Ethylene Biosynthesis Inhibition Combined with Cyanide Degradation Confer Resistance to Quinclorac in Echinochloa crus-galli var. mitis
Abstract
1. Introduction
2. Results
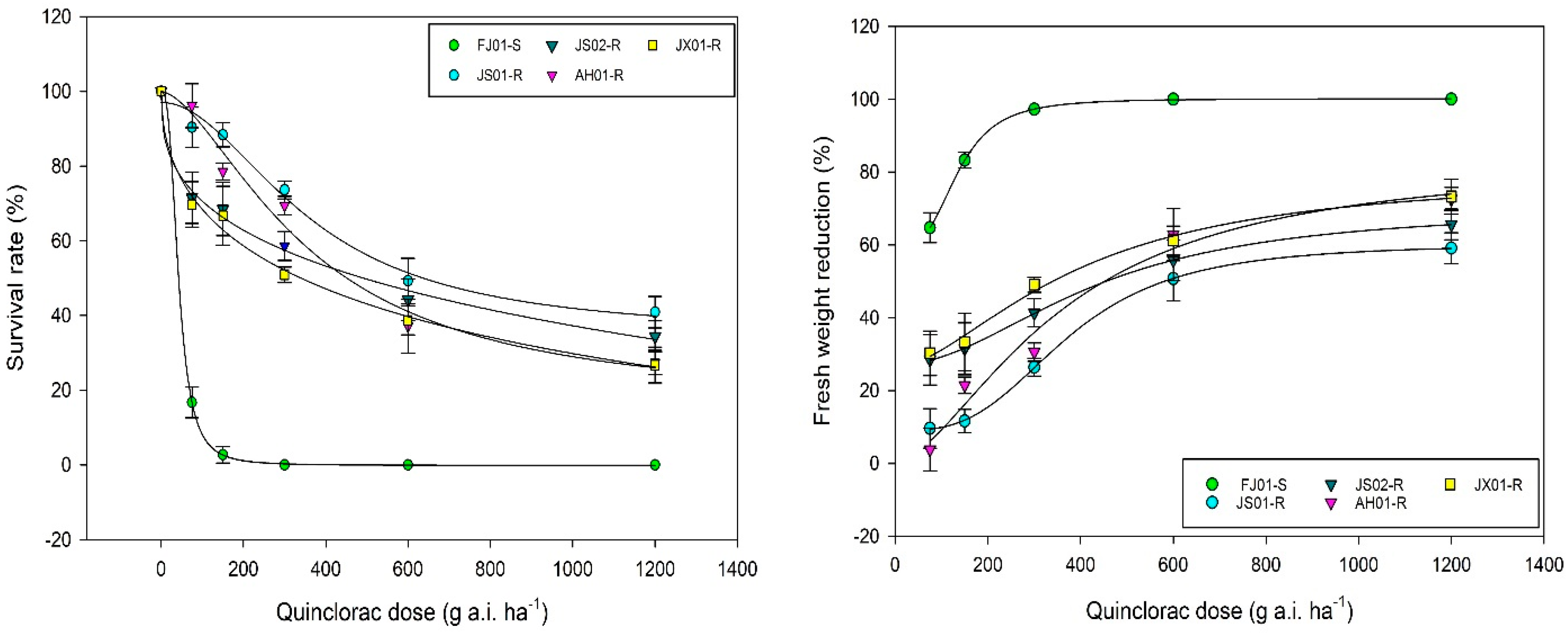
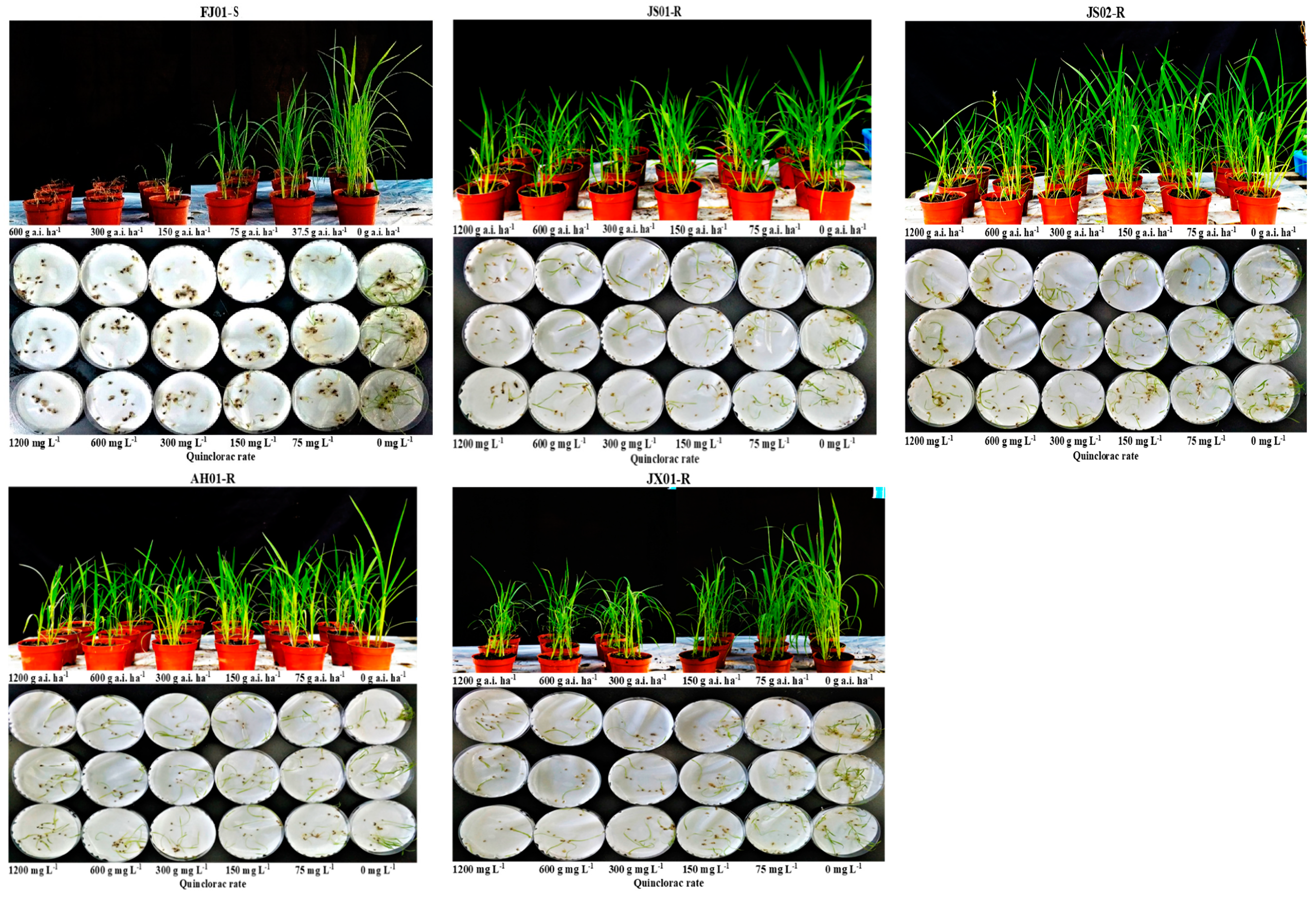
2.1. Dose–Response Analysis
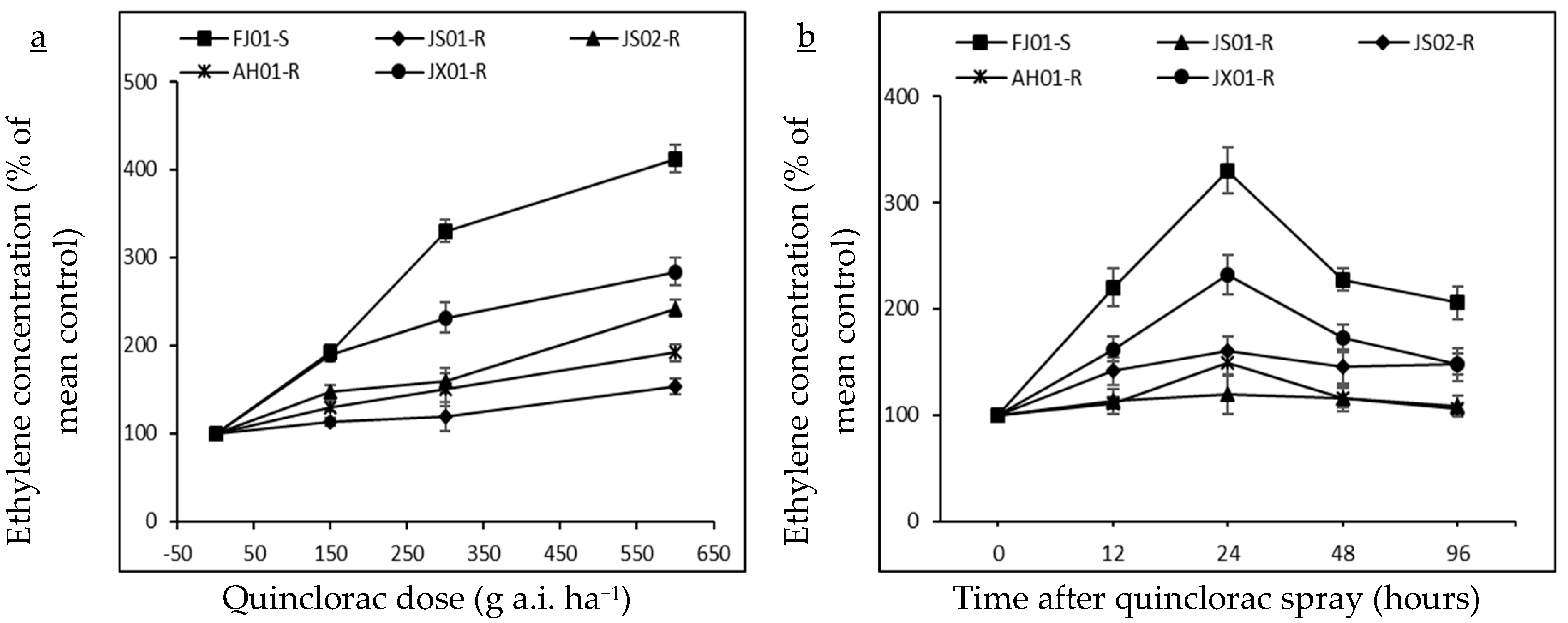
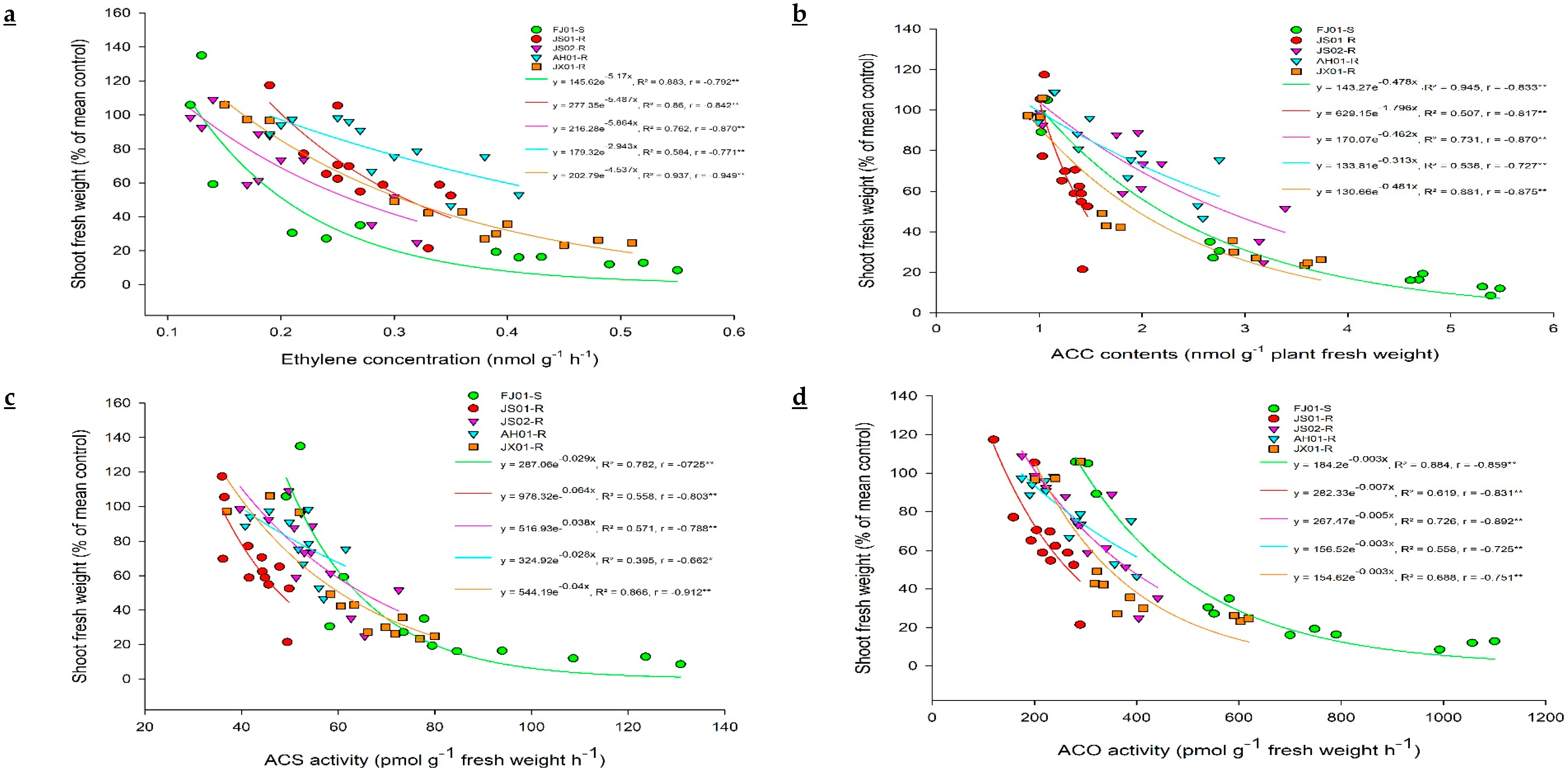
2.2. Effect on Ethylene Biosynthesis
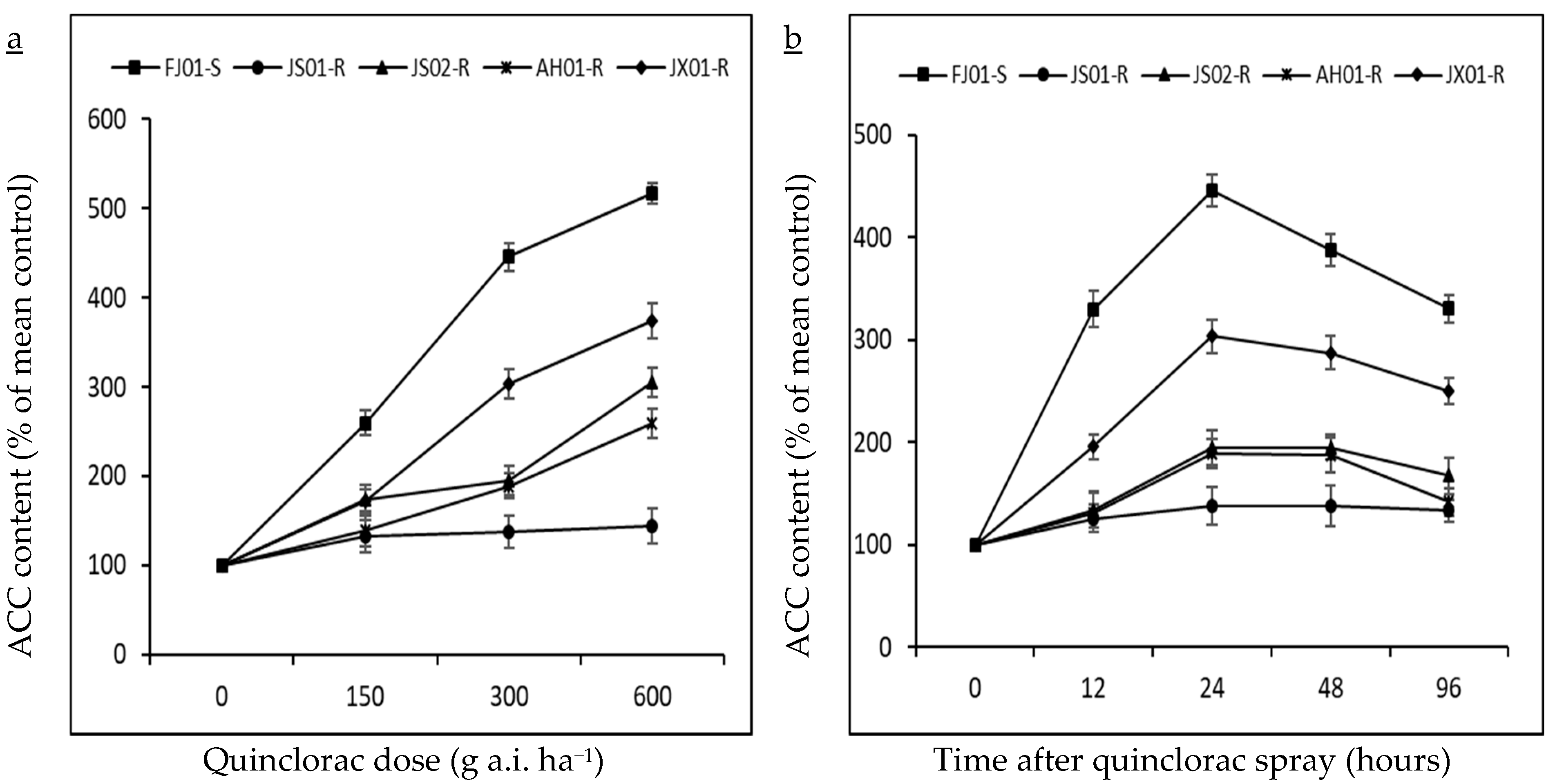
2.3. Effect on ACC (Precursor for Ethylene Biosynthesis)
2.4. ACS and ACO Enzymes Activity
2.4.1. Effect on ACS Activity
2.4.2. Effect on ACO Activity
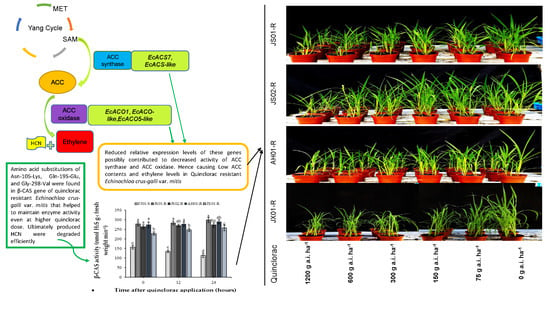
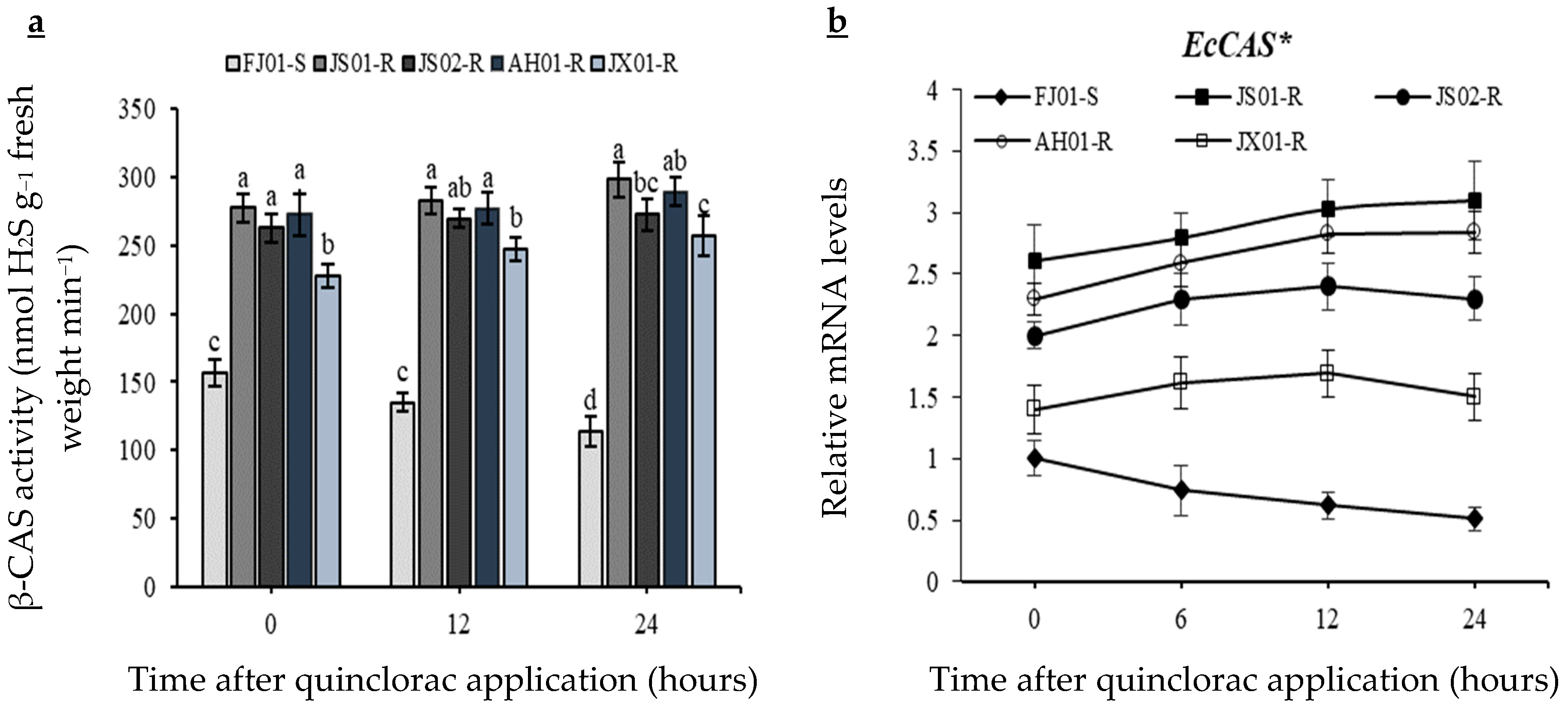
2.5. Effect on β-CAS Activity
2.6. EcCAS Gene Sequence Description, Comparison, and Mutation Identification
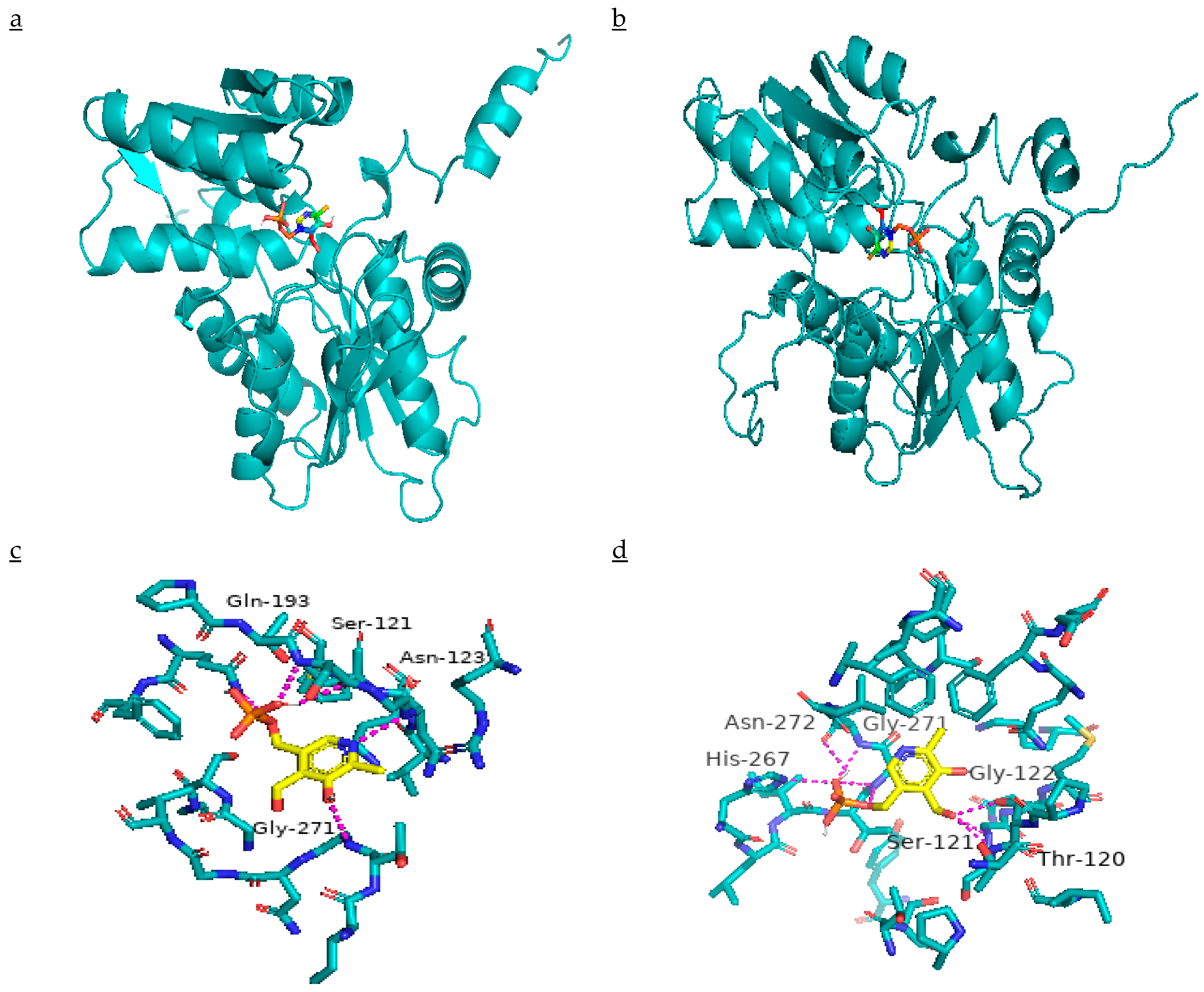
2.7. Effect of Mutation on β-CAS Structure and its Docking with PLP
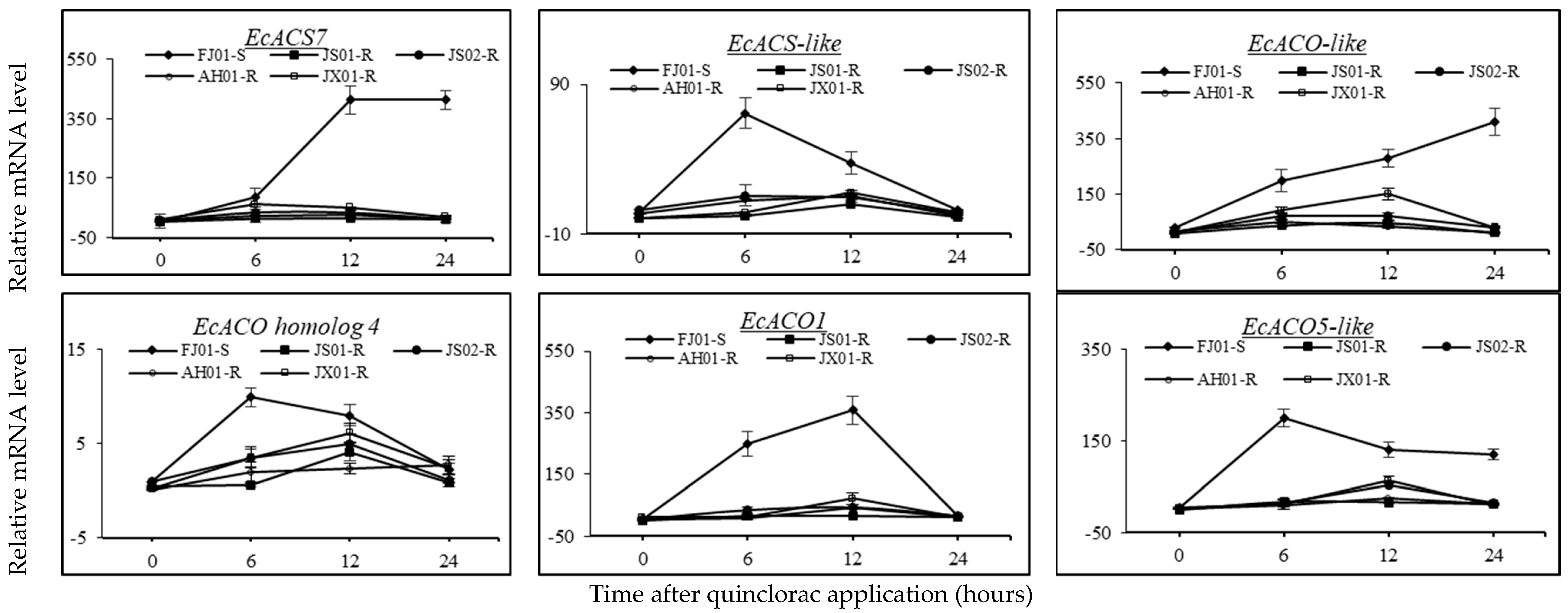
2.8. Effect on the Expression Pattern of ACS and ACO Genes
2.9. Effect on Transcript Level of EcCAS Gene
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical
4.3. Quinclorac Resistance
4.3.1. Petri Plate/Seed Bioassay
4.3.2. Resistance Confirmation in Pot Assay
4.4. Ethylene Production by R and S Biotypes
4.5. Effect on Endogenous ACC Levels
4.6. Effect on ACS Activity
4.7. Effect on ACO Activity
4.8. Cyanide Detoxification by β-CAS
4.9. EcCAS Gene Sequence
4.10. Computational Study of β-CAS Binding with PLP and Its Structure Stability
4.11. Real-Time PCR Based Expression Analysis of ACS, ACO, and CAS Genes
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ACS | 1-aminocyclopropane-1-carboxylic acid synthase |
| ACO | 1-aminocyclopropane-1-carboxylic acid oxidase |
| β-CAS | β-cyanoalanine synthase |
| EcCAS | Echinochloa crus-galli var. mitis β-cyanoalanine synthase |
| Asn | Asparagine |
| Lys | Lysine |
| Gln | Glutamine |
| Glu | Glutamic acid |
| Gly | Glycine |
| Val | Valine |
| TSR | Target-site resistance |
| NTSR | Non-target-site resistance |
| Aux/IAA | Auxin/Indole-3-Acetic Acid |
| HCN | Hydrogen cyanide |
| PLP | Pyridoxal phosphate |
| ALS | Acetolactate synthase |
| EC50 | Effective dose responsible for 50 % inhibitory response |
| GR50 | Effective dose responsible for 50% inhibition in growth |
| RI | Resistance Index |
| HAT | Hours after treatment |
| ORF | Open Reading Frame |
| Ile | Isoleucine |
| Thr | Threonine |
| Asp | Aspartic acid |
| Phe | Phenylalanine |
| Leu | Leucine |
| SDM | Site-Directed Mutator |
| ΔΔG | Gibbs free energy |
| SAM | S-Adenosyl-L-methionine |
| CN− | cyanides |
| SNP | Single nucleotide polymorphism |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| DDT | Dithiothreitol |
| h | hours |
| S | Susceptible |
| R | Resistant |
References
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: Waltham, MA, USA, 2005; p. 952. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–43. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, J.M.; Stinchcombe, J.R.; Wright, S.I. Population genomics of herbicide resistance: Adaptation via evolutionary rescue. Annu. Rev. Plant Biol. 2018, 69, 611–635. [Google Scholar] [CrossRef]
- Heap, I.M. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.com (accessed on 21 October 2019).
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Koo, D.H.; Molin, W.T.; Saski, C.A.; Jiang, J.; Putta, K.; Jugulam, M.; Friebe, B.; Gill, B.S. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri. Proc. Natl. Acad. Sci. USA 2018, 115, 3332–3337. [Google Scholar] [CrossRef]
- Patzoldt, W.L.; Hager, A.G.; McCormick, J.S.; Tranel, P.J. A codon deletion confers resistance to herbicides inhibiting protoporphyrinogen oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 12329–12334. [Google Scholar] [CrossRef]
- Yu, Q.; Jalaludin, A.; Han, H.; Chen, M.; Sammons, R.D.; Powles, S.B. Evolution of a double amino acid substitution in the 5-enolpyruvylshikimate- 3-phosphate synthase in Eleusine indica conferring high-level glyphosate resistance. Plant Physiol. 2015, 167, 1440–1447. [Google Scholar] [CrossRef]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest. Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef]
- Yuan, J.S.; Tranel, P.J.; Stewart, C.N.J. Non-target-site herbicide resistance: A family business. Trends Plant Sci. 2007, 12, 6–13. [Google Scholar] [CrossRef]
- Cummins, I.; Cole, D.J.; Edwards, R. A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 1999, 18, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cummins, I.; Wortley, D.J.; Sabbadin, F.; He, Z.; Coxon, C.R.; Straker, H.E.; Sellars, J.D.; Knight, K.; Edwards, L.; Hughes, D.; et al. Key role for a glutathione transferase in multiple-herbicide resistance in grass weeds. Proc. Natl. Acad. Sci. USA 2013, 110, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Endo, M.; Saika, H.; Okuno, J.; Nakamura, N.; Yokoyama, M.; Watanabe, H.; Toki, S.; Uchino, A.; Inamura, T. Cytochrome P450 CYP81A12 and CYP81A21 are associated with resistance to two acetolactate synthase inhibitors in Echinochloa phyllopogon. Plant Physiol. 2014, 165, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop. Protect. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Qiao, L.Y.; Wei, J.G.; Dong, L.Y.; Li, Y.H. A study on tolerance to pretilachlor in eight species of Echinochloa. Rice Sci. 2004, 11, 331–335. [Google Scholar]
- Xu, J.Y.; Lv, B.; Wang, G.; Li, J.; Dong, L.Y. A resistance mechanism dependent upon the inhibition of ethylene biosynthesis. Pest. Manag. Sci. 2013, 69, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Pan, X.; Liu, D.; Napier, R.; Dong, L. Quinclorac resistance induced by the suppression of the expression of 1- aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase genes in Echinochloa crus-galli var. zelayensis. Pestic. Biochem. Physiol. 2018, 146, 25–32. [Google Scholar] [CrossRef]
- Heap, I.M. International Survey of Herbicide-Resistant Weeds: Lessons and Limitations. In Proceedings of the Brighton Crop Protection Conference, Brighton, UK, 15–18 November 1999; pp. 769–776. [Google Scholar]
- He, H.B.; Wang, H.B.; Fang, C.X.; Lin, Z.H.; Yu, Z.M.; Lin, W.X. Separation of allelopathy from resource competition using rice/barnyardgrass mixed-cultures. PLoS ONE 2012, 7, e37201. [Google Scholar] [CrossRef]
- Grossmann, K. Quinclorac belongs to a new class of highly selective auxin herbicides. Weed Sci. 1998, 46, 707–716. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest. Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Westra, P.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Valverde, B.E.; Itoh, K. World rice and herbicide resistance. In Herbicide Resistance in World Grains; Powles, S.R., Shaner, D., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 145–249. [Google Scholar]
- Valverde, B.E. Status and management of grass–weed herbicide resistance in Latin America. Weed Technol. 2007, 21, 310–323. [Google Scholar] [CrossRef]
- Lopez-Martinez, N.; Marshall, G.; DePrado, R. Resistance of barnyardgrass to atrazine and quinclorac. Pestic. Sci. 1997, 51, 171–175. [Google Scholar] [CrossRef]
- Malik, M.S.; Burgos, N.R.; Talbert, R.E. Confirmation and control of propanil-resistant and quinclorac-resistant barnyardgrass (Echinochloa crus-galli) in rice. Weed Technol. 2010, 24, 226–233. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sahid, I.B.; Juraimi, A.Z. Study on resistant biotypes of Echinochloa crus-galli in Malaysia. Aust. J. Crop. Sci. 2010, 4, 107–115. [Google Scholar]
- Yasuor, H.; Milan, M.; Eckert, J.W.; Fischer, A.J. Quinclorac resistance: A concerted hormonal and enzymatic effort in Echinochloa phyllopogon. Pest. Manag. Sci. 2012, 68, 108–115. [Google Scholar] [CrossRef]
- Gomez de Barreda, D.; Carretero, J.L.; del Busto, A.; Asins, M.J.; Carbonell, E.A.; Lorenzo, E. Response of Echinochloa spp. (barnyardgrass) populations to quinclorac. In Proceedings of the International Symposium on Weed and Crop Resistance to Herbicides, Cordoba, Spain, 3–6 April 1995; pp. 157–158. [Google Scholar]
- Kepinski, S.; Leyser, O. The Arabidopsis TIR1 protein is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef]
- Ikegaya, H.; Iwase, H.; Hatanaka, K.; Sakurada, K.; Yoshida, K.; Takatori, T. Diagnosis of cyanide intoxication by measurement of cytochrome c oxidase activity. Toxicol. Lett. 2001, 119, 117–123. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Rev. Cell Dev. Biol. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Lai, K.W.; Yau, C.P.; Tse, Y.C.; Jiang, L.W.; Yip, Y.K. Heterologous expression analyses of rice OsCAS in Arabidopsis and in yeast provide evidence for its roles in cyanide detoxification rather than in cysteine synthesis in vivo. J. Exp. Bot. 2009, 60, 993–1008. [Google Scholar] [CrossRef]
- Grossmann, K.; Kwiatkowski, J. The mechanism of quinclorac selectivity in grasses. Pestic. Biochem. Physiol. 2000, 66, 83–91. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, L.; Sun, Y.; Zhang, T.; Dong, L.; Li, J. Resistance to quinclorac caused by the enhanced ability to detoxify cyanide and its molecular mechanism in Echinochloa crus-galli var. zelayensis. Pestic. Biochem. Physiol. 2017, 143, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chayapakdee, P.; Sunohara, Y.; Endo, M.; Yamaguchi, T.; Fan, L.; Uchino, A.; Matsumotoa, H.; Iwakamie, S. Quinclorac resistance in Echinochloa phyllopogon is associated with reduced ethylene synthesis rather than enhanced cyanide detoxification by β-cyanoalanine synthase. Pest. Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mdodana, N.T.; Jewell, J.F.; Phiri, E.E.; Smith, M.L.; Oberlander, K.; Mahmoodi, S.; Kossmann, J.; Lloyd, J.R. Mutations in glucan, water dikinase afect starch degradation and gametophore development in the moss Physcomitrella patens. Sci. Rep. 2019, 9, 15114. [Google Scholar] [CrossRef] [PubMed]
- Van Eerd, L.L.; Stephenson, G.R.; Kwiatkowski, J.; Grossmann, K.; Hall, J.C. Physiological and biochemical characterization of quinclorac resistance in a false cleaver (Galium spurium L.) biotype. J. Agric. Food Chem. 2005, 53, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.L.; Talbert, R.E.; Hoagland, R.E.; Scherder, E.F. Quinclorac absorption and translocation characteristics in quinclorac- and propanil-resistant and-susceptible barnyardgrass (Echinochloa crusgalli) biotypes. Weed Technol. 2007, 21, 683–687. [Google Scholar] [CrossRef]
- Tresch, S.; Grossmann, K. Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots. Pestic. Biochem. Physiol. 2003, 75, 73–78. [Google Scholar] [CrossRef]
- Bonow, J.F.L.; Lamego, F.P.; Andres, A.; Avila, L.A.; Teló, G.M.; Egewarth, K. Resistance of Echinochloa crusgalli var. mitis to imazapyr+imazapic herbicide an alternative control in irrigated rice. Planta Daninha 2018, 36, e018168627. [Google Scholar] [CrossRef]
- Grossmann, K.; Retzlaff, G. Bioregulatory effects of the fungicidal strobilurin kresoxim-methyl in wheat (Triticum aestivum L.). Pestic. Sci. 1997, 50, 11–20. [Google Scholar] [CrossRef]
- Vasilakoglou, I.B.; Eleftherohorinos, I.G.; Dhima, K.V. Propanil-resistant barnyardgrass (Echinochloa crus-galli) biotypes found in Greece. Weed Technol. 2000, 14, 524–529. [Google Scholar] [CrossRef]
- Grossmann, K.; Kwiatkowski, J. Evidence for a causative role of cyanide, derived from ethylene biosynthesis, in the herbicidal mode of action of quinclorac in barnyard grass. Pestic. Biochem. Physiol. 1995, 51, 150–160. [Google Scholar] [CrossRef]
- Abdallah, I.; Fischer, A.J.; Elmore, C.L.; Saltveit, M.E.; Zaki, M. Mechanism of resistance to quinclorac in smooth crabgrass (Digitaria ischaemum). Pestic. Biochem. Physiol. 2006, 84, 38–48. [Google Scholar] [CrossRef]
- Chutichude, P.; Chutichude, B.; Boontiang, K. Influence of 1-MCP fumigation on flowering weight loss, water uptake, longevity, anthocyanin content and colour of patumma (Curcuma alismatifolia) cv. Chiang Mai Pink. Int. J. Agric. Res. 2011, 6, 29–39. [Google Scholar] [CrossRef]
- Grossmann, K. A role for cyanide, derived from ethylene biosynthesis, in the development of stress symptoms. Physiol. Plant 1996, 97, 772–775. [Google Scholar] [CrossRef]
- Grossmann, K.; Scheltrup, F. Selective induction of 1- Aminocyclopropane-1-carboxylic acid (ACC) synthase activity is involved in the selectivity of the auxin herbicide quinclorac between barnyardgrass and rice. Pestic. Biochem. Physiol. 1997, 58, 145–153. [Google Scholar] [CrossRef]
- Grossmann, K. The mode of action of quinclorac: A case study of a new auxin-type herbicide. In Herbicides and their Mechanism of Action; Cobb, A.H., Kirkwood, R.C., Eds.; Sheffield Academic Press: Sheffield, UK, 2000; pp. 181–214. [Google Scholar]
- Yamagami, T.; Tsuchisaka, A.; Yamada, K.; Haddon, W.F.; Harden, L.A.; Theologis, A. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J. Biol. Chem. 2003, 278, 49102–49112. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, S.; Uchino, A.; Kataoka, Y.; Shibaike, H.; Watanabe, H.; Inamura, T. Cytochrome P450 genes induced by bispyribac- sodium treatment in a multiple herbicide- resistant biotype of Echinochloa phyllopogon. Pest. Manag. Sci. 2014, 70, 549–558. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007, 19, 2169–2185. [Google Scholar] [CrossRef]
- Yi, H.; Juergens, M.; Jez, J.M. Structure of soybean β-cyanoalanine synthase and the molecular basis for cyanide detoxification in plants. Plant Cell 2012, 24, 2696–2706. [Google Scholar] [CrossRef]
- Cargill, M.; Altshuler, D.; Ireland, J.; Sklar, P.; Ardlie, K.; Patil, N.; Lane, C.R.; Lim, E.P.; Kalyanaraman, N.; Nemesh, J.; et al. Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nat. Genet. 1999, 22, 231–238. [Google Scholar] [CrossRef]
- Zhang, X.; Myers, A.M.; James, M.G. Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol. 2005, 138, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Faure, F.; Kieber, J.J. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 2003, 15, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; de Paz, A.; Zamft, B.M.; Marlbestone, A.H.; Boyden, E.S.; Kording, K.P.; Tyo, K.E.J. DNA binding strength increases the processivity and activity of a Y-Family DNA polymerase. Sci. Rep. 2017, 7, 4756. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Tawfik, D.S. Stability effects of mutations and protein evolvability. Curr. Opin. Struct. Biol. 2009, 19, 596–604. [Google Scholar] [CrossRef]
- Lizada, M.C.C.; Yang, S.F. A simple and sensitive assay for 1- aminocyclopropane-1-carboxylic acid. Anal. Biochem. 1979, 100, 140–145. [Google Scholar]
- Bulens, I.; Van de Poel, B.; Hertog, M.L.A.T.M.; De Proft, M.P.; Geeraerd, A.H.; Nicolaï, B.M. Protocol: An updated integrated methodology for analysis of metabolites and enzyme activities of ethylene biosynthesis. Plant Methods 2011, 7, 17–26. [Google Scholar] [CrossRef]
- Chon, N.M.; Nishikawa-Koseki, N.; Takeuchi, Y.; Abe, H. Role of ethylene in abnormal shoot growth induced by high concentration of brassinolide in rice seedlings. J. Pestic. Sci. 2008, 33, 67–72. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kelly, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Pandurangan, A.P.; Ochoa-Montaño, B.; Ascher, D.B.; Blundell, T.L. SDM: A server for predicting effects of mutations on protein stability. Nucleic Acids Res. 2017, 45, W229–W235. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Populations | Dose–Response Analysis | |||||
|---|---|---|---|---|---|---|
| EC50 (mg L−1) | r2 | RI (EC50 Ratio) | GR50 (g a.i. ha−1) | r2 | RI (GR50 Ratio) | |
| FJ01-S | 118.69 (7.82) | 0.98 | 1 | 26.83 (1.02) | 0.999 | 1 |
| JS01-R | 4116.36 (76.65) | 0.99 | 35 | 733.73 (26.96) | 0.999 | 27 |
| JS02-R | 2604.65 (35.61) | 0.99 | 22 | 447.1 (14.73) | 0.999 | 17 |
| AH01-R | 2953.82 (37.78) | 0.96 | 25 | 484.22 (19.42) | 0.98 | 18 |
| JX01-R | 1605.25 (12.56) | 0.98 | 14 | 313.82 (11.08) | 0.99 | 12 |
| Dose (g a.i. ha−1) | Populations | ||||
|---|---|---|---|---|---|
| FJ01-S | JS01-R | JS02-R | AH01-R | JX01-R | |
| 0 | 54.51 ± 2.55 cA | 40.65 ± 2.99 aB | 45.33 ± 2.10 cAB | 42.74 ± 1.06 bB | 44.73 ± 3.10 cAB |
| 150 | 69.81 ± 4.19 cA | 44.69 ± 3.14 aC | 51.71 ± 1.03 bcBC | 49.72 ± 2.86 abBC | 58.28 ± 2.66 bAB |
| 300 | 86.20 ± 3.01 bA | 46.10 ± 2.44 aD | 58.37 ± 3.34 abC | 52.83 ± 1.26 aCD | 69.54 ± 1.48 aB |
| 600 | 120.69 ± 4.64 aA | 49.13 ± 1.57 aD | 66.42 ± 2.13 aBC | 56.26 ± 2.25 aCD | 76.43 ± 1.70 aB |
| Dose (g a.i. ha−1) | Populations | ||||
|---|---|---|---|---|---|
| FJ01-S | JS01-R | JS02-R | AH01-R | JX01-R | |
| 0 | 303.93 ± 8.58 dA | 158.72 ± 16.33 cC | 222.29 ± 24.77 bBC | 192.76 ± 7.33 cBC | 240.43 ± 18.53 dAB |
| 150 | 538.21 ± 21.05 cA | 204.82 ± 8.95 bC | 303.31 ± 18.52 abB | 222.87 ± 5.51 bcC | 322.69 ± 4.18 cB |
| 300 | 749.73 ± 18.69 bA | 230.83 ± 5.45 bD | 340.32 ± 37.29 aBC | 267.16 ± 12.92 bCD | 412.75 ± 27.77 bB |
| 600 | 1055.19 ± 22.68 aA | 276.18 ± 5.09 aD | 403.30 ± 13.61 aC | 369.14 ± 15.60 aC | 603.23 ± 5.89 aB |
| Gene | Forward Primer (5′-3′) | Reserve Primer (5′-3′) | Annealing Temperature (°C) | Length of Product |
|---|---|---|---|---|
| EcActin | TACTCCTTCACCACAACCGC | TGATGACCTGTCCATCAGGC | 60 | 154 |
| EcACS7 | TCGCGAGGATGAGCAGATTC | ACCCAAGGTAGTATTATTTACCCTC | 60 | 124 |
| EcACS-like | GATGCTGTCGGACCACGAG | GTCCATCCACGAGAAGAGCC | 60 | 148 |
| EcACO homolog 4 | CATCTTCCCGCACACGGAC | GATGGAAACCCTTGGCTCGT | 60 | 250 |
| EcACO1 | AGTCCCAGGTTTGGAGTTTCTG | ATTATGGCGTCAGCACCAGG | 60 | 207 |
| EcACO-like | CCGGAGTTCAAGGAGACCAT | TGACCTTGGTGCCGAAGAAG | 60 | 163 |
| EcACO5-like | GCACATGGCGGTGAACTACT | CACGCTCTTGTAGCGGTCAT | 60 | 229 |
| EcCAS | TGCCGTCATACACCAGTCTT | CCAATCCCCATCACARAAA | 56 | 253 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zia Ul Haq, M.; Zhang, Z.; Wei, J.; Qiang, S. Ethylene Biosynthesis Inhibition Combined with Cyanide Degradation Confer Resistance to Quinclorac in Echinochloa crus-galli var. mitis. Int. J. Mol. Sci. 2020, 21, 1573. https://doi.org/10.3390/ijms21051573
Zia Ul Haq M, Zhang Z, Wei J, Qiang S. Ethylene Biosynthesis Inhibition Combined with Cyanide Degradation Confer Resistance to Quinclorac in Echinochloa crus-galli var. mitis. International Journal of Molecular Sciences. 2020; 21(5):1573. https://doi.org/10.3390/ijms21051573
Chicago/Turabian StyleZia Ul Haq, Muhammad, Zheng Zhang, Jiajia Wei, and Sheng Qiang. 2020. "Ethylene Biosynthesis Inhibition Combined with Cyanide Degradation Confer Resistance to Quinclorac in Echinochloa crus-galli var. mitis" International Journal of Molecular Sciences 21, no. 5: 1573. https://doi.org/10.3390/ijms21051573
APA StyleZia Ul Haq, M., Zhang, Z., Wei, J., & Qiang, S. (2020). Ethylene Biosynthesis Inhibition Combined with Cyanide Degradation Confer Resistance to Quinclorac in Echinochloa crus-galli var. mitis. International Journal of Molecular Sciences, 21(5), 1573. https://doi.org/10.3390/ijms21051573