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Ginaldi, L.; De Martinis, M.; Ciccarelli, F.; Saitta, S.; Imbesi, S.; Mannucci, C.; Gangemi, S. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Ginaldi, L.; De Martinis, M.; Saitta, S.; Sirufo, M.M.; Mannucci, C.; Casciaro, M.; Ciccarelli, F.; Gangemi, S. Interleukin-33 serum levels in postmenopausal women with osteoporosis. Sci. Rep. 2019, 9, 3786. [Google Scholar] [CrossRef] [Green Version]
- Deschildre, A.; Lejeune, S. How to cope with food allergy symptoms? Curr. Opin. Allergy Clin. Immunol. 2018, 18, 234–242. [Google Scholar] [CrossRef]
- Ruiter, B.; Shreffler, W.G. Innate immunostimulatory properties of allergens and their relevance to food allergy. Semin. Immunopathol. 2012, 34, 617–632. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef]
- Yu, W.; HusseyFreeland, D.M.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Tupa, D.; Graham, M.T.; Chatila, T.A.; Spergel, J.M.; Nadeau, K.C. Deciphering the black box of food allergymechanisms. Ann. Allergy Asthma Immunol. 2017, 118, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma, Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.C. Intestinal epithelial barrier dysfunction in food hypersensitivity. J. Allergy 2012, 2012, 596081. [Google Scholar] [CrossRef] [Green Version]
- Iweala, O.I.; Nagler, C.R. The Microbiome and Food Allergy. Annu. Rev. Immunol. 2019, 37, 377–403. [Google Scholar] [CrossRef]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Apoptosis remodeling in immunosenescence: Implications for strategies to delay ageing. Curr. Med. Chem. 2007, 14, 1389–1397. [Google Scholar] [CrossRef]
- Corazza, G.R.; Ginaldi, L.; Quaglione, G.; Ponzielli, F.; Vecchio, L.; Biagi, F.; Quaglino, D. Proliferating cell nuclear antigen expression is increased in small bowel epithelium in the elderly. Mech. Ageing Dev. 1998, 104, 1–9. [Google Scholar] [CrossRef]
- Nakajima-Adachi, H.; Shibahara, K.; Fujimura YTakeyama, J.; Hiraide, E.; Kikuchi, A.; Murakami, H.; Hosono, A.; Nochi, T.; Wakatsuki, Y.; Shimojo, N.; et al. Critical role of intestinal interleukin-4 modulatingregulatory T cells for desensitization, tolerance, and inflammation of food allergy. PLoS ONE 2017, 12, e0172795. [Google Scholar] [CrossRef]
- Leyva-Castillo, J.M.; Galand, C.; Kam, C.; Burton, O.; Gurish, M.; Musser, M.A.; Goldsmith, J.D.; Hait, E.; Nurko, S.; Brombacher, F.; et al. Mechanical skin injury promotes food anaphylaxis by driving intestinal mast cell expansion. Immunity 2019, 50, 1262–1275. [Google Scholar] [CrossRef]
- Ruiter, B.; Shreffler, W.G. The role of dendritic cells in food allergy. J. Allergy Clin. Immunol. 2012, 129, 921–928. [Google Scholar] [CrossRef]
- Shik, D.; Tomar, S.; Lee, J.B.; Chen, C.Y.; Smith, A.; Wang, Y.H. IL-9–producingcells in the development of IgE-mediated food allergy. Semin. Immunopathol. 2017, 39, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabanillas, B.; Brehler, A.C.; Novak, N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular mechanisms of cutaneous inflammatory disorder: Atopic dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Pioggia, G.; Calapai, G.; Gangemi, S.; Mannucci, C. Alarmins in osteoporosis, RAGE and IL-33 pathways: A Literature Review. Medicina 2020, in press. [Google Scholar]
- De Martinis, M.; Sirufo, M.M.; Suppa, M. Ginaldi IL-33/IL-31 axis in osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Peng, J.; Zhao, S.; Zhang, Y.; Su, X.; Wang, Y. Lactic acid bacteria-specific induction of CD4+Foxp3+ T cells ameliorates shrimp tropomyosin induced allergic response in mice via suppression of mTOR signaling. Sci. Rep. 2017, 7, 1987. [Google Scholar] [CrossRef] [Green Version]
- Irelli, A.; Sirufo, M.M.; Scipioni, T.; De Pietro, F.; Pancotti, A.; Ginaldi, L.; De Martinis, M. mTOR links tumor immunity and bone metabolism: What are the clinical implications? Int. J. Mol. Sci. 2019, 20, 5841. [Google Scholar] [CrossRef] [Green Version]
- Berni Canani, R.; Gilbert, J.A.; Nagler, C.R. The role of the commensal microbiota in the regulation of tolerance to dietary allergens. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Diesner, S.C.; Bergmayr, C.; Pfitzner, B.; Assmann, V.; Krishnamurthy, D.; Starkl, P.; Endesfelder, D.; Rothballer, M.; Welzl, G.; Rattei, T.; et al. A distinct microbiota composition is associated with protection from food allergy in an oral mouse immunization model. Clin. Immunol. 2016, 173, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, A.B.; Berin, M.C. Microbiome and food allergy. Transl. Res. 2017, 179, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Garn, H.; Neves, J.F.; Blumberg, R.S.; Renz, H. Effect of barrier microbes on organ-based inflammation. J. Allergy Clin. Immunol. 2013, 131, 1465–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrs, T.; Sim, K. Demystifying dysbiosis: Can the gut microbiome promote oral tolerance over IgE-mediated food allergy? Curr. Pediatr. Rev. 2018, 14, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xu, L.Z.; Peng, K.; Wu, W.; Wu, R.; Liu, Z.Q.; Yang, G.; Geng, X.R.; Liu, J.; Liu, Z.G.; et al. Specific immunotherapy in combination with Clostridium butyricum inhibits allergic inflammation in the mouse intestine. Sci. Rep. 2015, 5, 17651. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.A.; Yuen, A.W.T.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, G.X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Berin, M.C. Food allergy and the microbiome: Current understandings and future directions. J. Allergy Clin. Immunol. 2019, 144, 1468–1477. [Google Scholar] [CrossRef]
- Platts-Mills, T.A. The Allergy Epidemics: 1870–2010. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, T.; Lum, S.Z.C.; Nagata, Y.; Kawamoto, S.; Oyoshi, M.K. Influences of maternal factors over offspring allergies and the application for food allergy. Front. Immunol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Pastor-Vargas, C.; Maroto, A.S.; Díaz-Perales AVillalba, M.; Esteban, V.; Ruiz-Ramos, M.; de Alba, M.R.; Vivanco, F.; Cuesta-Herranz, J. Detection of major food allergens in amnioticfluid: Initial allergenic encounter during pregnancy. Pediatr. Allergy Immunol. 2016, 27, 716–720. [Google Scholar] [CrossRef]
- Fiocchi, A.; Assa’ad, A.; Bahna, S. Food allergy and the introduction of solid foods to infants: A consensus document. Adverse Reactions to Foods Committee, American College of Allergy, Asthma and Immunology. Ann. Allergy Asthma Immunol. 2006, 97, 10–20. [Google Scholar] [CrossRef]
- Wershil, B.K.; Butzner, D.; Sabra, A.; Savilahti, E.; Seidman, E.; Strobel, S.; Yamashiro, Y. Allergy and immunologicdisease: Working Group Report of the First World Congress of PediatricGastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2002, 35, S74–S77. [Google Scholar] [CrossRef]
- Baumgart, K.; Brown, S.; Gold, M.; Kemp, A.; Loblay, R.; Loh, R.; Mitrou, D.; Mullins, R.; Peake, J.; Ruhno, J.; et al. Australasian Society of Clinical Immunology and Allergy Anaphylaxis Working Party. ASCIA guidelines for prevention of food anaphylactic reactions in schools, preschools and child-care centres. J. Paediatr. Child. Health. 2004, 40, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Vale, S.; Smith, J.; Said, M.; Mullins, R.J.; Loh, R. ASCIA guidelines for prevention of anaphylaxis in schools, pre-schools and childcare: 2015 update. J. Paediatr. Child. Health. 2015, 51, 949–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Keet, C.A.; Wood, R.A. Emerging therapies for food allergy. J. Clin. Investig. 2014, 124, 1880–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuToit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhawt, M.J. The learning early about peanut allergy study: The benefits of early peanut introduction, and a new horizon in fighting the food allergy epidemic. Pediatr. Clin. N. Am. 2015, 62, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sicherer, S.H. Timing of food introduction and atopy prevention. Clin. Dermatol. 2017, 35, 398–405. [Google Scholar] [CrossRef]
- Fleischer, D.M. Life after LEAP: How to implement advice on introducing peanuts in early infancy. J. Paediatr. Child. Health 2017, 53, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Ebisawa, M.; Ito, K.; Fujisawa, T. Committee for Japanese Pediatric Guideline for Food Allergy, The Japanese Society of Pediatric Allergy and Clinical Immunology, The Japanese Society of Allergology. Japanese guidelines for food allergy 2017. Allergol. Int. 2017, 66, 248–264. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Netting, M.J.; Campbell, D.E.; Koplin, J.J.; Beck, K.M.; McWilliam, V.; Dharmage, S.C.; Tang, M.L.K.; Ponsonby, A.L.; Prescott, S.L.; Vale, S.; et al. Centre for Food and Allergy Research, the Australasian Society of Clinical Immunology and Allergy, the National Allergy Strategy, and the Australian Infant Feeding Summit Consensus Group. An Australian Consensus on Infant Feeding Guidelines to Prevent Food Allergy: Outcomes From the Australian Infant Feeding Summit. J. Allergy Clin. Immunol. Pract. 2017, 5, 1617–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halken, S.; Larenas-Linnemann, D.; Roberts, G.; Calderón, M.A.; Angier, E.; Pfaar, O.; Ryan, D.; Agache, I.; Ansotegui, I.J.; Arasi, S.; et al. EAACI guidelines on allergen immunotherapy: Prevention of allergy. Pediatr. Allergy Immunol. 2017, 28, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Nicklaus, S.; Divaret-Chauveau, A.; Chardon, M.L.; Roduit, C.; Kaulek, V.; Ksiazek, E.; Dalphin, M.L.; Karvonen, A.M.; Kirjavainen, P.; Pekkanen, J.; et al. The protective effect of cheese consumption at 18 months on allergic diseases in the first 6 years. Allergy 2019, 74, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.R.; DuToit, G.; Bahnson, H.T.; Lack, G. The challenges of preventing food allergy: Lessons learned from LEAP and EAT. Ann. Allergy Asthma Immunol. 2018, 121, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Ohsaki, A.; Venturelli, N.; Buccigrosso, T.M.; Osganian, S.K.; Lee, J.; Blumberg, R.S.; Oyoshi, M.K. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. J. Exp. Med. 2018, 215, 91–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Immune Tolerance Network Learning Early About Peanut Allergy Study Team. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About PeanutAllergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Gonipeta, B.; Kim, E.; Gangur, V. Mouse models of food allergy: How well do they simulate the human disorder? Crit. Rev. Food Sci. Nutr. 2015, 55, 437–452. [Google Scholar] [CrossRef]
- Albuhairi, S.; Rachid, R. Novel Therapies for Treatment of Food Allergy. Immunol. Allergy Clin. North Am. 2020, 40, 175–186. [Google Scholar] [CrossRef]
- Virkud, Y.V.; Vickery, B.P. Advances in immunotherapy for food allergy. Discov. Med. 2012, 14, 159–165. [Google Scholar]
- Waldron, J.; Kim, E.H. Sublingual and Patch Immunotherapy for Food Allergy. Immunol. Allergy Clin. N. Am. 2020, 40, 135–148. [Google Scholar] [CrossRef]
- Henson, M.; Burks, A.W. The future of food allergy therapeutics. Semin. Immunopathol. 2012, 34, 703–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berin, M.C.; Mayer, L. Can we produce true tolerance in patients with food allergy? J. Allergy Clin. Immunol. 2013, 131, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanser, B.J.; Wright, B.L.; Orgel, K.A.; Vickery, B.P.; Fleischer, D.M. Current Options for the Treatment of Food Allergy. Pediatr. Clin. North Am. 2015, 62, 1531–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott-Taylor, T.H.; Axinia, S.C.; Amin, S.; Pettengell, R. Immunoglobulin G: Structure and functional implications of different subclass modifications in initiation and resolution of allergy. Immun. Inflamm. Dis. 2018, 6, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi SRoberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; Eigenmann, P.; et al. EAACI Guidelines on allergenimmunotherapy: IgE-mediated food allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraro, A.; Roberts, G.; Halken, S.; Agache, I.; Angier, E.; Fernandez-Rivas, M.; Gerth van Wijk, R.; Jutel, M.; Lau, S.; Pajno, G.; et al. EAACI guidelines on allergen immunotherapy: Executive statement. Allergy 2018, 73, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Mondoulet, L.; Blazquez, A.B.; Benhamou, P.H.; Sampson, H.A.; Berin, M.C. Epicutaneous immunotherapy induces gastrointestinal LAP+ Tregs and prevents food-induced anaphylaxis. J. Allergy Clin. Immunol. 2017, 139, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Aceves, S.S. Food and aeroallergens in eosinophilic esophagitis: Role of the allergist in patient management. Curr. Opin. Gastroenterol. 2014, 30, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Williamson, P.; Aceves, S. Allergies and Eosinophilic Esophagitis-Current Updates for the Pediatric Gastroenterologist. Curr. Gastroenterol. Rep. 2019, 21, 56. [Google Scholar] [CrossRef]
- Greenhawt, M.J.; Vickery, B.P. Allergist-reported trends in the practice of food allergen oral immunotherapy. J. Allergy Clin. Immunol. Pract. 2015, 3, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.; Wood, R.A.; French, S.; Fiocchi, A.; Jordana, M.; Waserman, S.; Brożek, J.L.; Schünemann, H.J. Oral immunotherapy for peanut allergy (PACE): A systematic review and meta-analysis of efficacy and safety. Lancet 2019, 393, 2222–2232. [Google Scholar] [CrossRef]
- Upton, J.; Nowak-Wegrzyn, A. The Impact of baked egg and baked milk diets on IgE and non-IgE-mediated allergy. Clin. Rev. Allergy Immunol. 2018, 55, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, H.; Alyasin, S.; Haghighat, M.; Nabavizadeh, H.; Esmaeilzadeh, E.; Mosavat, F. The effect of baked milk on accelerating unheated cow’s milk tolerance: A control randomized clinical trial. Pediatr. Allergy Immunol. 2018, 29, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rangel, I.; Rodriguez Del Rio, P.; Escudero, C.; Sanchez-Garcia, S.; Sanchez-Hernandez, J.J.; Ibanez, M.D. Efficacy and safety of high-dose rush oral immunotherapy in persistent egg allergic children: A randomized clinical trial. Ann. Allergy Asthma Immunol. 2017, 118, 356–364. [Google Scholar] [CrossRef]
- Keet, C. Recognition and management of food induced anaphylaxis. Pediatr. Clin. North Am. 2011, 58, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Simons, F.E.R.; Sanchez-Borges, M.; Thong, B.Y.; Worm, M.; Tanno, L.K.; Lockey, R.F.; El-Gamal, Y.M.; Brown, S.G.; Park, H.S.; Sheikh, A. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Q.; Hu, H.J.; Liu, C.Y.; Zhang, Q.; Shakya, S.; Li, Z.Y. Probiotics for prevention of atopy and food hypersensitivity in early childhood. A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e2562. [Google Scholar] [CrossRef]
- Bauer, R.N.; Manohar, M.; Singh AMJay, D.C.; Nadeau, K.C. The future of biologics: Applications for food allergy. J. Allergy Clin. Immunol. 2015, 135, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Fiocchi, A.; Burks, W.; Bahna, S.L.; Bielory, L.; Boyle, R.J.; Cocco, R.; Dreborg, S.; Goodman, R.; Kuitunen, M.; Haahtela, T.; et al. WAO Special Committee on Food Allergy and Nutrition. On behalf of the WAO Special Committee on Food Allergy and Nutrition. Clinical Use of Probiotics in PediatricAllergy (CUPPA): A World Allergy Organization Position Paper. World Allergy Organ. J. 2012, 5, 148–167. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.Y.; Yang, Z.Y.; Dai, W.K.; Huang, J.Q.; Li, Y.H.; Zhang, J.; Qiu, C.Z.; Wei, C.; Zhou, Q.; Sun, X.; et al. Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-inducedairwayasthma and b-lactoglobulininducedintestinal food allergy mouse models. World J. Gastroenterol. 2017, 23, 2149–2158. [Google Scholar] [CrossRef]
- Wesemann, D.R.; Nagler, C.R. Commensal bacteria, timing and barrier function in the context of allergic disease. Immunity 2016, 44, 728–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martinis, M.; Sirufo, M.M.; Ginaldi, L. A “Stadium” Urticaria, Cold Urticaria Is Still a Mostly Unknown Disease, with a Wide Spectrum of Severity Degrees and Few Therapeutic Certainties: Is Omalizumab One of These? Reflections from a Clinical Case Report. Iran. Red. Cresc. Med. J. 2019, 21, e84250. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, M.; Turner, P.J. Improving the safety of oral immunotherapy for food allergy. Pediatr. Allergy Immunol. 2016, 27, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocchi, A.; Pecora, V.; Valluzzi, R.L.; Fierro, V.; Mennini, M. Use of biologics in severe food allergies. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Chehade, M. Biological therapies for eosinophilic esophagitis: Where do we stand? Clin. Rev. Allergy Immunol. 2018, 55, 205–216. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Stephen-Victor, E.; Gerber, G.K.; Noval Rivas, M.; Wang, S.; Harb, H.; Wang, L.; Li, N.; Crestani, E.; Spielman, S.; et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med. 2019, 1164–1174. [Google Scholar] [CrossRef]
- Zhao, W.; Ho, H.E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Bunyavanich, S. Food allergy: Could the gut microbiota hold the key? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 201–202. [Google Scholar] [CrossRef]
- Ho, H.E.; Bunyavanich, S. Microbial adjuncts for food allergen immunotherapy. Curr. Allergy Asthma Rep. 2019, 19, 25. [Google Scholar] [CrossRef]
- Ho, H.E.; Bunyavanich, S. Role of the microbiome in food allergy. Curr. Allergy Asthma Rep. 2018, 18, 27. [Google Scholar] [CrossRef]
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, C.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 2017, 9, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and preventive effects in allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. https://doi.org/10.3390/ijms21041474
De Martinis M, Sirufo MM, Suppa M, Ginaldi L. New Perspectives in Food Allergy. International Journal of Molecular Sciences. 2020; 21(4):1474. https://doi.org/10.3390/ijms21041474
Chicago/Turabian StyleDe Martinis, Massimo, Maria Maddalena Sirufo, Mariano Suppa, and Lia Ginaldi. 2020. "New Perspectives in Food Allergy" International Journal of Molecular Sciences 21, no. 4: 1474. https://doi.org/10.3390/ijms21041474
APA StyleDe Martinis, M., Sirufo, M. M., Suppa, M., & Ginaldi, L. (2020). New Perspectives in Food Allergy. International Journal of Molecular Sciences, 21(4), 1474. https://doi.org/10.3390/ijms21041474






