Short-Term Responses to Fatty Acids on Lipid Metabolism and Adipogenesis in Rainbow Trout (Oncorhynchus mykiss)
Abstract
:1. Introduction
2. Results
2.1. FA Effects in Rainbow Trout Preadipocytes In Vitro
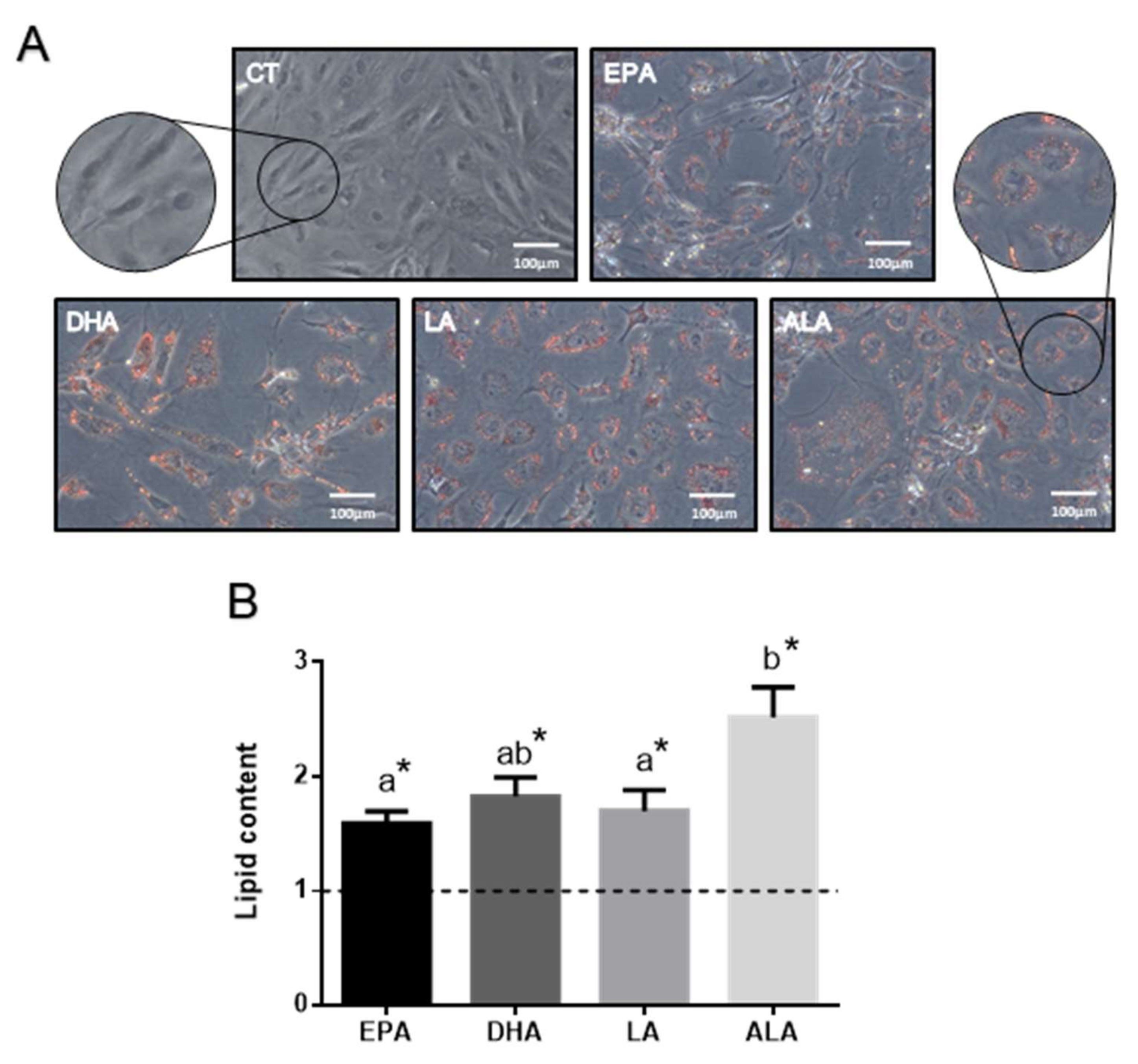
2.1.1. FA Effects on Cell Lipid Content
2.1.2. FA Effects on Adipogenesis and Lipid Metabolism-Related Preadipocyte mRNA Levels
2.2. FA Effects in Rainbow Trout In Vivo
2.2.1. FA Effects on Plasma Metabolites
2.2.2. FA Effects in Adipogenesis and Lipid Metabolism-Related Genes and Protein Levels in Adipose Tissue
2.2.3. FA Effects in Lipid Metabolism-Related Genes and Protein Levels in Liver
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Primary Culture of Adipocyte Cells and FA Treatment
4.3. Oil Red O Staining
4.4. In Vivo FA Treatment by Gavage
4.5. Plasma Analyses
4.6. RNA Extraction and cDNA Synthesis
4.7. Quantitative PCR Analyses
4.8. Protein Extraction and Western Blot Analysis
4.9. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LC-PUFA | long-chain polyunsaturated fatty acids |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| FA | Fatty acid |
| LA | Linoleic acid |
| ALA | Alpha-linolenic acid |
| PPAR | peroxisome proliferator-activated receptor |
| FAS | fatty acid synthase |
| CD36 | Fatty acid translocase/cluster of differentiation 36 |
| FO | fish oil |
| VO | vegetable oil |
| OA | oleic acid |
| PA | palmitic acid |
| AA | arachidonic acid |
| C/EBP | CCAAT/enhancer binding protein |
| HSL | hormone sensitive lipase |
| FABP11 | fatty acid binding protein 11 |
| FATP1 | fatty acid transporter protein 1 |
| LD | lipid droplets |
| ORO | Oil red O |
| LXR | liver X receptor |
| NEFA | non-esterified fatty acids |
| RXR | retinoid X receptor |
| LPL | lipoprotein lipase |
| Ef1a | elongation factor 1 alfa |
| Ub | ubiquitin |
| β-actin | beta-actin |
| ABCA1 | ATP-binding cassette transporter 1 |
References
- Pike, I.; Jackson, A. Fish oil: Production and use now and in the future. Lipid Technol. 2010, 22, 59–61. [Google Scholar] [CrossRef]
- Tocher, D. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Glencross, B.; Booth, M.; Allan, G. A feed is only as good as its ingredients: A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Saele, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Tocher, D. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B. 2010, 365, 2897–2912. [Google Scholar] [CrossRef]
- Torstensen, B.; Lie, Ø.; Frøyland, L. Lipid metabolism and tissue composition in Atlantic salmon (Salmo salar L.)—Effects of capelin oil, palm oil, and oleic acid-enriched sunflower oil as dietary lipid sources. Lipids 2000, 35, 653–664. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Navarro, J.C.; Sitjà-Bobadilla, A.; Gordon Bell, J.; Kaushik, S.; Pérez-Sánchez, J. High levels of vegetable oils in plant protein-rich diets fed to gilthead sea bream (Sparus aurata L.): Growth performance, muscle fatty acid profiles and histological alterations of target tissues. Br. J. Nutr. 2008, 100, 992. [Google Scholar] [CrossRef] [Green Version]
- Benedito-Palos, L.; Navarro, J.C.; Bermejo-Nogales, A.; Saera-Vila, A.; Kaushik, S.; Pérez-Sánchez, J. The time course of fish oil wash-out follows a simple dilution model in gilthead sea bream (Sparus aurata L.) fed graded levels of vegetable oils. Aquaculture 2009, 288, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Benedito-Palos, L.; Navarro, J.C.; Kaushik, S.; Pérez-Sánchez, J. Tissue-specific robustness of fatty acid signatures in cultured gilthead sea bream (Sparus aurata L.) fed practical diets with a combined high replacement of fish meal and fish oil. J. Anim. Sci. 2010, 88, 1759–1770. [Google Scholar] [CrossRef] [Green Version]
- Sprague, M.; Dick, J.; Tocher, D. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Lee, S.; Cleveland, B.; Romano, N.; Lalgudi, R.; Benito, M.; McGraw, B.; Hardy, R. Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): Effects on growth performance and growth-related genes. Aquaculture 2020, 516. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H.; Lucas, P.M. Apparent phosphorus availabilities of selected traditional and alternative feedstuffs for channel catfish. N. Am. J. Aquac. 2015, 77, 136–140. [Google Scholar] [CrossRef]
- Voorhees, J.; Barnes, M.; Chipps, S.; Brown, M. Bioprocessed soybean meal replacement of fish meal in rainbow trout (Oncorhynchus mykiss) diets. Cogent Food Agric. 2019, 5. [Google Scholar] [CrossRef]
- FAO-Food and Agriculture Organization of the United Nations. The State of Mediterranean and Black Sea Fisheries; General Fisheries Commission for the Mediterranean: Rome, Italy, 2018. [Google Scholar]
- Turchini, G.; Ng, W.; Tocher, D. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bouraoui, L.; Sánchez-Gurmaches, J.; Cruz-Garcia, L.; Gutiérrez, J.; Benedito-Palos, L.; Pérez-Sánchez, J.; Navarro, I. Effect of dietary fish meal and fish oil replacement on lipogenic and lipoprotein lipase activities and plasma insulin in gilthead sea bream (Sparus aurata). Aquac. Nutr. 2011, 17, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Garcia, L.; Sánchez-Gurmaches, J.; Bouraoui, L.; Saera-Vila, A.; Pérez-Sánchez, J.; Gutiérrez, J.; Navarro, I. Changes in adipocyte cell size, gene expression of lipid metabolism markers, and lipolytic responses induced by dietary fish oil replacement in gilthead sea bream (Sparus aurata L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.; Huh, J.; Hwang, I.; Kim, J.; Kim, J. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Otto, T.C.; Lane, M.D. Adipose development: From stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 229–242. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Madsen, L.; Petersen, R.; Kristiansen, K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta-Mol. Bas. Dis. 2005, 1740, 266–286. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Chen, H. Effects of different fatty acids on cell differentiation and lipid accumulation in preadipocytes of warm water fish cobia (Rachycentron canadum Linnaeus, 1766). Aquac. Res. 2013, 46, 590–601. [Google Scholar] [CrossRef]
- Riera-Heredia, N.; Lutfi, E.; Gutiérrez, J.; Navarro, I.; Capilla, E. Fatty acids from fish or vegetable oils promote the adipogenic fate of mesenchymal stem cells derived from gilthead sea bream bone potentially through different pathways. PLoS ONE 2019, 14, e0215926. [Google Scholar] [CrossRef] [PubMed]
- Todorčević, M.; Vegusdal, A.; Gjøen, T.; Sundvold, H.; Torstensen, B.E.; Kjær, M.A.; Ruyter, B. Changes in fatty acids metabolism during differentiation of Atlantic salmon preadipocytes; Effects of n-3 and n-9 fatty acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Torstensen, B.; Espe, M.; Stubhaug, I.; Lie, Ø. Dietary plant proteins and vegetable oil blends increase adiposity and plasma lipids in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2011, 106, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Bou, M.; Todorčević, M.; Rodríguez, J.; Capilla, E.; Gutiérrez, J.; Navarro, I. Interplay of adiponectin, TNFα and insulin on gene expression, glucose uptake and PPARγ, AKT and TOR pathways in rainbow trout cultured adipocytes. Gen. Comp. Endocrinol. 2014, 205, 218–225. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- Bouraoui, L.; Gutiérrez, J.; Navarro, I. Regulation of proliferation and differentiation of adipocyte precursor cells in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2008, 198, 459–469. [Google Scholar] [CrossRef]
- Oku, H.; Tokuda, M.; Okumura, T.; Umino, T. Effects of insulin, triiodothyronine and fat soluble vitamins on adipocyte differentiation and LPL gene expression in the stromal-vascular cells of red sea bream. Pagrus major. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 144, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Salmerón, C.; Acerete, L.; Gutiérrez, J.; Navarro, I.; Capilla, E. Characterization and endocrine regulation of proliferation and differentiation of primary cultured preadipocytes from gilthead sea bream (Sparus aurata). Domest. Anim. Endocrinol. 2013, 45, 1–10. [Google Scholar] [CrossRef]
- Vegusdal, A.; Sundvold, H.; Gjøen, T.; Ruyter, B. An in vitro method for studying the proliferation and differentiation of Atlantic salmon preadipocytes. Lipids 2003, 38, 289–296. [Google Scholar] [CrossRef]
- Matsubara, Y.; Sato, K.; Ishii, H.; Akiba, Y. Changes in mRNA expression of regulatory factors involved in adipocyte differentiation during fatty acid induced adipogenesis in chicken. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Della-Fera, M.; Lin, J.; Baile, C.A. Docosahexaenoic Acid Inhibits Adipocyte Differentiation and Induces Apoptosis in 3T3-L1 Preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bou, M.; Montfort, J.; Le Cam, A.; Rallière, C.; Lebret, V.; Gabillard, J.-C.; Weil, C.; Gutierrez, J.; Rescan, P.-Y.; Capilla, E.; et al. Gene expression profile during proliferation and differentiation of rainbow trout adipocyte precursor cells. BMC Genom. 2017, 18, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riera-Heredia, N. Fatty Acids and Temperature Effects in Fish Adipogenesis and Osteogenesis: In Vitro and in Vivo Approaches. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Barber, E.; Sinclair, A.; Cameron-Smith, D. Comparative actions of omega-3 fatty acids on in-vitro lipid droplet formation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-S.; Todorčević, M.; Ruyter, B.; Torstensen, B.E. Altered expression of CCAAT/enhancer binding protein and FABP11 genes during adipogenesis in vitro in Atlantic salmon (Salmo salar). Aquac. Nutr. 2010, 16, 72–80. [Google Scholar] [CrossRef]
- Prostek, A.; Gajewska, M.; Kamola, D.; Bałasińska, B. The influence of EPA and DHA on markers of inflammation in 3T3-L1 cells at different stages of cellular maturation. Lipids Health Dis. 2014, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Murali, G.; Desouza, C.; Clevenger, M.; Ramalingam, R.; Saraswathi, V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 13–21. [Google Scholar] [CrossRef]
- Wójcik, C.; Lohe, K.; Kuang, C.; Xiao, Y.; Jouni, Z.; Poels, E. Modulation of adipocyte differentiation by omega-3 polyunsaturated fatty acids involves the ubiquitin-proteasome system. J. Cell. Mol. Med. 2014, 18, 590–599. [Google Scholar] [CrossRef]
- Todorčević, M.; Hodson, L. The Effect of Marine Derived n-3 Fatty Acids on Adipose Tissue Metabolism and Function. J. Clin. Med. 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Todorčević, M.; Škugor, S.; Krasnov, A.; Ruyter, B. Gene expression profiles in Atlantic salmon adipose-derived stromo-vascular fraction during differentiation into adipocytes. BMC Genom. 2010, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Salmerón, C.; Riera-Heredia, N.; Gutiérrez, J.; Navarro, I.; Capilla, E. Adipogenic gene expression in gilthead sea bream mesenchymal stem cells from different origin. Front. Endocrinol. 2016, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Gurmaches, J.; Cruz-Garcia, L.; Gutiérrez, J.; Navarro, I. Adiponectin effects and gene expression in rainbow trout: An in vivo and in vitro approach. J. Exp. Biol. 2012, 215, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librán-Pérez, M.; Figueiredo-Silva, A.C.; Panserat, S.; Geurden, I.; Míguez, J.M.; Polakof, S.; Soengas, J.L. Response of hepatic lipid and glucose metabolism to a mixture or single fatty acids: Possible presence of fatty acid-sensing mechanisms. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2012, 164, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Librán-Pérez, M.; López-Patiño, M.; Míguez, J.; Soengas, J. In vitro response of putative fatty acid-sensing systems in rainbow trout liver to increased levels of oleate or octanoate. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2013, 165, 288–294. [Google Scholar] [CrossRef]
- Riccardi, G.; Giacco, R.; Rivellese, A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef]
- Wang, X.; Chan, C. n-3 polyunsaturated fatty acids and insulin secretion. J. Endocrinol. 2014, 224, R97–R106. [Google Scholar] [CrossRef] [Green Version]
- Flattem, N.; Igawa, K.; Shiota, M.; Emshwiller, M.G.; Neal, D.W.; Cherrington, A.D. α- and β-cell responses to small changes in plasma glucose in the conscious dog. Diabetes 2001, 50, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Gnoni, A.; Giudetti, A.M. Dietary long-chain unsaturated fatty acids acutely and differently reduce the activities of lipogenic enzymes and of citrate carrier in rat liver. J. Physiol. Biochem. 2016, 72, 485–494. [Google Scholar] [CrossRef]
- Nakamura, M.; Cheon, Y.; Li, Y.; Nara, T. Mechanisms of regulation of gene expression by fatty acids. Lipids 2004, 39, 1077–1083. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [Green Version]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Ogata, H.; Oku, H. Effect of dietary fatty acids on lipoprotein lipase gene expression in the liver and visceral adipose tissue of fed and starved red sea bream Pagrus major. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2002, 132, 913–919. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000, 84, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raclot, T.; Langin, D.; Lafontan, M.; Groscolas, R. Selective release of human adipocyte fatty acids according to molecular structure. Biochem. J. 1997, 324, 911–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urrutia, O.; Mendizabal, J.; Insausti, K.; Soret, B.; Purroy, A.; Arana, A. Effects of Addition of Linseed and Marine Algae to the Diet on Adipose Tissue Development, Fatty Acid Profile, Lipogenic Gene Expression, and Meat Quality in Lambs. PLoS ONE 2016, 11, e0156765. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.; Barnard, R. Effect of diet on fat cell size and hormone-sensitive lipase activity. J. Appl. Physiol. 1999, 87, 227–232. [Google Scholar] [CrossRef]
- Farnier, C.; Krief, S.; Blache, M.; Diot-Dupuy, F.; Mory, G.; Ferre, P.; Bazin, R. Adipocyte functions are modulated by cell size change: Potential involvement of an integrin/ERK signalling pathway. Int. J. Obes. 2003, 27, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Thematic review series: Adipocyte Biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007, 48, 1905–1914. [Google Scholar] [CrossRef] [Green Version]
- Feingold, K.; Grunfeld, C. Introduction to Lipids and Lipoproteins. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305896/ (accessed on 10 June 2019).
- Pegorier, J.P.; May, C.L.; Girard, J. Control of gene expression by fatty acids. J. Nutr. 2004, 134, 2444S–24449S. [Google Scholar] [CrossRef]
- Alvarez, M.; Díez, A.; López-Bote, C.; Gallego, M.; Bautista, J. Short-term modulation of lipogenesis by macronutrients in rainbow trout (Oncorhynchus mykiss) hepatocytes. Br. J. Nutr. 2000, 84, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coburn, C.; Hajri, T.; Ibrahimi, A.; Abumrad, N. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J. Mol. Neurosci. 2001, 16, 117–122. [Google Scholar] [CrossRef]
- Torstensen, B.; Nanton, D.; Olsvik, P.; Sundvold, H.; Stubhaug, I. Gene expression of fatty acid-binding proteins, fatty acid transport proteins (cd36 and FATP) and β-oxidation-related genes in Atlantic salmon (Salmo salar L.) fed fish oil or vegetable oil. Aquac. Nutr. 2009, 15, 440–451. [Google Scholar] [CrossRef]
- Weil, C.; Lefèvre, F.; Bugeon, J. Characteristics and metabolism of different adipose tissues in fish. Rev. Fish Biol. Fish. 2012, 23, 157–173. [Google Scholar] [CrossRef]
- Librán-Pérez, M.; López-Patiño, M.; Míguez, J.; Soengas, J. Oleic acid and octanoic acid sensing capacity in rainbow trout Oncorhynchus mykiss is direct in hypothalamus and Brockmann bodies. PLoS ONE 2013, 8, e59507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librán-Pérez, M.; Polakof, S.; López-Patiño, M.A.; Míguez, J.M.; Soengas, J.L. Evidence of a metabolic fatty-acid sensing system in the hypothalamus and Brockmann bodies of rainbow trout: Implications in food intake regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1340–R1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo-Silva, A.; Panserat, S.; Kaushik, S.; Geurden, I.; Polakof, S. High levels of dietary fat impair glucose homeostasis in rainbow trout. J. Exp. Biol. 2012 215, 169–178. [CrossRef] [Green Version]
- Lutfi, E.; Riera-Heredia, N.; Córdoba, M.; Porte, C.; Gutiérrez, J.; Capilla, E.; Navarro, I. Tributyltin and triphenyltin exposure promotes in vitro adipogenic differentiation but alters the adipocyte phenotype in rainbow trout. Aquat. Toxicol. 2017, 188, 148–158. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29. [Google Scholar] [CrossRef]
- Lansard, M.; Panserat, S.; Plagnes-Juan, E.; Seiliez, I.; Skiba-Cassy, S. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: Role of TOR. Amino Acids 2010, 39, 801–810. [Google Scholar] [CrossRef]
- Belghit, I.; Skiba-Cassy, S.; Geurden, I.; Dias, K.; Surget, A.; Kaushik, S.; Panserat, S.; Seiliez, I. Dietary methionine availability affects the main factors involved in muscle protein turnover in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2014, 112, 493–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| A | EPA | DHA | LA | ALA |
|---|---|---|---|---|
| cebpa | 0.54 ± 0.144 * | 0.63 ± 0.079 | 0.71 ± 0.077 | 0.73 ± 0.055 |
| pparg | 0.51 ± 0.109 * | 0.66 ± 0.044 | 0.70 ± 0.128 | 0.81 ± 0.094 |
| lxr | 0.91 ± 0.112 | 0.96 ± 0.064 | 0.88 ± 0.068 | 0.95 ± 0.046 |
| fas | 0.69 ± 0.138 | 0.80 ± 0.109 | 0.74 ± 0.100 | 0.89 ± 0.107 |
| lpl | 0.97 ± 0.119 | 1.02 ± 0.066 | 0.98 ± 0.067 | 1.03 ± 0.059 |
| hsl | 1.12 ± 0.040 | 1.07 ± 0.059 | 0.94 ± 0.065 | 1.06 ± 0.051 |
| cd36 | 0.70 ± 0.197 | 0.90 ± 0.091 | 0.83 ± 0.034 | 0.93 ± 0.067 |
| fatp1 | 1.38 ± 0.158 a.b | 1.29 ± 0.029 b | 1.64 ± 0.116 *a | 1.78 ± 0.077 *a |
| fabp11a | 1.27 ± 0.160 | 1.13 ± 0.155 | 1.14 ± 0.138 | 1.11 ± 0.106 |
| abca1 | 0.85 ± 0.353 | 0.84 ± 0.057 | 0.80 ± 0.079 | 0.92 ± 0.093 |
| B | EPA + DHA | EPA + LA | EPA + ALA | DHA + LA | DHA + ALA | LA + ALA |
|---|---|---|---|---|---|---|
| cebpa | 1.28 ± 0.208 | 0.78 ± 0.141 | 0.72 ± 0.091 | 0.80 ± 0.173 | 0.91 ± 0.079 | 0.84 ± 0.070 |
| pparg | 0.24 ± 0.027 * | 0.31 ± 0.035 * | 0.30 ± 0.036 * | 0.41 ± 0.057 * | 0.42 ± 0.046 * | 0.42 ± 0.092 * |
| lxr | 0.71 ± 0.040 * | 1.06 ± 0.151 | 0.93 ± 0.126 | 0.94 ± 0.209 | 0.93 ± 0.078 | 0.87 ± 0.044 |
| fas | 1.23 ± 0.117 | 1.45 ± 0.200 | 1.29 ± 0.252 | 1.05 ± 0.113 | 1.28 ± 0.214 | 1.34 ± 0.222 |
| lpl | 0.95 ± 0.128 | 1.06 ±0.134 | 0.95 ± 0.106 | 1.09 ± 0.198 | 0.94 ± 0.118 | 0.79 ± 0.112 |
| hsl | 1.2 ± 0.159 | 1.21 ± 0.145 | 1.09 ± 0.116 | 1.13 ± 0.234 | 1.13 ± 0.051 | 1.02 ± 0.099 |
| cd36 | 1.08 ± 0.051 | 1.46 ± 0.236 | 1.38 ± 0.197 | 1.02 ± 0.157 | 1.25 ± 0.122 | 1.33 ± 0.219 |
| fatp1 | 1.04 ± 0.064 a | 1.73 ± 0.178 *b | 1.62 ± 0.160 ab | 1.47 ± 0.169 ab | 1.41 ± 0.105 ab | 1.76 ± 0.180 *b |
| fabp11 | 1.02 ± 0.078 | 1.32 ± 0.152 | 1.09 ± 0.054 | 1.12 ± 0.176 | 1.05 ± 0.123 | 1.25 ± 0.129 |
| abca1 | 1.32 ± 0.189 | 1.52 ± 0.296 | 1.25 ± 0.184 | 1.2 ± 0.337 | 1.1 ± 0.170 | 1.09 ± 0.203 |
| Gene | Primer Sequence (5’→3’) | Ta (°C) | Acc. Num. |
|---|---|---|---|
| Cebpa | F: TGTGGCGATAAAGCAAGAGC | 57 | DQ423469.1 |
| R: CTGGTGGGAATGGTGGTAGG | |||
| Cebpb | F: CACAAAGTGCTGGAACTGGC | 60 | FR904306.1 |
| R: TGGCACAGCGATAAATGGGT | |||
| Ppara | F: CTGGAGCTGGATGACAGTGA | 54 | AY494835 |
| R: GGCAAGTTTTTGCAGCAGAT | |||
| Pparb | F: CTGGAGCTGGATGACAGTGA | 59 | AY356399.1 |
| R: GTCAGCCATCTTGTTGAGCA | |||
| Pparg | F: GCCAGTACTGTCGCTTTCAG | 60 | HM536192.1 |
| R: TCCATAAACTCAGCCAGCAG | |||
| Lxr | F: TGCAGCAGCCGTATGTGGA | 62 | NM_001159338 |
| R: GCGGCGGGAGCTTCTTGTC | |||
| Rxr | F: AAAGAGCGCAGTGAGAACGA | 55 | AJ969439.1 |
| R: TGTAGGTCTCGGTCTTGGGT | |||
| Fas | F: GAGACCTAGTGGAGGCTGTC | 54 | tcaa0001c.m.06_5.1.om.4 |
| R: TCTTGTTGATGGTGAGCTGT | |||
| Lpl | F: TAATTGGCTGCAGAAAACAC | 59 | AJ224693 |
| R: CGTCAGCAAACTCAAAGGT | |||
| hsl | F: AGGGTCATGGTCATCGTCTC | 58 | TC172767 |
| R: CTTGACGGAGGGACAGCTAC | |||
| cd36 | F: CAAGTCAGCGACAAACCAGA | 62 | AY606034 |
| R: ACTTCTGAGCCTCCACAGGA | |||
| fatp1 | F: AGGAGAGAACGTCTCCACCA | 60 | CA373015 |
| R: CGCATCACAGTCAAATGTCC | |||
| fabp11 | F: CATTTGAGGAGACCACCGCT | 60 | NM_001124713.1 |
| R: ACTTGAGTTTGGTGGTACGCT | |||
| abca1 | F: CAGGAAAGACGAGCACCTTC | 58 | TC169876 |
| R: TCTGCCACCTCACACACTTC | |||
| 18s | F: GGCGCCCCCTCGATGCTCTTA | 65 | AF308735.1 |
| R: CCCCCGGCCGTCCCTCTTAAT | |||
| ef1a | F: TCCTCTTGGTCGTTTCGCTG | 58 | AF498320 |
| R: ACCCGAGGGACATCCTGTG | |||
| b-actin | F: ATCCTGACAGAGCGCGGTTACAGT | 61 | AJ438158 |
| R: TGCCCATCTCCTGCTCAAAGTCAA | |||
| Ub | F: ACAACATCCAGAAAGAGTCCAC | 58 | AB036060 |
| R: AGGCGAGCGTAGCACTTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riera-Heredia, N.; Lutfi, E.; Sánchez-Moya, A.; Gutiérrez, J.; Capilla, E.; Navarro, I. Short-Term Responses to Fatty Acids on Lipid Metabolism and Adipogenesis in Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2020, 21, 1623. https://doi.org/10.3390/ijms21051623
Riera-Heredia N, Lutfi E, Sánchez-Moya A, Gutiérrez J, Capilla E, Navarro I. Short-Term Responses to Fatty Acids on Lipid Metabolism and Adipogenesis in Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences. 2020; 21(5):1623. https://doi.org/10.3390/ijms21051623
Chicago/Turabian StyleRiera-Heredia, Natàlia, Esmail Lutfi, Albert Sánchez-Moya, Joaquim Gutiérrez, Encarnación Capilla, and Isabel Navarro. 2020. "Short-Term Responses to Fatty Acids on Lipid Metabolism and Adipogenesis in Rainbow Trout (Oncorhynchus mykiss)" International Journal of Molecular Sciences 21, no. 5: 1623. https://doi.org/10.3390/ijms21051623
APA StyleRiera-Heredia, N., Lutfi, E., Sánchez-Moya, A., Gutiérrez, J., Capilla, E., & Navarro, I. (2020). Short-Term Responses to Fatty Acids on Lipid Metabolism and Adipogenesis in Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences, 21(5), 1623. https://doi.org/10.3390/ijms21051623








