Biomarkers of Liver Injury during Transplantation in an Era of Machine Perfusion
Abstract
:1. Introduction
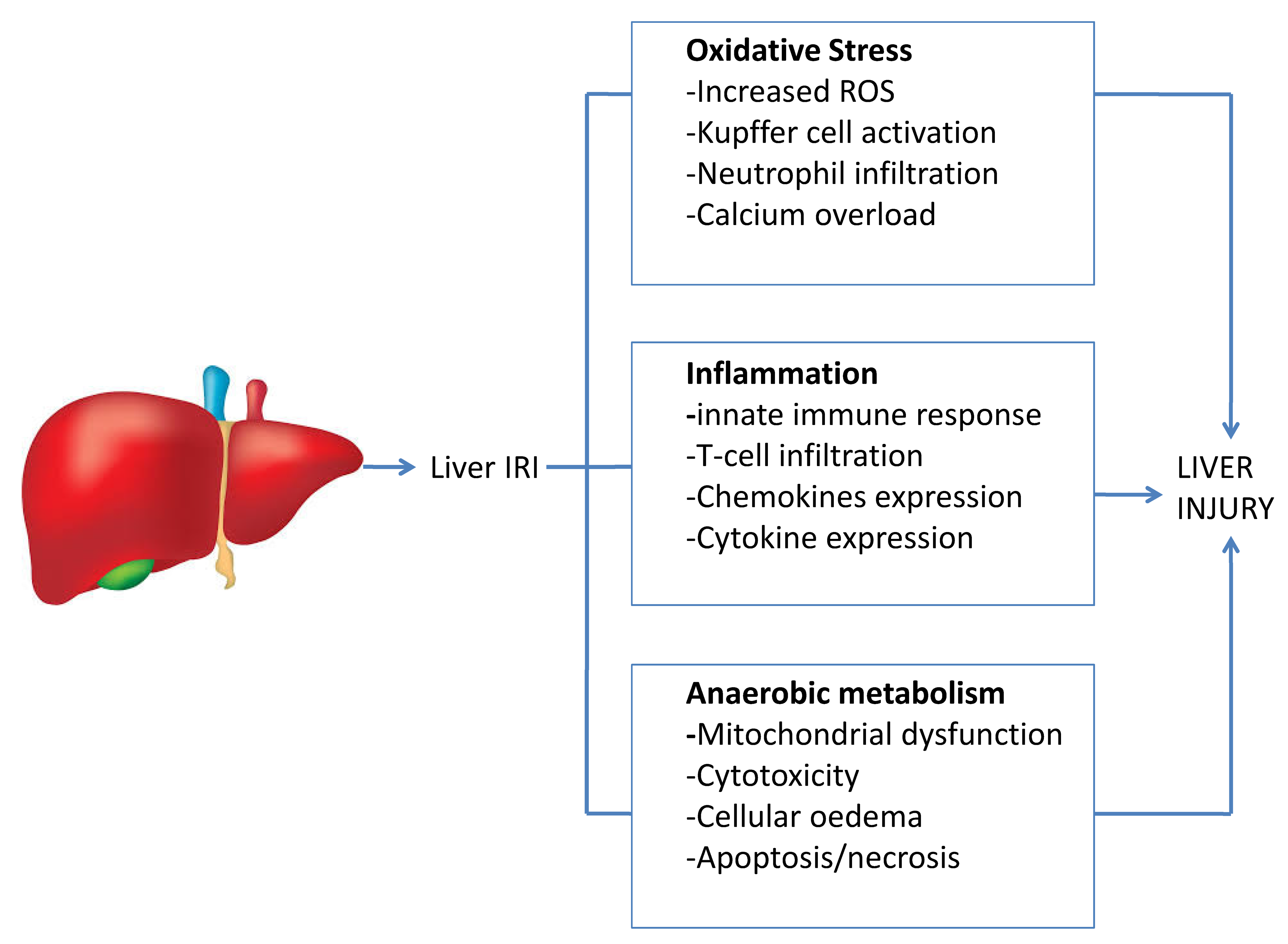
2. The Mechanism of the Liver Ischaemia–Reperfusion Injury
3. Overview of Different Techniques of Liver Machine Perfusion
4. Biomarkers of Liver Ischaemia–Reperfusion Injury
5. MicroRNAs
6. Interleukin 17
7. Interleukin 33 and Cyclin D1
8. Fibroblast Growth Factor 21
9. Endothelin-1
10. Biomarkers of Ischaemia–Reperfusion Injury during Machine Perfusion
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LT | liver transplantation |
| DCD | donation after cardiac death |
| MP | machine perfusion |
| IRI | ischaemia–reperfusion injury |
| LSEC | liver sinusoidal endothelial cells |
| ROS | reactive oxygen species |
| EAD | early allograft dysfunction |
| PNF | primary graft non-function |
| IC | ischaemic cholangiopathy |
| NMP | normothermic machine perfusion |
| HMP | hypothermic machine perfusion |
| TNF-α | tumour necrosis factor alpha |
References
- Barshes, N.R.; Horwitz, I.B.; Franzini, L.; Vierling, J.M.; Goss, J.A. Waitlist Mortality Decreases with Increased Use of Extended Criteria Donor Liver Grafts at Adult Liver Transplant Centers. Am. J. Transpl. 2007, 7, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, P.; Mangus, R.; Ekser, B.; Cabrales, A.; Timsina, L.; Fridell, J.; Lacerda, M.; Ghabril, M.; Nephew, L.; Chalasani, N.; et al. Expanding the Donor Pool with the Use of Extended Criteria Donation after Circulatory Death Livers. Liver Transpl. 2019, 25, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Nozdrin, M.; Yiu, J.; Papalois, V. Machine Perfusion for Abdominal Organ Preservation: A Systematic Review of Kidney and Liver Human Grafts. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mergental, H.; Perera, M.T.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.T.; Cilliers, H.; Neil, D.A.; Hubscher, S.G.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transpl. 2016, 16, 3235–3245. [Google Scholar] [CrossRef] [Green Version]
- Desai, C.S.; Gerber, D.A. Concise Review of Machine Perfusion in Liver Transplantation. World J. Hepatol. 2020, 12, 6–9. [Google Scholar] [CrossRef]
- O’neill, S.; Srinivasa, S.; Callaghan, C.J.; Watson, C.J.; Dark, J.H.; Fisher, A.J.; Wilson, C.H.; Friend, P.J.; Johnson, R.; Forsythe, J.L.; et al. Novel Organ Perfusion and Preservation Strategies in Transplantation—Where Are We Going in the UK? Transplantation 2020. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Afford, S.C. Machine Perfusion of the Liver: Which Is the Best Technique to Mitigate Ischaemia-Reperfusion Injury? World J. Transpl. 2019, 9, 14–20. [Google Scholar] [CrossRef]
- Toledo-Pereya, L.H.; Whitten, J.; Baskin, S.; Mcnichol, L.; Thavarajah, K.; Lin, W. Treatment of Multiple Rejection Episodes with Alg or Atgam after Cadaveric Renal Transplantation. Bol. Asoc. Med. P. R. 1984, 76, 293–296. [Google Scholar]
- Jennings, R.B.; Sommers, H.M.; Smyth, G.A.; Flack, H.A.; Linn, H. Myocardial Necrosis Induced by Temporary Occlusion of a Coronary Artery in the Dog. Arch. Pathol. 1960, 70, 68–78. [Google Scholar]
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia Reperfusion Injury in Liver Transplantation: Cellular and Molecular Mechanisms. Liver Int. 2019, 39, 788–801. [Google Scholar] [CrossRef] [Green Version]
- Li, D.Y.; Shi, X.J.; Li, W.; Du, X.H.; Wang, G.Y. Key Points in Establishing a Model of Mouse Liver Transplantation. Transpl. Proc. 2015, 47, 2683–2689. [Google Scholar] [CrossRef]
- Konishi, T.; Lentsch, A.B. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017, 17, 277–287. [Google Scholar] [CrossRef]
- Prieto, I.; Monsalve, M. Ros Homeostasis, a Key Determinant in Liver Ischemic-Preconditioning. Redox Biol. 2017, 12, 1020–1025. [Google Scholar] [CrossRef] [Green Version]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef]
- Oliveira, T.H.C.; Marques, P.E.; Proost, P.; Teixeira, M.M.M. Neutrophils: A Cornerstone of Liver Ischemia and Reperfusion Injury. Lab. Investig. 2018, 98, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Guo, J.; Gu, J.; Chen, K.; Li, H.; Wang, J. Protective Role of Mtor in Liver Ischemia/Reperfusion Injury: Involvement of Inflammation and Autophagy. Oxid. Med. Cell Longev. 2019, 2019, 7861290. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Song, H.L. [Toll-Like Receptor 4 in Liver Ischemia-Reperfusion Injury]. Zhonghua Gan Zang Bing Za Zhi 2019, 27, 473–476. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, L.; Zhou, Y.; Sheng, J.; Long, D.; Li, S.; Li, Y. Systematic Review with Meta-Analysis: Hif-1alpha Attenuates Liver Ischemia-Reperfusion Injury. Transpl. Rev. 2015, 29, 127–134. [Google Scholar] [CrossRef]
- Schlegel, A.; De Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective Mechanisms of End-Ischemic Cold Machine Perfusion in Dcd Liver Grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; Garcia-Valdecasas, J.C.; Heaton, N.; et al. A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Angelico, R.; Perera, M.T.; Ravikumar, R.; Holroyd, D.; Coussios, C.; Mergental, H.; Isaac, J.R.; Iqbal, A.; Cilliers, H.; Muiesan, P.; et al. Normothermic Machine Perfusion of Deceased Donor Liver Grafts Is Associated with Improved Postreperfusion Hemodynamics. Transplant. Direct 2016, 2, E97. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of Dcd Liver Transplantation Using Organs Treated by Hypothermic Oxygenated Perfusion before Implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- Cannistra, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic Ischemia Reperfusion Injury: A Systematic Review of Literature and the Role of Current Drugs and Biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef]
- Wyllie, S.; Seu, P.; Gao, F.Q.; Gros, P.; Goss, J.A. Disruption of the Nramp1 (Also Known As Slc11a1) Gene in Kupffer Cells Attenuates Early-Phase, Warm Ischemia-Reperfusion Injury in the Mouse Liver. J. Leukoc. Biol. 2002, 72, 885–897. [Google Scholar]
- Shen, X.; Wang, Y.; Gao, F.; Ren, F.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Zhai, Y. Cd4 T Cells Promote Tissue Inflammation via Cd40 Signaling without De Novo Activation in a Murine Model of Liver Ischemia/Reperfusion Injury. Hepatology 2009, 50, 1537–1546. [Google Scholar] [CrossRef] [Green Version]
- Eggenhofer, E.; Sabet-Rashedi, M.; Lantow, M.; Renner, P.; Rovira, J.; Koehl, G.E.; Schlitt, H.J.; Geissler, E.K.; Kroemer, A. Rorgammat(+) Il-22-Producing Nkp46(+) Cells Protect from Hepatic Ischemia Reperfusion Injury in Mice. J. Hepatol. 2016, 64, 128–134. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Wang, Z.; Yang, J. Microrna-155 Deficiency in Kupffer Cells Ameliorates Liver Ischemia-Reperfusion Injury in Mice. Transplantation 2017, 101, 1600–1608. [Google Scholar] [CrossRef]
- Ito, T.; Kuriyama, N.; Kato, H.; Matsuda, A.; Mizuno, S.; Usui, M.; Sakurai, H.; Isaji, S. Sinusoidal Protection by Sphingosine-1-Phosphate Receptor 1 Agonist in Liver Ischemia-Reperfusion Injury. J. Surg. Res. 2018, 222, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, J.; Ochiya, T. Extracellular Micrornas and Oxidative Stress in Liver Injury: A Systematic Mini Review. J. Clin. Biochem. Nutr. 2018, 63, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific Micrornas from Mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Andersson, P.; Gidlof, O.; Braun, O.O.; Gotberg, M.; Van Der Pals, J.; Olde, B.; Erlinge, D. Plasma Levels of Liver-Specific Mir-122 Is Massively Increased in a Porcine Cardiogenic Shock Model and Attenuated by Hypothermia. Shock 2012, 37, 234–238. [Google Scholar] [CrossRef]
- Roderburg, C.; Benz, F.; Vargas Cardenas, D.; Koch, A.; Janssen, J.; Vucur, M.; Gautheron, J.; Schneider, A.T.; Koppe, C.; Kreggenwinkel, K.; et al. Elevated Mir-122 Serum Levels Are An Independent Marker of Liver Injury in Inflammatory Diseases. Liver Int. 2015, 35, 1172–1184. [Google Scholar] [CrossRef]
- Yang, M.; Antoine, D.J.; Weemhoff, J.L.; Jenkins, R.E.; Farhood, A.; Park, B.K.; Jaeschke, H. Biomarkers Distinguish Apoptotic and Necrotic Cell Death during Hepatic Ischemia/Reperfusion Injury in Mice. Liver Transpl. 2014, 20, 1372–1382. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Q.; Zhang, W.; Chen, M.; Ju, W.; Wu, L.; Han, M.; Ma, Y.; Zhu, X.; Wang, D.; et al. Microrna-146b-5p Identified in Porcine Liver Donation Model Is Associated with Early Allograft Dysfunction in Human Liver Transplantation. Med. Sci. Monit. 2017, 23, 5876–5884. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Gao, Y.; Qin, J.; Lu, S. The Role Of Mir-34a in the Hepatoprotective Effect of Hydrogen Sulfide on Ischemia/Reperfusion Injury in Young and Old Rats. PLoS ONE 2014, 9, E113305. [Google Scholar] [CrossRef]
- Schueller, F.; Roy, S.; Loosen, S.H.; Alder, J.; Koppe, C.; Schneider, A.T.; Wandrer, F.; Bantel, H.; Vucur, M.; Mi, Q.S.; et al. Mir-223 Represents a Biomarker in Acute and Chronic Liver Injury. Clin. Sci. 2017, 131, 1971–1987. [Google Scholar] [CrossRef] [Green Version]
- Li, S.P.; Wang, F.F.; Zhang, W.K.; Bian, M.Z.; Zhang, S.Y.; Yan, H.; Fang, Y.; Zhang, H.M. Characteristics of Changes in Inflammatory Cytokines as a Function of Hepatic Ischemia-Reperfusion Injury Stage in Mice. Inflammation 2019, 42, 2139–2147. [Google Scholar] [CrossRef]
- Marvie, P.; Lisbonne, M.; L’helgoualc’h, A.; Rauch, M.; Turlin, B.; Preisser, L.; Bourd-Boittin, K.; Theret, N.; Gascan, H.; Piquet-Pellorce, C.; et al. Interleukin-33 Overexpression Is Associated with Liver Fibrosis in Mice and Humans. J. Cell Mol. Med. 2010, 14, 1726–1739. [Google Scholar] [CrossRef] [Green Version]
- Nunez, K.G.; Frank, A.; Gonzalez-Rosario, J.; Galliano, G.; Bridle, K.; Crawford, D.; Seal, J.; Abbruscato, F.; Vashistha, H.; Thevenot, P.T.; et al. Interleukin-33/Cyclin D1 Imbalance in Severe Liver Steatosis Predicts Susceptibility to Ischemia Reperfusion Injury. PLoS ONE 2019, 14, 14. [Google Scholar] [CrossRef]
- Mergental, H.; Stephenson, B.T.F.; Laing, R.W.; Kirkham, A.J.; Neil, D.A.H.; Wallace, L.L.; Boteon, Y.L.; Widmer, J.; Bhogal, R.H.; Perera, M.; et al. Development of Clinical Criteria for Functional Assessment to Predict Primary Nonfunction of High-Risk Livers Using Normothermic Machine Perfusion. Liver Transpl. 2018, 24, 1453–1469. [Google Scholar] [CrossRef] [Green Version]
- Ye, D.; Li, H.; Wang, Y.; Jia, W.; Zhou, J.; Fan, J.; Man, K.; Lo, C.; Wong, C.; Wang, Y.; et al. Circulating Fibroblast Growth Factor 21 Is a Sensitive Biomarker for Severe Ischemia/Reperfusion Injury in Patients with Liver Transplantation. Sci. Rep. 2016, 6, 19776. [Google Scholar] [CrossRef] [Green Version]
- Mourad, M.M.; Algarni, A.; Liossis, C.; Bramhall, S.R. Aetiology and Risk Factors of Ischaemic Cholangiopathy after Liver Transplantation. World J. Gastroenterol. 2014, 20, 6159–6169. [Google Scholar] [CrossRef]
- Dorobantu, B.; Popescu, I.; Dima, S.; Nastase, A.; Iacob, S. Molecular Biomarkers as Predictors for Biliary Complications Following Liver Transplantation. A Prospective Study. J. Gastrointestin. Liver Dis. 2017, 26, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Pulitano, C.; Joseph, D.; Sandroussi, C.; Verran, D.; Ho, P.; Debiasio, A.; Luongo, A.; Mccaughan, G.W.; Shackel, N.A.; Crawford, M. Postreperfusion Microcirculatory Derangements after Liver Transplantation: Relationship to Hemodynamics, Serum Mediators, and Outcome. Liver Transpl. 2017, 23, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Laing, R.W.; Bhogal, R.H.; Wallace, L.; Boteon, Y.; Neil, D.A.H.; Smith, A.; Stephenson, B.T.F.; Schlegel, A.; Hubscher, S.G.; Mirza, D.F.; et al. The Use of an Acellular Oxygen Carrier in a Human Liver Model of Normothermic Machine Perfusion. Transplantation 2017, 101, 2746–2756. [Google Scholar] [CrossRef]
- De Vries, Y.; Berendsen, T.A.; Fujiyoshi, M.; Van Den Berg, A.P.; Blokzijl, H.; De Boer, M.T.; Van Der Heide, F.; De Kleine, R.H.J.; Van Leeuwen, O.B.; Matton, A.P.M.; et al. Transplantation Of High-Risk Donor Livers after Resuscitation and Viability Assessment Using a Combined Protocol of Oxygenated Hypothermic, Rewarming and Normothermic Machine Perfusion: Study Protocol for a Prospective, Single-Arm Study (Dhope-Cor-Nmp Trial). BMJ Open 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, J.V.; Henry, S.D.; Chen, S.W.; Brown, T.; Nachber, E.; Arrington, B.; Boykin, J.; Samstein, B.; Brown, R.S.; Emond, J.C.; et al. Hypothermic Machine Preservation Attenuates Ischemia/Reperfusion Markers after Liver Transplantation: Preliminary Results. J. Surg. Res. 2011, 167, E365–E373. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, X.; He, W.; Wang, Y.; Li, L.; Xiong, Y.; Ye, Q. Hypothermic Machine Perfusion Ameliorates Inflammation during Ischemiareperfusion Injury via Sirtuin1mediated Deacetylation of Nuclear Factorkappab P65 in Rat Livers Donated after Circulatory Death. Mol. Med. Rep. 2017, 16, 8649–8656. [Google Scholar] [CrossRef] [Green Version]
- Linares-Cervantes, I.; Echeverri, J.; Cleland, S.; Kaths, J.M.; Rosales, R.; Goto, T.; Kollmann, D.; Hamar, M.; Urbanellis, P.; Mazilescu, L.; et al. Predictor Parameters of Liver Viability during Porcine Normothermic Ex Situ Liver Perfusion in a Model of Liver Transplantation with Marginal Grafts. Am. J. Transpl. 2019, 19, 2991–3005. [Google Scholar] [CrossRef]
- Op Den Dries, S.; Karimian, N.; Westerkamp, A.C.; Sutton, M.E.; Kuipers, M.; Wiersema-Buist, J.; Ottens, P.J.; Kuipers, J.; Giepmans, B.N.; Leuvenink, H.G.; et al. Normothermic Machine Perfusion Reduces Bile Duct Injury and Improves Biliary Epithelial Function in Rat Donor Livers. Liver Transpl. 2016, 22, 994–1005. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Wurdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-Time Prediction of Liver Graft Function during Hypothermic Oxygenated Machine Perfusion before Liver Transplantation. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Karangwa, S.A.; Burlage, L.C.; Adelmeijer, J.; Karimian, N.; Westerkamp, A.C.; Matton, A.P.; Van Rijn, R.; Wiersema-Buist, J.; Sutton, M.E.; Op Den Dries, S.; et al. Activation of Fibrinolysis, but Not Coagulation, during End-Ischemic Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2017, 101, E42–E48. [Google Scholar] [CrossRef] [PubMed]
- Selten, J.W.; Verhoeven, C.J.; Heedfeld, V.; Roest, H.P.; De Jonge, J.; Pirenne, J.; Van Pelt, J.; Ijzermans, J.N.M.; Monbaliu, D.; Van Der Laan, L.J.W. The Release of Microrna-122 during Liver Preservation Is Associated with Early Allograft Dysfunction and Graft Survival after Transplantation. Liver Transpl. 2017, 23, 946–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Nassar, A.; Buccini, L.; Iuppa, G.; Soliman, B.; Pezzati, D.; Hassan, A.; Blum, M.; Baldwin, W.; Bennett, A.; et al. Lipid Metabolism and Functional Assessment of Discarded Human Livers with Steatosis Undergoing 24 H of Normothermic Machine Perfusion. Liver Transpl. 2018, 24, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the Ex Situ Perfusion of Livers for Transplantation. Am. J. Transpl. 2018, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matton, A.P.M.; De Vries, Y.; Burlage, L.C.; Van Rijn, R.; Fujiyoshi, M.; De Meijer, V.E.; De Boer, M.T.; De Kleine, R.H.J.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, Ph, and Glucose Are Suitable Biomarkers of Biliary Viability during Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019, 103, 1405–1413. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Nassar, A.; Farias, K.; Buccini, L.; Baldwin, W.; Mangino, M.; Bennett, A.; O’rourke, C.; Okamoto, T.; Uso, T.D.; et al. Sanguineous Normothermic Machine Perfusion Improves Hemodynamics and Biliary Epithelial Regeneration in Donation after Cardiac Death Porcine Livers. Liver Transpl. 2014, 20, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Li, L. Pre-Conditions for Eliminating Mitochondrial Dysfunction and Maintaining Liver Function after Hepatic Ischaemia Reperfusion. J. Cell Mol. Med. 2017, 21, 1719–1731. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic Machine Preservation of the Liver: State of the Art. Curr. Transpl. Rep. 2018, 5, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Boteon, Y.L.; Laing, R.W.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.H.; Hubscher, S.; Perera, M.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018, 24, 1699–1715. [Google Scholar] [CrossRef] [Green Version]
| Biomarker | Experimental Model Used | Validated in Transplant | Validated in MP | Injured Assessed | Reference | |
|---|---|---|---|---|---|---|
| IRI/EAD | Biliary | |||||
| miRNA | ||||||
| miRNA-122 | Porcine | DCD transplant | No | √ | X | [29] * |
| miRNA46-5p | Human | DCD transplant | No | √ | X | [34] |
| miRNA-34 | Rodent | No | No | √ | X | [35] |
| miRNA-223 | Murine | No | No | √ | X | [36] |
| Interleukins | ||||||
| IL-b | Human | Yes | Yes | √ | X | [47] |
| IL-6 | Rodent | Yes | No | √ | X | [48] |
| IL-8 | Human | Yes | Yes | √ | X | [47] |
| IL-17 | Murine | No | No | √ | X | [37] |
| IL-33 | Human/Murine | No | No | √ | X | [38] |
| Cytokines and Chemokines | ||||||
| Eotaxin | Murine | No | No | √ | X | [37] |
| Eotaxin-2 | Murine | No | No | √ | X | [37] |
| TNF-α | Human | Yes | Yes | X | √ | [43] |
| Metabolites | ||||||
| Lactate | Human | Yes | Yes | √ | X | [40] |
| Urea | Human | Yes | Yes | X | √ | [49] |
| Bile/glucose ratio | Human | Yes | Yes | X | √ | [49] |
| Lactate dehydrogenase | Human | Yes | Yes | X | √ | [50] |
| Mitochondrial Flavin | Human | Yes | Yes | √ | X | [51] |
| Miscellaneous | ||||||
| D-Dimers | Human | Yes | Yes | √ | X | [52] |
| Endothelin-1 | Human | Yes | Yes | √ | √ | [43] |
| FGF21 | Human | Yes | No | √ | X | [41] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhogal, R.H.; Mirza, D.F.; Afford, S.C.; Mergental, H. Biomarkers of Liver Injury during Transplantation in an Era of Machine Perfusion. Int. J. Mol. Sci. 2020, 21, 1578. https://doi.org/10.3390/ijms21051578
Bhogal RH, Mirza DF, Afford SC, Mergental H. Biomarkers of Liver Injury during Transplantation in an Era of Machine Perfusion. International Journal of Molecular Sciences. 2020; 21(5):1578. https://doi.org/10.3390/ijms21051578
Chicago/Turabian StyleBhogal, Ricky H., Darius F. Mirza, Simon C. Afford, and Hynek Mergental. 2020. "Biomarkers of Liver Injury during Transplantation in an Era of Machine Perfusion" International Journal of Molecular Sciences 21, no. 5: 1578. https://doi.org/10.3390/ijms21051578




