Micronucleus Assay: The State of Art, and Future Directions
Abstract
1. Introduction
2. Why Do We Need Genotoxicity Testing, Biomonitoring, Cytogenetic Tests and Biological Dosimetry?
3. How Micronuclei are Formed?
4. Different Types of Micronucleus Assays
4.1. Cytokinesis-Block Micronucleus Assay (CBMN)
4.2. Erythrocyte Micronucleus Assay–the Most Popular In Vivo MN
4.3. Buccal MN (BMm)–Mature but Underused Assay
4.4. Other Types of MN
5. Visibility by International Organization
6. Automation of MN
6.1. Automatic/Semiautomatic Scoring by Microscope Aided Systems
6.2. Flow Cytometry and Imaging Flow Cytometry Aided Mn Scoring
6.3. Automatization of EMn
7. Chromothripsis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMn | Buccal cells micronucleus assay; |
| CBMN | Cytokinesis-block micronucleus assay; |
| CRA | Coordinated Research Activities; |
| DB | Double minutes; |
| EMn | Mammalian erythrocyte micronucleus assay; |
| HUMN | International Human Micronucleus Project; |
| IAEA | International Atomic Energy Agency |
| ISO | International Organization for Standardization; |
| IR | Ionizing radiation; |
| Mn | Micronucleus, micronuclei; |
| MN | Micronucleus assay; |
| OECD | The Organization for Economic Co-operation and Development; |
| RENEB | Running the European Network of Biological and retrospective Physical dosimetry |
| RT | Radiation therapy, radiotherapy; |
References
- Heddle, J.A.; Fenech, M.; Hayashi, M.; MacGregor, J.T. Reflections on the development of micronucleus assays. Mutagenesis 2011, 26, 3–10. [Google Scholar] [CrossRef]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guideline for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2016. Available online: https://doi.org/10.1787/9789264264762-en (accessed on 20 February 2020).
- Kirsch-Volders, M.; Fenech, M.; Bolognesi, C. Validity of the Lymphocyte Cytokinesis-Block Micronucleus Assay (L-CBMN) as biomarker for human exposure to chemicals with different modes of action: A synthesis of systematic reviews. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 47–52. [Google Scholar] [CrossRef]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guideline for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2016. Available online: https://doi.org/10.1787/9789264264861-en (accessed on 20 February 2020).
- Fenech, M. The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat. Res. 1997, 392, 11–18. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar]
- Nersesyan, A.; Fenech, M.; Bolognesi, C.; Mišík, M.; Setayesh, T.; Wultsch, G.; Bonassi, S.; Thomas, P.; Knasmüller, S. Use of the lymphocyte cytokinesis-block micronucleus assay in occupational biomonitoring of genome damage caused by in vivo exposure to chemical genotoxins: Past, present and future. Mutat. Res. 2016, 770, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maluf, S.W.; Mergener, M.; Dalcanale, L.; Costa, C.C.; Pollo, T.; Kayser, M.; da Silva, L.B.; Pra, D.; Teixeira, P.J. DNA damage in peripheral blood of patients with chronic obstructive pulmonary disease (COPD). Mutat. Res. 2007, 626, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Coppedè, F.; Fenech, M.; Thomas, P. Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis 2011, 26, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Smajdova, L. The use of genotoxicity biomarkers in molecular epidemiology: Applications in environmental, occupational and dietary studies. AIMS Genet. 2017, 4, 166–191. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Arslan, M.; Ozdemir, O. Genotoxicity testing: Progress and prospects for the next decade. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1089–1098. [Google Scholar] [CrossRef]
- Bolognesi, C.; Bruzzi, P.; Gismondi, V.; Volpi, S.; Viassolo, V.; Pedemonte, S.; Varesco, L. Clinical Application of Micronucleus Test: A Case-Control Study on the Prediction of Breast Cancer Risk/Susceptibility. PLoS ONE 2014, 9, 1–18. [Google Scholar] [CrossRef]
- Smith, D.F.; MacGregor, J.T.; Hiatt, R.A.; Hooper, N.K.; Wehr, C.M.; Peters, B.; Goldman, L.R.; Yuan, L.A.; Smith, P.A.; Becker, C.E. Micronucleated erythrocytes as an index of cytogenetic damage in humans: Demographic and dietary factors associated with micronucleated erythrocytes in splenectomized subjects. Cancer Res. 1990, 50, 5049–5054. [Google Scholar] [PubMed]
- Marcon, A.E.; Navoni, J.A.; de Oliveira Galvão, M.F.; Garcia, A.C.F.S.; do Amaral, V.S.; Petta, R.A.; Campos, T.F.D.C.; Panosso, R.; Quinelato, A.L.; de Medeiros, S.R.B. Mutagenic potential assessment associated with human exposure to natural radioactivity. Chemosphere 2017, 167, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Giri, S.; Mazumdar, M.; Giri, A.; Roy, P.; Dhar, P. Micronucleus and other nuclear abnormalities among betel quid chewers with or without sadagura, a unique smokeless tobacco preparation, in a population from North-East India. Mutat. Res. 2009, 677, 72–75. [Google Scholar] [CrossRef]
- Cavalcante, D.N.; Sposito, J.C.; Crispim, B.D.; Nascimento, A.V.; Grisolia, A.B. Genotoxic and mutagenic effects of passive smoking and urban air pollutants in buccal mucosa cells of children enrolled in public school. Toxicol. Mech. Methods 2017, 27, 346–351. [Google Scholar] [CrossRef]
- Saks, M.; Upreti, S.; Rajendra, S.V.; Dang, R. Genotoxicity: Mechanisms, Testing Guidelines and Methods. Glob. J. Pharmaceu. Sci. 2017, 555575, 1–6. [Google Scholar] [CrossRef]
- Umbuzeiro, G.A.; Heringa, M.; Zeiger, E. In Vitro Genotoxicity Testing: Significance and Use in Environmental Monitoring. Adv. Biochem. Eng. Biotechnol. 2017, 157, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kishi, M.; Sofuni, T.; Ishidate, M., Jr. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem. Toxicol. 1988, 26, 487–500. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 7th ed.; Wolters Kluver/Lippincott Williams & Wilkins: Philadelfia, PA, USA, 2012; ISBN 978-1-60831-193-4. [Google Scholar]
- Ainsbury, E.A.; Bakhanova, E.; Barquinero, J.F.; Brai, M.; Chumak, V.; Correcher, V.; Darroudi, F.; Fattibene, P.; Gruel, G.; Guclu, I.; et al. Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat. Prot. Dosimetry 2011, 147, 573–592. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies; IAEA: Vienna, Austria, 2011. [Google Scholar]
- Sproull, M.T.; Camphausen, K.A.; Koblentz, G.D. Biodosimetry: A Future Tool for Medical Management of Radiological Emergencies. Health Secur. 2017, 15, 599–610. [Google Scholar] [CrossRef]
- Franciesa, F.Z.; Wainwrightc, R.; Pooled, J.; de Leeneere, K.; Coenee, I.; Wiemee, G.; Poirelg, H.A.; Brichardh, B.; Vermeuleni, S.; Vral, A.; et al. Diagnosis of Fanconi Anaemia by ionising radiation- or mitomycin C—Induced micronuclei. DNA Repair. 2018, 61, 17–24. [Google Scholar] [CrossRef]
- Claes, K.; Depuydt, J.; Taylor, A.M.; Last, J.I.; Baert, A.; Schietecatte, P.; Vandersickel, V.; Poppe, B.; de Leeneer, K.; D’Hooghe, M.; et al. Variant ataxia telangiectasia: Clinical and molecular findings and evaluation of radiosensitive phenotypes in a patient and relatives. Neuromolecular Med. 2013, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bugarín, O.; Macriz Romero, N.; Ramos Ibarra, M.L.; Flores-García, A.; Valdez Aburto, P.; Zavala-Cerna, M.G. Genotoxic Effect in Autoimmune Diseases Evaluated by the Micronucleus Test Assay: Our Experience and Literature Review. Biomed. Res. Int. 2015, 194031, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Padjas, A.; Kedzierawski, P.; Florek, A.; Kukolowicz, P.; Kuszewski, T.; Góźdz, S.; Lankoff, A.; Wojcik, A.; Lisowska, H. Comparative analysis of three functional predictive assays in lymphocytes of patients with breast and gynaecological cancer treated by radiotherapy. J. Contemp. Brachytherapy. 2012, 4, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Encheva, E.; Deleva, S.; Hristova, R.; Hadjidekova, V.; Hadjieva, T. Investigating micronucleus assay applicability for prediction of normal tissue intrinsic radiosensitivity in gynecological cancer patients. Rep. Pract. Oncol. Radiother. 2011, 17, 24–31. [Google Scholar] [CrossRef][Green Version]
- Vinnikov, V.; Belyakov, O. Clinical applications of biomarkers of radiation exposure: Limitations and possible solutions through coordinated research. Radiat. Prot. Dosimetry 2019, 1–6. [Google Scholar] [CrossRef]
- Fenech, M.; Morley, A. Measurement of micronuclei in lymphocytes. Mutat. Res. 1985, 147, 29–36. [Google Scholar] [CrossRef]
- Terradas, M.; Martín, M.; Genescà, A. Impaired nuclear functions in micronuclei results in genome instability and chromothripsis. Arch. Toxicol. 2016, 90, 2657–2667. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Bonassi, S.; Knasmueller, S.; Holland, N.; Bolognesi, C.; Fenech, M.F. Commentary: Critical questions, misconceptions and a road map for improving the use of the lymphocyte cytokinesis-block micronucleus assay for in vivo biomonitoring of human exposure to genotoxic chemicals-a HUMN project perspective. Mutat. Res. Rev. Mutat. Res. 2014, 759, 49–58. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, L.H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Gisselsson, D.; Jonson, T.; Petersén, A.; Strömbeck, B.; dal Cin, P.; Höglund, M.; Mitelman, F.; Mertens, F.; Mandahl, N. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 12683–12688. [Google Scholar] [CrossRef]
- Gisselsson, D.; Björk, J.; Höglund, M.; Mertens, F.; dal Cin, P.; Akerman, M.; Mandahl, N. Abnormal nuclear shape in solid tumors reflects mitotic instability. Am. J. Pathol. 2001, 158, 199–206. [Google Scholar] [CrossRef]
- Barker, P.E. Double minutes in human tumor cells. Cancer Genet. Cytogenet. 1982, 5, 81–94. [Google Scholar] [CrossRef]
- Masters, J.; Keeley, B.; Gay, H.; Attardi, G. Variable content of double minute chromosomes is not correlated with degree of phenotype instability in methotrexate-resistant human cell lines. Mol. Cell. Biol. 1982, 2, 498–507. [Google Scholar] [CrossRef]
- Baskin, F.; Rosenberg, R.N.; Dev, V. Correlation of double-minute chromosomes with unstable multidrug cross-resistance in uptake mutants of neuroblastoma cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3654–3658. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef]
- Kanda, T.; Sullivan, K.F.; Wahl, G.M. Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1988, 8, 377–385. [Google Scholar] [CrossRef]
- Kanda, T.; Wahl, G.M. The dynamics of acentric chromosomes in cancer cells revealed by GFP-based chromosome labeling strategies. J. Cell Biochem. Suppl. 2000, 35, 107–114. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Natural. Product. Reports 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Theodoropoulos, P.A.; Gravanis, A.; Tsapara, A.; Margioris, A.N.; Papadogiorgaki, E.; Galanopoulos, V.; Stournaras, C. Cytochalasin B may shorten actin filaments by a mechanism independent of barbed end capping. Biochem. Pharmacol. 1994, 47, 1875–1881. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Sofuni, T.; Aardema, M.; Albertini, S.; Eastmond, D.; Fenech, M.; Ishidate, M.; Lorge, E.; Norppa, H.; Surralles, J.; et al. Report from the In Vitro Micronucleus Assay Working Group. Environ. Mol. Mutagen. 2000, 35, 167–172. [Google Scholar] [CrossRef]
- Fenech, M.; Morley, A.A. Cytokinesis-block micronucleus method in human lymphocytes: Effect of in vivo ageing and low-dose x-irradiation. Mutat. Res. 1986, 161, 193–198. [Google Scholar] [CrossRef]
- Wakata, A.; Sasaki, M.S. Measurement of micronuclei by cytokinesis-block method in cultured Chinese hamster cells: Comparison with types and rates of chromosome aberrations. Mutat. Res. 1987, 190, 51–57. [Google Scholar] [CrossRef]
- Prosser, J.S.; Moquet, J.E.; Lloyd, D.C.; Edwards, A.A. Radiation induction of micronuclei in human lymphocytes. Mutat. Res. 1988, 199, 37–45. [Google Scholar] [CrossRef]
- Lindholm, C.; Norrpa, H.; Hayashi, M.; Sorsa, M. Induction of micronuclei and anaphase aberrations by cytochalasin-B in human lymphocyte cultures. Mutat. Res. 1991, 260, 369–375. [Google Scholar] [CrossRef]
- Fenech, M. A mathematical model of the in vitro micronucleus assay predicts false negative results if micronuclei are not specifically scored in binucleated cells or in cells that have completed one nuclear division. Mutagenesis 2000, 15, 329–336. [Google Scholar] [CrossRef][Green Version]
- Kim, S.R.; Kim, T.H.; Ryu, S.Y.; Lee, H.J.; Oh, H.; Jo, S.K.; Oh, K.S.; Park, I.C.; Kim, J.C.; Kang, C.M.; et al. Measurement of micronuclei by cytokinesis-block method in human, cattle, goat, pig, rabbit, chicken and fish peripheral blood lymphocytes irradiated in vitro with gamma radiation. In Vivo 2003, 17, 433–438. [Google Scholar]
- Catena, C.; Conti, D.; Villani, P.; Nastasi, R.; Archilei, R.; Righi, E. Micronuclei and 3AB index in human and canine lymphocytes after in vitro X-irradiation. Mutat. Res. 1994, 312, 1–8. [Google Scholar] [CrossRef]
- Resendes, A.S.; dos Santos, D.S.; França, F.M.; Petesse, M.L.; Badaró-Pedroso, C.; Ferreira, C.M. Acute toxic and genotoxic effects of formalin in Danio rerio (zebrafish). Ecotoxicology 2018, 27, 1379–1386. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Beaton-Green, L.A.; Wilkins, R.C.; Fenech, M.F. The potential for complete automated scoring of the cytokinesis block micronucleus cytome assay using imaging flow cytometry. Mutat. Res. Gen. Tox. En. 2018, 836, 53–64. [Google Scholar] [CrossRef]
- Wang, Q.; Rodrigues, M.A.; Repin, M.; Pampou, S.; Beaton-Green, L.A.; Perrier, J.; Garty, G.; Brenner, D.J.; Turner, H.C.; Wilkins, R.C. Automated Triage Radiation Biodosimetry: Integrating Imaging Flow Cytometry with High-Throughput Robotics to Perform the Cytokinesis-Block Micronucleus Assay. Radiat. Res. 2019, 191, 342–351. [Google Scholar] [CrossRef]
- Thierens, H.; Vral, A.; Vandevoorde, C.; Vandersickel, V.; de Gelder, V.; Romm, H.; Oestreicher, U.; Rothkamm, K.; Barnard, S.; Ainsbury, E.; et al. Is a semi-automated approach indicated in the application of the automated micronucleus assay for triage purposes? Radiat. Prot. Dosimetry 2014, 159, 87–94. [Google Scholar] [CrossRef]
- Kirsch-Volders, M. Towards a validation of the micronucleus test. Mutat. Res. 1997, 392, 1–4. [Google Scholar] [CrossRef]
- Parry, J.M.; Sors, A. The detection and assessment of the aneugenic potential of environmental chemicals: The European Community aneuploidy project. Mutat. Res. 1993, 287, 3–15. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Oliveira, N.G.; Gil, O.M.; Léonard, A.; Rueff, J. Use of cytogenetic indicators in radiobiology. Radiat. Prot. Dosim. 2005, 115, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.J.; Sharpe, Z.; Alemara, S.; Mackenzie, S.; Liu, G.; Abdallah, B.; Horne, S.; Regan, S.; Heng, H.H. Micronuclei and Genome Chaos: Changing the System Inheritance. Genes 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Jeggo, P.A.; West, C.; Gomolka, M.; Quintens, R.; Badie, C.; Laurent, O.; Aerts, A.; Anastasov, N.; Azimzadeh, O.; et al. Ionizing radiation biomarkers for epidemiological studies—An update. Mutat. Res. 2017, 771, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Baert, A.; Depuydt, J.; van Maerken, T.; Poppe, B.; Malfait, F.; Storm, K.; van den Ende, J.; Van Damme, T.; De Nobele, S.; Perletti, G.; et al. Increased chromosomal radiosensitivity in asymptomatic carriers of a heterozygous BRCA1 mutation. Breast Cancer Res. 2016, 18, 52. [Google Scholar] [CrossRef]
- Vral, A.; Thierens, H.; de Ridder, L. Micronucleus induction by 60Co γ-rays and fast neutrons in ataxia telangiectasia lymphocytes. Int. J. Radiat. Biol. 1996, 70, 171–176. [Google Scholar] [CrossRef]
- Okunieff, P.; Chen, Y.; Maguire, D.J.; Huser, A.K. Molecular markers of radiation related normal tissue toxicity. Cancer Metastasis Rev. 2008, 27, 363–374. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Parliament, M.; Deasy, J.O.; Dicker, A.; Curran, W.J.; Williams, J.P.; Rosenstein, B.S. Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: The importance of dose-volume effects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 145–150. [Google Scholar] [CrossRef]
- Bonassi, S.; Znaor, A.; Ceppi, M.; Lando, C.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Ban, S.; Barale, R.; et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2007, 28, 625–631. [Google Scholar] [CrossRef]
- Bolognesi, C.; Bonassi, S.; Knasmueller, S.; Fenech, M.; Bruzzone, M.; Lando, C.; Ceppi, M. Clinical application of micronucleus test in exfoliated buccal cells: A systematic review and metanalysis. Mutat. Res. 2015, 766, 20–31. [Google Scholar] [CrossRef]
- McClelland, S.E. Role of chromosomal instability in cancer progression. Endocr. Relat. Cancer 2017, 24, T23–T31. [Google Scholar] [CrossRef]
- Vargas-Rondón, N.; Villegas, V.E.; Rondón-Lagos, M. The role of chromosomal instability in cancer and therapeutic responses. Cancers 2017, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Scalbert, A.; Herceg, Z. Measuring the exposome: A powerful basis for evaluating environmental exposures and cancer risk. Environ. Mol. Mutagen. 2013, 54, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Smith, M.T. Epidemiology, Environment and disease risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, V.A.; Nersesyan, A.K.; Atefie, K.; Hoelzl, C.; Ferk, F.; Bichler, J.; Valic, E.; Schaffer, A.; Schulte-Hermann, R.; Fenech, M.; et al. Inhalativeexposure to vanadium pentoxide causes DNA damage in workers: Results of amultiple end point study. Environ. Health Perspect. 2008, 116, 1689–1693. [Google Scholar]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Von Ledebur, M.; Schmid, W. The micronucleus test: Methodological aspects. Mutat. Res. 1973, 19, 109–117. [Google Scholar] [CrossRef]
- MacGregor, J.T.; Wehr, C.M.; Gould, D.H. Clastogen-induced micronuclei in peripheral blood erythrocytes: The basis of an improved micronucleus test. Environ. Mutagen. 1980, 2, 509–514. [Google Scholar] [CrossRef]
- Abe, T.; Isemura, T.; Kikuchi, Y. Micronuclei in human bone-marrow cells: Evaluation of the micronucleus test using human leukemia patients treated with antileukemic agents. Mutat. Res. 1984, 130, 113–120. [Google Scholar] [CrossRef]
- Goetz, P.; Šrám, R.J.; Dohnalová, J. Relationship between experimental results in mammals and man: Cytogenetic analysis of bone marrow injury induced by a single dose of cyclophosphamide. Mutat. Res. 1975, 31, 247–254. [Google Scholar] [CrossRef]
- Udroiu, I. Feasibility of conducting the micronucleus test in circulating erythrocytes from different mammalian species: An anatomical perspective. Environ. Mol. Mutagen. 2006, 47, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Polard, T.; Jean, S.; Merlina, G.; Laplanche, C.; Pinelli, E.; Gauthier, L. Giemsa versus acridine orange staining in the fish micronucleus assay and validation for use in water quality monitoring. Ecotoxicol. Environ. Saf. 2011, 74, 144–149. [Google Scholar] [CrossRef][Green Version]
- Sato, S.M.; Taketomi, M.; Nakajima, M. Effect of aging on spontaneous micronucleus frequencies in peripheral blood of nine mouse strains: The results of the 7th collaborative study organized by CSGMT/MMS. Mutat. Res. 1995, 338, 51–57. [Google Scholar] [CrossRef]
- Dobrzyńska, M.; Gajowik, A.; Radzikowska, J.; Lankoff, A.; Dušinská, M.; Kruszewski, M. Genotoxicity of silver and titanium dioxide nanoparticles in bone marrow cells of rats in vivo. Toxicology 2014, 315, 86–91. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Q.; Jin, Z.D.; Zhou, Z.; Nie, J.H.; Tong, J. Induction of adaptive response: Pre-exposure of mice to 900 MHz radiofrequency fields reduces hematopoietic damage caused by subsequent exposure to ionising radiation. Int. J. Radiat. Biol. 2011, 87, 720–728. [Google Scholar] [CrossRef]
- Dertinger, S.D.; Torous, D.K.; Hayashi, M.; MacGregor, J.T. Flow cytometric scoring of micronucleated erythrocytes: An efficient platform for assessing in vivo cytogenetic damage. Mutagenesis 2011, 26, 139–145. [Google Scholar] [CrossRef]
- Schlegel, R.; MacGregor, J.T.; Everson, R.B. Assessment of cytogenetic damage by quantitation of micronuclei in human peripheral blood erythrocytes. Cancer Res. 1986, 46, 3717–3721. [Google Scholar]
- Abramsson-Zetterberg, L.; Zetterberg, G.; Bergqvist, M.; Grawé, J. Human cytogenetic biomonitoring using flow-cytometric analysis of micronuclei in transferrin-positive immature peripheral blood reticulocytes. Environ. Mol. Mutagen. 2000, 36, 22–31. [Google Scholar] [CrossRef]
- Hayashi, M.; MacGregor, J.T.; Gatehouse, D.G.; Blakey, D.H.; Dertinger, S.D.; Abramsson-Zetterberg, L.; Krishna, G.; Morita, T.; Russo, A.; Asano, N.; et al. In Vivo Micronucleus Assay Working Group, IWGT. In vivo erythrocyte micronucleus assay III. Validation and regulatory acceptance of automated scoring and the use of rat peripheral blood reticulocytes, with discussion of non-hematopoietic target cells and a single dose-level limit test. Mutat. Res. 2007, 627, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Stich, H.F.; San, R.H.; Rosin, M.P. Adaptation of the DNA-repair and micronucleus tests to human cell suspensions and exfoliated cells. Ann. N. Y. Acad. Sci. 1983, 407, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Stich, H.F.; Rosin, M.P. Quantitating the synergistic effect of smoking and alcohol consumption with the micronucleus test on human buccal mucosa cells. Int. J. Cancer. 1983, 31, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal micronucleus cytome assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef]
- Thomas, P.; Fenech, M. Buccal micronucleus cytome assay. Methods Mol. Biol. 2011, 682, 235–248. [Google Scholar] [CrossRef]
- Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat. Res. 2008, 659, 93–108. [Google Scholar] [CrossRef]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay—An update and expanded photogallery. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef]
- Bolognesi, C.; Fenech, M. Micronucleus Cytome Assays in Human Lymphocytes and Buccal Cells. Methods Mol. Biol. 2019, 2031, 147–163. [Google Scholar] [CrossRef]
- De Oliveira, F.M.; Carmona, A.M.; Ladeira, C. Is mobile phone radiation genotoxic? An analysis of micronucleus frequency in exfoliated buccal cells. Mutat. Res. 2017, 822, 41–46. [Google Scholar] [CrossRef]
- Ceppi, M.; Biasotti, B.; Fenech, M.; Bonassi, S. Human population studies with the exfoliated buccal micronucleus assay: Statistical and epidemiological issues. Mutat Res. 2010, 705, 11–19. [Google Scholar] [CrossRef]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Roggieri, P.; Ceppi, M.; Bruzzone, M.; Blaszczyk, E.; Mielzynska-Svach, D.; Milic, M.; Bonassi, S.; et al. Inter-laboratory consistency and variability in the buccal micronucleus cytome assay depends on biomarker scored and laboratory experience: Results from the HUMNxl international inter-laboratory scoring exercise. Mutagenesis 2017, 32, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Holland, N.; Zeiger, E.; Chang, W.P.; Burgaz, S.; Thomas, P.; Bolognesi, C.; Knasmueller, S.; Kirsch-Volders, M.; Bonassi, S. The HUMN and HUMNxL international collaboration projects on human micronucleus assays in lymphocytes and buccal cells—Past, present and future. Mutagenesis 2011, 26, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Linhares, D.P.S.; Garcia, P.V.; Silva, C.; Barroso, J.; Kazachkova, N.; Pereira, R.; Lima, M.; Camarinho, R.; Ferreira, T.; Dos Santos Rodrigues, A. DNA damage in oral epithelial cells of individuals chronically exposed to indoor radon (222Rn) in a hydrothermal area. Environ. Geochem. Health 2018, 40, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Torres, L.; Dos Santos Rodrigues, A.; Linhares, D.; Camarinho, R.; Nunes Páscoa Soares Rego, Z.M.; Ventura Garcia, P. Buccal epithelial cell micronuclei: Sensitive, non-invasive biomarkers of occupational exposure to low doses of ionizing radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 838, 54–58. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Fenech, M.; Bolognesi, C.; Misik, M.; Wultsch, G.; Hartmann, M.; Knasmueller, S. Micronucleus assay with urine derived cells (UDC): A review of its application in human studies investigating genotoxin exposure and bladder cancer risk. Mutat. Res. Rev. Mutat. Res. 2014, 762, 37–51. [Google Scholar] [CrossRef]
- Kesimci, E.; Çoşkun, E.; Uğur, G.; Müderris, T.; İzdeş, S.; Karahalil, B. Can Sevoflurane Induce Micronuclei Formation in Nasal Epithelial Cells of Adult Patients? Turk. J. Anaesthesiol. Reanim. 2017, 45, 264–269. [Google Scholar] [CrossRef]
- Bonassi, S.; Milić, M.; Neri, M. Frequency of micronuclei and other biomarkers of DNA damage in populations exposed to dusts, asbestos and other fibers. A systematic review. Mutat. Res. 2016, 770, 106–118. [Google Scholar] [CrossRef]
- Kulka, U.; Abend, M.; Ainsbury, E.; Badie, C.; Barquinero, J.F.; Barrios, L.; Beinke, C.; Bortolin, E.; Cucu, A.; De Amicis, A.; et al. RENEB—Running the European Network of biological dosimetry and physical retrospective dosimetry. Int. J. Radiat. Biol. 2017, 93, 2–14. [Google Scholar] [CrossRef]
- ISO 17099:2014. Radiological Protection—Performance Criteria for Laboratories Using the Cytokinesis Block Micronucleus (CBMN) Assay in Peripheral Blood Lymphocytes for Biological Dosimetry. 2014. Available online: https://www.iso.org/standard/59141.html (accessed on 20 February 2020).
- International Atomic Energy Agency. Biological Dosimetry: Chromosomal Aberration Analysis for Dose Assessment, Technical Reports Series No. 260; IAEA: Vienna, Austria, 1986. [Google Scholar]
- International Atomic Energy Agency. Cytogenetic Analysis for Radiation Dose Assessment, Technical Reports Series No. 405; IAEA: Vienna, Austria, 2001. [Google Scholar]
- International Atomic Energy Agency. The Radiological Accident in Istanbul; IAEA: Vienna, Austria, 2000. [Google Scholar]
- International Atomic Energy Agency Factsheet. IAEA Coordinated Research Activities, IAEA Office of Public Information and Communication, August 2019. Available online: https://www.iaea.org/sites/default/files/19/09/iaea-coordinated-research-activities.pdf (accessed on 20 February 2020).
- Applications of Biological Dosimetry Methods in Radiation Oncology, Nuclear Medicine, and Diagnostic and Interventional Radiology (MEDBIODOSE). Available online: https://www.iaea.org/projects/crp/e35010 (accessed on 20 February 2020).
- Depuydt, J.; Baeyens, A.; Barnard, S.; Beinke, C.; Benedek, A.; Beukes, P.; Buraczewska, I.; Darroudi, F.; De Sanctis, S.; Dominguez, I.; et al. RENEB intercomparison exercises analyzing micronuclei (Cytokinesis-block Micronucleus Assay). Int. J. Radiat. Biol. 2017, 93, 36–47. [Google Scholar] [CrossRef]
- Brzozowska, B.; Ainsbury, E.; Baert, A.; Beaton-Green, L.; Barrios, L.; Barquinero, J.F.; Bassinet, C.; Beinke, C.; Benedek, A.; Beukes, P.; et al. RENEB accident simulation exercise. Int J Radiat Biol. 2017, 93, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ainsbury, E.A.; Al-Hafidh, J.; Bajinskis, A.; Barnard, S.; Barquinero, J.F.; Beinke, C.; de Gelder, V.; Gregoire, E.; Jaworska, A.; Lindholm, C.; et al. Inter- and intra-laboratory comparison of a multibiodosimetric approach to triage in a simulated, large scale radiation emergency. Int. J. Radiat. Biol. 2014, 90, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kulka, U.; Ainsbury, L.; Atkinson, M.; Barnard, S.; Smith, R.; Barquinero, J.F.; Barrios, L.; Bassinet, C.; Beinke, C.; Cucu, A.; et al. Realising the European network of biodosimetry: RENEB-status quo. Radiat Prot Dosim. 2015, 164, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Decordier, I.; Papine, A.; Vande Loock, K.; Plas, G.; Soussaline, F.; Kirsch-Volders, M. Automated image analysis of micronuclei by IMSTAR for biomonitoring. Mutagenesis 2011, 26, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Frieauff, W.; Martus, H.J.; Suter, W.; Elhajouji, A. Automatic analysis of the micronucleus test in primary human lymphocytes using image analysis. Mutagenesis 2013, 28, 15–23. [Google Scholar] [CrossRef]
- Schunck, C.; Johannes, T.; Varga, D.; Lörch, T.; Plesch, A. New developments in automated cytogenetic imaging: Unattended scoring of dicentric chromosomes, micronuclei, single cell gel electrophoresis, and fluorescence signals. Cytogenet Genome Res. 2004, 104, 383–389. [Google Scholar] [CrossRef]
- Rossnerova, A.; Spatova, M.; Schunck, C.; Sram, R.J. Automated scoring of lymphocyte micronuclei by the MetaSystems Metafer image cytometry system and its application in studies of human mutagen sensitivity and biodosimetry of genotoxin exposure. Mutagenesis 2011, 26, 169–175. [Google Scholar] [CrossRef]
- Seager, A.L.; Shah, U.K.; Brüsehafer, K.; Wills, J.; Manshian, B.; Chapman, K.E.; Thomas, A.D.; Scott, A.D.; Doherty, A.T.; Doak, S.H.; et al. Recommendations, evaluation and validation of a semi-automated, fluorescent-based scoring protocol for micronucleus testing in human cells. Mutagenesis 2014, 29, 155–164. [Google Scholar] [CrossRef]
- Decordier, I.; Papine, A.; Plas, G.; Roesems, S.; Vande Loock, K.; Moreno-Palomo, J.; Cemeli, E.; Anderson, D.; Fucic, A.; Marcos, R.; et al. Automated image analysis of cytokinesis-blocked micronuclei: An adapted protocol and a validated scoring procedure for biomonitoring. Mutagenesis 2009, 24, 85–93. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Smolewski, P.; Holden, E.; Luther, E.; Henriksen, M.; François, M.; Leifert, W.; Fenech, M. Laser scanning cytometry for automation of the micronucleus assay. Mutagenesis 2011, 26, 153–161. [Google Scholar] [CrossRef]
- Nüsse, M.; Marx, K. Flow cytometric analysis of micronuclei in cell cultures and human lymphocytes: Advantages and disadvantages. Mutat. Res. 1997, 392, 109–115. [Google Scholar] [CrossRef]
- Schreiber, G.A.; Beisker, W.; Bauchinger, M.; Nüsse, M. Multiparametric flow cytometric analysis of radiation-induced micronuclei in mammalian cell cultures. Cytometry 1992, 13, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Avlasevich, S.L.; Bryce, S.M.; Cairns, S.E.; Dertinger, S.D. In vitro micronucleus scoring by flow cytometry: Differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability. Environ. Mol. Mutagen. 2006, 47, 56–66. [Google Scholar] [CrossRef]
- Hintzsche, H.; Hemmann, U.; Poth, A.; Utesch, D.; Lott, J.; Stopper, H.; Working Group “In vitro micronucleus test”; Gesellschaft für Umwelt-Mutationsforschung (GUM, German-speaking section of the European Environmental Mutagenesis and Genomics Society EEMGS). Fate of micronuclei and micronucleated cells. Mutat. Res. 2017, 771, 85–98. [Google Scholar] [CrossRef]
- Romagna, F.; Staniforth, C.D. The automated bone marrow micronucleus test. Mutat. Res. 1989, 213, 91–104. [Google Scholar] [CrossRef]
- Frieauff, W.; Romagna, F. Technical aspects of automatic micronucleus analysis in rodent bone marrow assays. Cell. Biol. Toxicol. 1994, 10, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Dertinger, S.D.; Torous, D.K.; Tometsko, K.R. Simple and reliable enumeration of micronucleated reticulocytes with a single-laser flow cytometer. Mutat. Res. 1996, 371, 283–292. [Google Scholar] [CrossRef]
- Dertinger, S.D.; Miller, R.K.; Brewer, K.; Smudzin, T.; Torous, D.K.; Roberts, D.J.; Avlasevich, S.L.; Bryce, S.M.; Sugunan, S.; Chen, Y. Automated human blood micronucleated reticulocyte measurements for rapid assessment of chromosomal damage. Mutat. Res. 2007, 626, 111–119. [Google Scholar] [CrossRef][Green Version]
- Zeiger, E.; Recio, L.; Fennell, T.R.; Haseman, J.K.; Snyder, R.W.; Friedman, m. Investigation of the low-dose response in the in vivo induction of micronuclei and adducts by acrylamide. Toxicol. Sci. 2009, 107, 247–257. [Google Scholar] [CrossRef]
- Cammerer, Z.; Schumacher, M.M.; Kirsch-Volders, M.; Suter, W.; Elhajouji, A. Flow cytometry peripheral blood micronucleus test in vivo: Determination of potential thresholds for aneuploidy induced by spindle poisons. Environ. Mol. Mutagen. 2010, 51, 278–284. [Google Scholar] [CrossRef]
- Wakata, A.; Miyamae, Y.; Sato, S.; Suzuki, T.; Morita, T.; Asano, N.; Awogi, T.; Kondo, K.; Hayashi, M. Evaluation of the rat micronucleus test with bone marrow and peripheral blood: Summary of the 9th collaborative study by CSGMT/JEMS. MMS. Collaborative Study Group for the Micronucleus Test. Environmental Mutagen Society of Japan. Mammalian Mutagenicity Study Group. Environ. Mol. Mutagen. 1998, 32, 84–100. [Google Scholar]
- Hamada, S.; Sutou, S.; Morita, T.; Wakata, A.; Asanami, S.; Hosoya, S.; Ozawa, S.; Kondo, K.; Nakajima, M.; Shimada, H.; et al. Evaluation of the rodent micronucleus assay by a 28-day treatment protocol: Summary of the 13th Collaborative Study by the Collaborative Study Group for the Micronucleus Test (CSGMT)/Environmental Mutagen Society of Japan (JEMS)-Mammalian Mutagenicity Study Group (MMS). Environ Mol. Mutagen. 2001, 37, 93–110. [Google Scholar] [PubMed]
- Maher, C.A.; Wilson, R.K. Chromothripsis and Human Disease: Piecing Together the Shattering Process. Cell 2012, 148, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, W.P.; Cuppen, E. Chromothripsis in congenital disorders and cancer: Similarities and differences. Curr Opin Cell Biol. 2013, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.L.; Zhang, C.Z.; Pellman, D. Chromothripsis: A New Mechanism for Rapid Karyotype Evolution. Annu. Rev. Genet. 2015, 49, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Forment, J.V.; Kaidi, A.; Jackson, S.P. Chromothripsis and cancer: Causes and consequences of chromosome shattering. Nature Reviews Cancer. 2012, 12, 663–670. [Google Scholar] [CrossRef]
- Molenaar, J.J.; Koster, J.; Zwijnenburg, D.A.; van Sluis, P.; Valentijn, L.J.; van der Ploeg, I.; Hamdi, M.; van Nes, J.; Westerman, B.A.; van Arkel, J.; et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012, 483, 589–593. [Google Scholar] [CrossRef]
- Cai, H.; Kumar, N.; Bagheri, H.C.; von Mering, C.; Robinson, M.D.; Baudis, M. Chromothripsis-like patterns are recurring but heterogeneously distributed features in a survey of 22,347 cancer genome screens. BMC Genom. 2014, 15, 82. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 527, 398. [Google Scholar] [CrossRef]
- Meyerson, M.; Pellman, D. Cancer genomes evolve by pulverizing single chromosomes. Cell 2011, 144, 9–10. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Leibowitz, M.L.; Pellman, D. Chromothripsis and beyond: Rapid genome evolution from complex chromosomal rearrangements. Genes Dev. 2013, 27, 2513–2530. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef]
- Huang, Y.; Hou, H.; Yi, Q.; Zhang, Y.; Chen, D.; Jiang, E.; Xia, Y.; Fenech, M.; Shi, Q. The fate of micronucleated cells post X-irradiation detected by live cell imaging. DNA Repair (Amst). 2011, 10, 629–638. [Google Scholar] [CrossRef] [PubMed]

| Diagnose genetic disorders like Fanconi anemia, ataxia telangiectasia or various autoimmune diseases [24,25,26]. |
| Evaluation of individual susceptibility to the effects of exogenous or endogenous genotoxic agents [3,12]. |
| Assessment of the risk of developing cancer and other chronic diseases [12]. |
| Prediction assay for a radiation side effect (normal tissue reaction) of patients with different cancers subjected to RT and post RT follow up [27,28,29]. |
| Quantification of in vitro genotoxicity of different schemes of RT [29]. |
| Acentric chromosome fragments |
| Acentric chromosome fragments result from unrepaired DNA strand breaks or misrepaired DNA strand breaks, DNA strand cross-links or adducts leading to chromosome or chromatid type aberrations, e.g., polycentric chromosomes usually accompanied with acentric fragments; [22,31,32,33]. |
| Malsegregation of chromosomes |
| A whole chromosome lagging behind during mitosis or numerical chromosome aberrations, as a result of centromere dysfunction; kinetochore dysfunction, spindle dysfunction. Aging of women, when some chromosomes X are excluded from the nucleus [22,31,32,33]. |
| Dicentric chromosome breakage |
| Polycentric chromosomes, when spread between opposite cells, may break in many pieces, giving rise to Mn or/and broken unprotected chromatid ends [31,32,33,34]. Unprotected chromatid ends are susceptible to different reorganization processes and can start breakage–fusion–bridge cycles [34], that give rise to Mn formation and are considered as one of the mechanisms of chromosome instability of cancer cells [34]. |
| Chromosome instability |
| Chromosome instability is a condition in which cells gain changes in their genome at a high rate. Chromosome instability is often displayed by pre-neoplastic and cancerous cells, which usually show a high frequency of Mn [31,35]. It is accepted that Mn is a good indicator of chromosome instability [31]. |
| Aggregation of double minutes (DB) |
| DB are small acentric and telomere-free extrachromosomal bodies composed of circular DNA [31,36,37]. They have been observed in many kinds of tumors including breast, lung, ovary, colon and neuroblastoma [36,37,38]. DB are the manifestation of genomic instability and recently they have been linked to chromothripsis phenomenon [39]. They carry multiple copies of amplified genes, usually oncogenes or genes involved in drug resistance. Many copies of DB can be found in a single cell often stuck to the chromosomes [40]. DB, when detached from chromosomes, can aggregate and form Mn [41]. |
| Type of Test | Cells Used | Purpose | Short Characteristics |
|---|---|---|---|
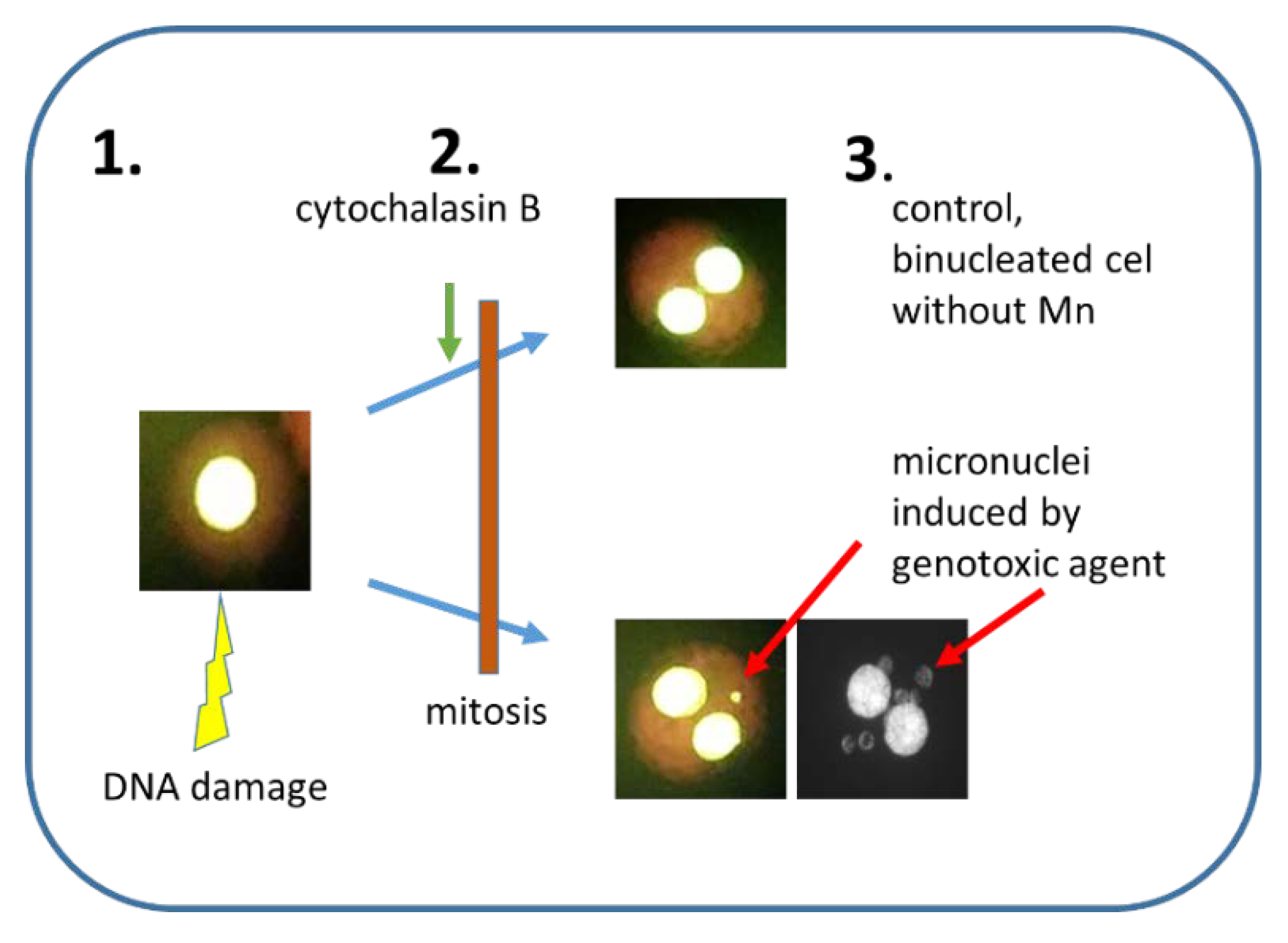
| Cytokinesis-block micro-nucleus assay (CBMN) | human, rodents, rabbit, fish, dogs, primates, etc. lymphocytes or cell lines. |
| Mn is scored in binucleated cells, where cytokinesis is stopped by addition of cytochalasin B. The most popular in vitro and in vivo MN. |
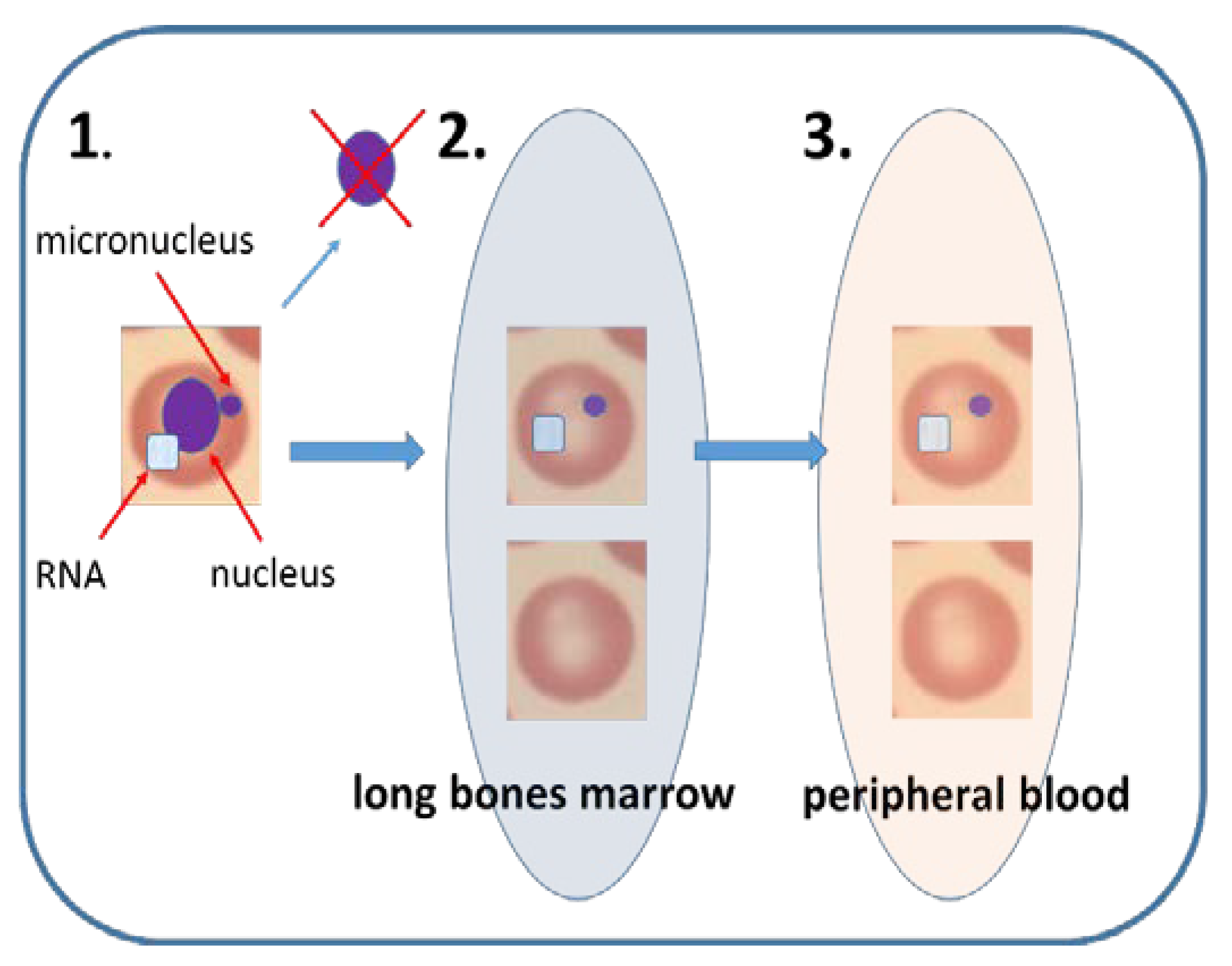
| Mammalian Erythrocyte MN | human, rodents, rabbit, fish, dogs, primates, immature erythrocytes. |
| Test performed usually on young rodents, but biomonitoring of the human population based on peripheral blood is possible. When performed in peripheral blood erythrocytes splenic selection must be considered. |
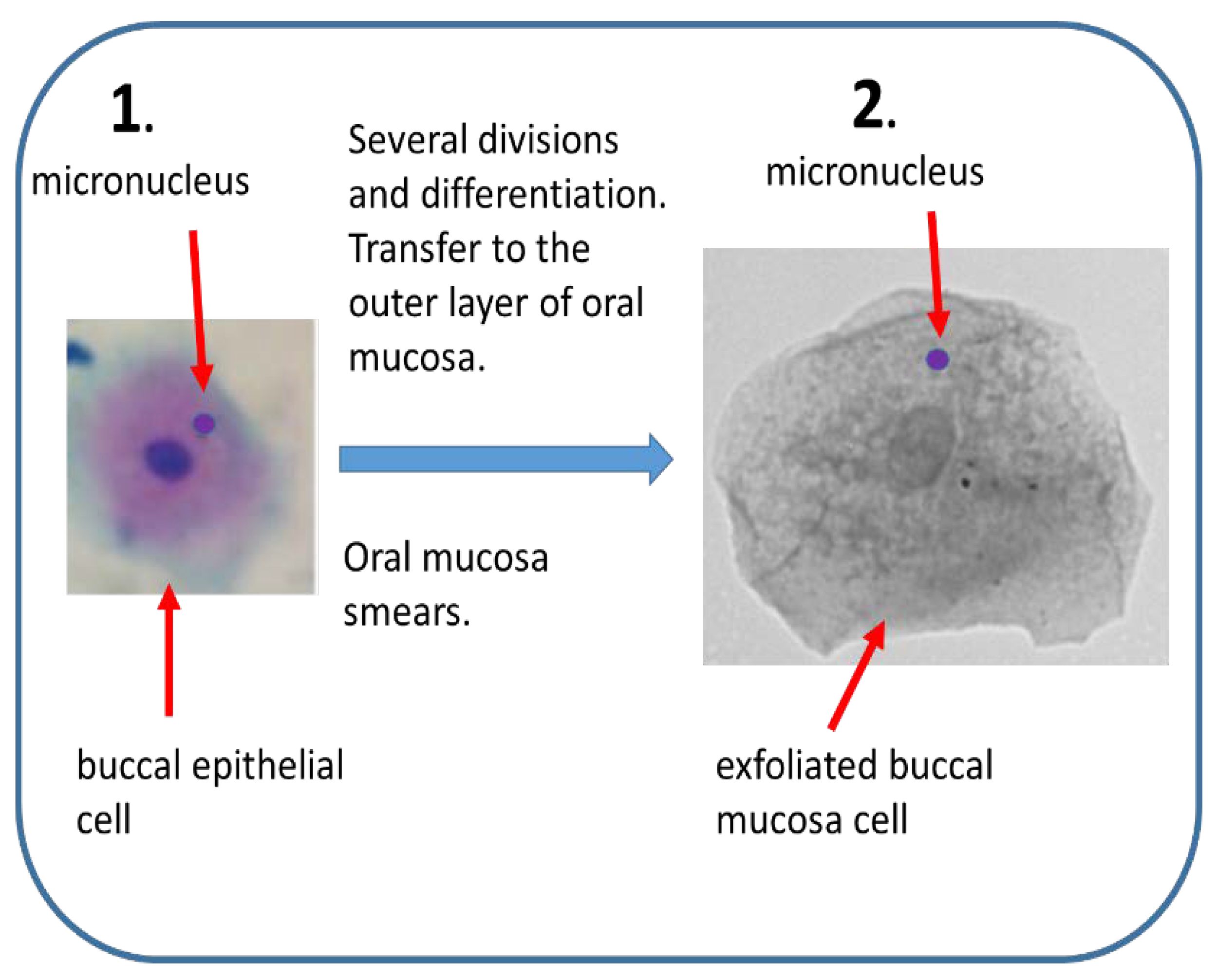
| Buccal MN | human epithelial buccal cells. |
| Incoming, little invasive in vivo test. Suitable for biomonitoring. |
| MN in other cell types | nasal mucosa cells, urine-derived cells. |
| Not very popular, although there are new publications. |
| basic research on DNA damage and repair [58,59]; |
| radiosensitivity studies of various groups, whether healthy or with genetic disorders [24,60,61,62]; |
| attempts to link the radiosensitivity with the radiation reaction of normal tissues in persons undergoing radiotherapy [63,64]; |
| predictive tests of neoplastic disease [60,65,66]; |
| characterizations of cytogenetic damage during chemo- and radiotherapy [67,68]; |
| biomonitoring of the environment or occupational exposure [32,69,70,71]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. https://doi.org/10.3390/ijms21041534
Sommer S, Buraczewska I, Kruszewski M. Micronucleus Assay: The State of Art, and Future Directions. International Journal of Molecular Sciences. 2020; 21(4):1534. https://doi.org/10.3390/ijms21041534
Chicago/Turabian StyleSommer, Sylwester, Iwona Buraczewska, and Marcin Kruszewski. 2020. "Micronucleus Assay: The State of Art, and Future Directions" International Journal of Molecular Sciences 21, no. 4: 1534. https://doi.org/10.3390/ijms21041534
APA StyleSommer, S., Buraczewska, I., & Kruszewski, M. (2020). Micronucleus Assay: The State of Art, and Future Directions. International Journal of Molecular Sciences, 21(4), 1534. https://doi.org/10.3390/ijms21041534






