Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
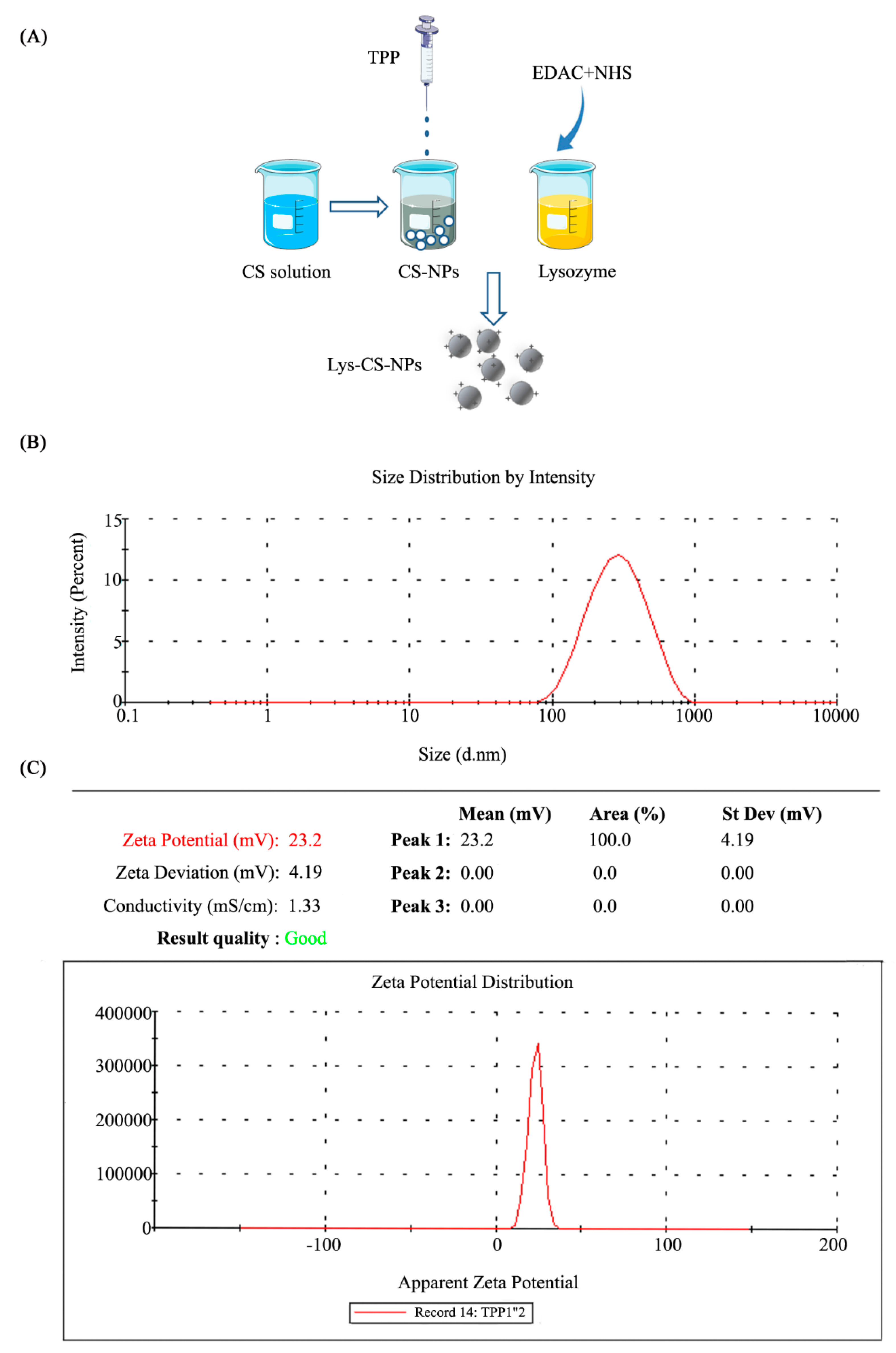
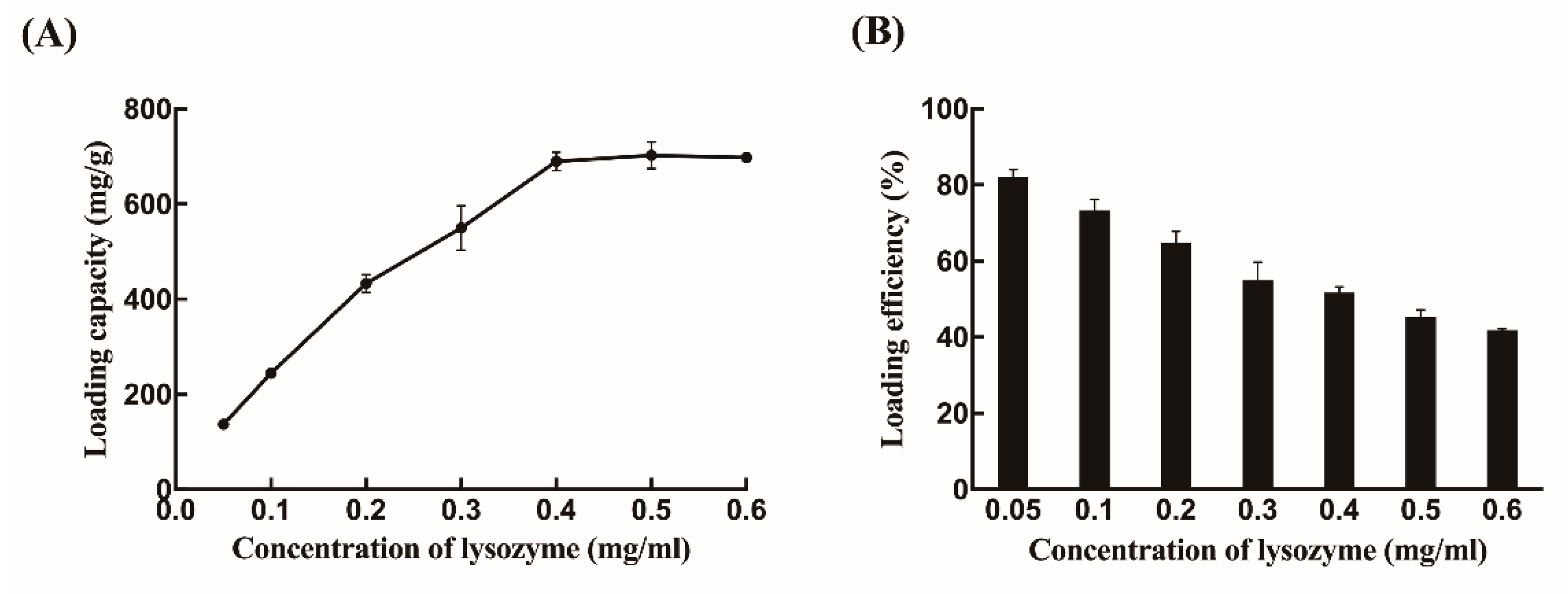
2.1. Synthesis and Morphology of Chitosan Nanoparticles
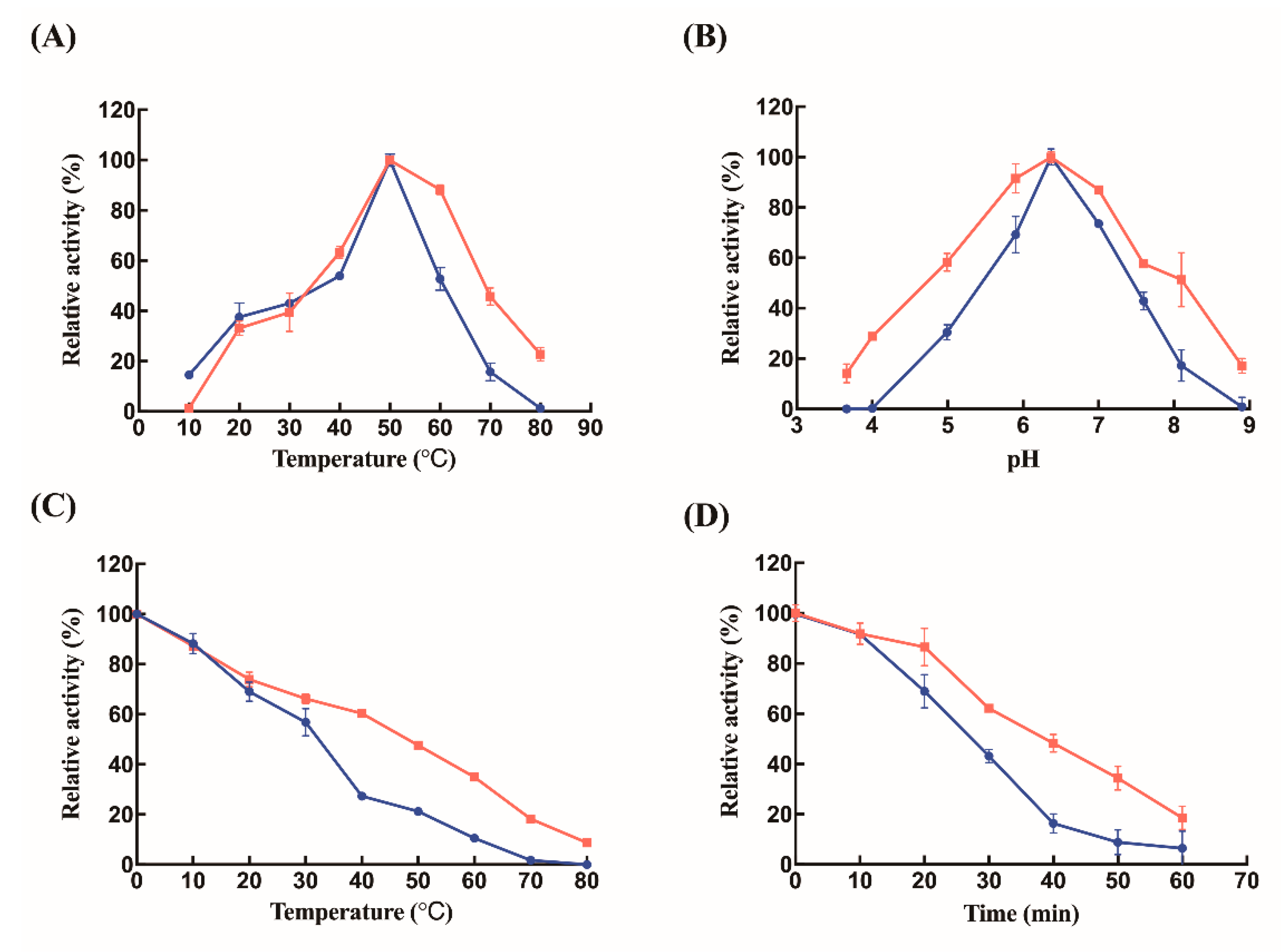
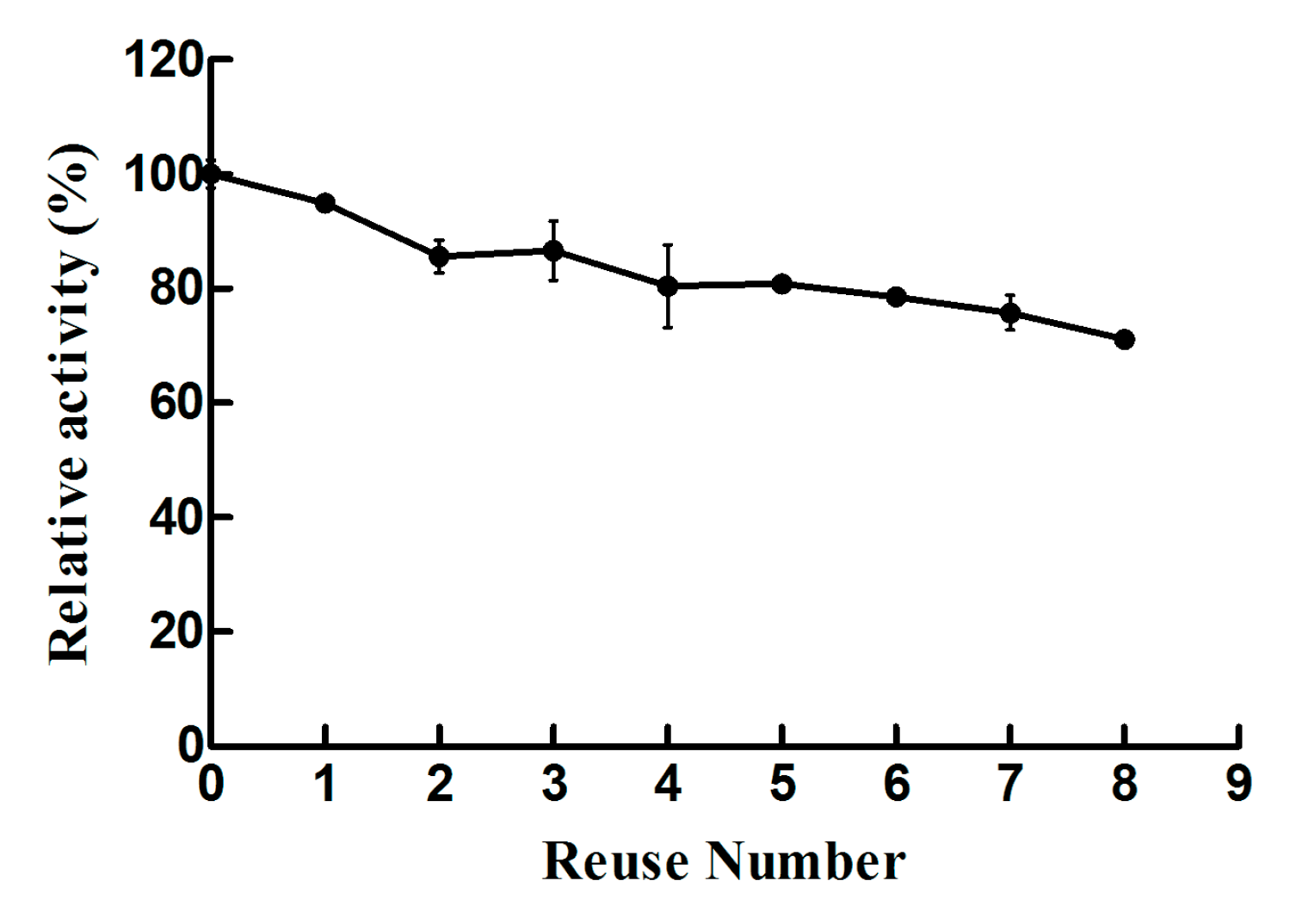
2.2. Biochemical Characterizations of Free Lysozyme and Immobilized Lysozyme
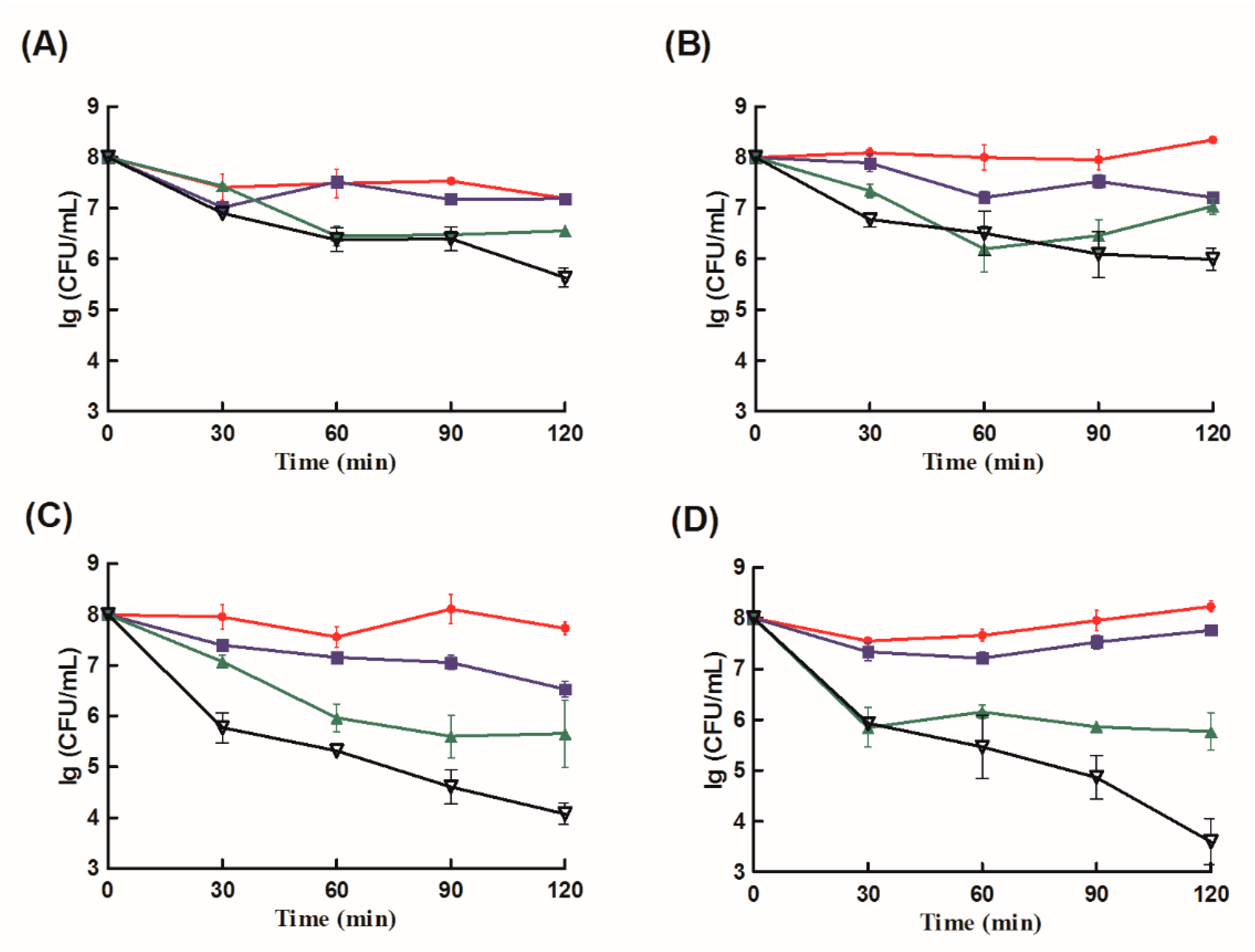
2.3. Antibacterial Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Chitosan Nanoparticles
4.3. Evaluation of the Particle Properties
4.4. Enzyme Activity Assay
4.5. Characterization of Free and Immobilized Enzymes
4.6. Antibacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CS-NPs | chitosan nanoparticles |
| Lys-CS-NPs | lysozyme immobilized chitosan nanoparticles |
| TEM | transmission electron microscopy |
| DLS | dynamic light scattering |
| MIC | minimum inhibitory concentration |
| P. aeruginosa | Pseudomonas aeruginosa |
| K. pneumoniae | Klebsiella pneumoniae |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus. |
| LC | loading capacity |
| %LE | loading efficiency |
| DD | degree of deacetylation |
| ICU | intensive care unit |
| VAP | ventilator-associated pneumonia |
| QS | quorum sensing |
| EDAC | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| NHS | N-Hydroxysuccinimide |
| TPP | Tri-polyphosphate |
References
- Wen, S.; Yao, D.; Liu, X.; Wang, F. A novel fluorescence resonance energy transfer-based high-throughput screening method for generation of lysozyme with improved antimicrobial activity against Escherichia coli strains. J. Agric. Food. Chem. 2019, 67, 12584–12589. [Google Scholar] [CrossRef]
- Brott, A.S.; Clarke, A.J. Peptidoglycan o-acetylation as a virulence factor: Its effect on lysozyme in the innate immune system. Antibiotics 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Callewaert, L.; Van Herreweghe, J.M.; Vanderkelen, L.; Leysen, S.; Voet, A.; Michiels, C.W. Guards of the great wall: Bacterial lysozyme inhibitors. Trends. Microbiol. 2012, 20, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Griswold, K.E.; Bement, J.L.; Teneback, C.C.; Scanlon, T.C.; Wargo, M.J.; Leclair, L.W. Bioengineered lysozyme in combination therapies for Pseudomonas aeruginosa lung infections. Bioengineered 2014, 5, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, N.A.; Brandelli, A. Nanostructures for delivery of natural antimicrobials in food. Crit. Rev. Food. Sci. Nutr. 2018, 58, 2202–2212. [Google Scholar] [CrossRef]
- Aminlari, L.; Hashemi, M.M.; Aminlari, M. Modified lysozymes as novel broad spectrum natural antimicrobial agents in foods. J. Food. Sci. 2014, 79, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Retamozo, S.; Sisó-Almirall, A.; Pérez-Alvarez, R.; Pallarés, L.; Brito-Zerón, P. Clinically-useful serum biomarkers for diagnosis and prognosis of sarcoidosis. Expert. Rev. Clin. Immunol. 2019, 15, 391–405. [Google Scholar] [CrossRef]
- Bergamo, A.; Gerdol, M.; Pallavicini, A.; Greco, S.; Schepens, I.; Hamelin, R.; Armand, F.; Dyson, P.J.; Sava, G. Lysozyme-induced transcriptional regulation of TNF-α pathway genes in cells of the monocyte lineage. Int. J. Mol. Sci. 2019, 20, 5502. [Google Scholar] [CrossRef] [Green Version]
- Galar, A.; Weil, A.A.; Dudzinski, D.M.; Muñoz, P.; Siedner, M.J. Methicillin-resistant staphylococcus aureus prosthetic valve endocarditis: Pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin. Microbiol. Rev. 2019, 32, e00041-18. [Google Scholar] [CrossRef] [Green Version]
- Unertl, K.E.; Lenhart, F.P.; Forst, H.; Peter, K. Systemic antibiotic treatment of nosocomial pneumonia. Intensive Care. Med. 1992, 18, 28–34. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaître, N.; Liang, X.; Najeeb, J.; Lee, C.J.; Titecat, M.; Leteurtre, E.; Simonet, M.; Toone, E.J.; Zhou, P.; Sebbane, F. Curative treatment of severe Gram-negative bacterial infections by a new class of antibiotics targeting lpxC. mBio 2017, 8, e00674-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, A.R.; Mecsas, J.; Moir, D.T. Beyond antibiotics: New therapeutic approaches for bacterial infections. Clin. Infect. Dis. 2016, 63, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hukić, M.; Seljmo, D.; Ramovic, A.; Ibrišimović, M.A.; Dogan, S.; Hukic, J.; Bojic, E.F. The effect of lysozyme on reducing biofilms by Staphylococcus aureus, Pseudomonas aeruginosa, and Gardnerella vaginalis: An in vitro examination. Microb. Drug. Resist. 2018, 24, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Elkordy, A.A.; Forbes, R.T.; Barry, B.W. Stability of crystallised and spray-dried lysozyme. Int. J. Pharm. 2004, 278, 209–219. [Google Scholar] [CrossRef]
- Mohtashami, M.; Fooladi, J.; Haddad-Mashadrizeh, A.; Housaindokht, M.R.; Monhemi, H. Molecular mechanism of enzyme tolerance against organic solvents: Insights from molecular dynamics simulation. Int. J. Biol. Macromol. 2019, 122, 914–923. [Google Scholar] [CrossRef]
- Steiert, E.; Radi, L.; Fach, M.; Wich, P.R. Protein-based nanoparticles for the delivery of enzymes with antibacterial activity. Macromol. Rapid. Commun. 2018, 39, 1800186. [Google Scholar] [CrossRef]
- Wahba, M.I. Porous chitosan beads of superior mechanical properties for the covalent immobilization of enzymes. Int. J. Biol. Macromol. 2017, 105, 894–904. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; Segura-Ceniceros, P.; López, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases immobilization on chitosan-coated magnetic nanoparticles: Application for agave atrovirens lignocellulosic biomass hydrolysis. Bioprocess. Biosyst. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Munemura, O.; Masumoto, K.; Ueda, T.; Imoto, T. Thermostability of doubly glycosylated recombinant lysozyme. Biol. Pharm. Bull. 2001, 24, 1102–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, R.; Kim, J.Y.; Hong, E.Y.; Lee, S.G.; Seo, J.H.; Kim, B.G. RiSLnet: Rapid identification of smart mutant libraries using protein structure network. application to thermal stability enhancement. Biotechnol. Bioeng. 2019, 116, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sanders, J.P. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Es, I.; Vieira, J.D.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Tarhan, T.; Ulu, A.; Saricam, M.; Culha, M.; Ates, B. Maltose functionalized magnetic core/shell Fe3O4@Au nanoparticles for an efficient L-asparaginase immobilization. Int. J. Biol. Macromol. 2020, 142, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Li, X.; Lee, B.S.; Jung, S.; Lee, M.S. Enhancing the thermo-stability and anti-biofilm activity of alginate lyase by immobilization on low molecular weight chitosan nanoparticles. Int. J. Mol. Sci. 2019, 20, 4565. [Google Scholar] [CrossRef] [Green Version]
- Giamarellou, H.; Poulakou, G. Multidrug-resistant Gram-negative infections: What are the treatment options? Drugs 2009, 69, 1879–1901. [Google Scholar] [CrossRef]
- Cigana, C.; Bianconi, I.; Baldan, R.; De Simone, M.; Riva, C.; Sipione, B.; Rossi, G.; Cirillo, D.M.; Bragonzi, A. Staphylococcus aureus impacts Pseudomonas aeruginosa chronic respiratory disease in murine models. J. Infect Dis. 2018, 217, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Fihman, V.; Messika, J.; Hajage, D.; Tournier, V.; Gaudry, S.; Magdoud, F.; Barnaud, G.; Billard-Pomares, T.; Branger, C.; Dreyfuss, D.; et al. Five-year trends for ventilator-associated pneumonia: Correlation between microbiological findings and antimicrobial drug consumption. Int. J. Antimicrob Agents. 2015, 46, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Epaud, R.; Delestrain, C.; Weaver, T.E.; Akinbi, H.T. Bacterial killing is enhanced by exogenous administration of lysozyme in the lungs. Respir. Med. Res. 2019, 76, 22–27. [Google Scholar] [CrossRef]
- Alpar, H.O.; Somavarapu, S.; Atuah, K.N.; Bramwell, V.W. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 411–430. [Google Scholar] [CrossRef]
- Islam, M.A.; Park, T.-E.; Reesor, E.; Cherukula, K.; Hasan, A.; Firdous, J.; Singh, B.; Kang, S.-K.; Choi, Y.-J.; Park, I.-K.; et al. Mucoadhesive Chitosan Derivatives as Novel Drug Carriers. Curr. Pharm. Des. 2015, 21, 4285–4309. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubini, D.; Banu, S.F.; Subramani, P.; Hari, B.N.V.; Gowrishankar, S.; Pandian, S.K.; Wilson, A.; Nithyanand, P. Extracted chitosan disrupts quorum sensing mediated virulence factors in Urinary tract infection causing pathogens. Pathog. Dis. 2019, 77. [Google Scholar] [CrossRef] [PubMed]
- Seviour, T.; Derlon, N.; Dueholm, M.S.; Flemming, H.C.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M.C.M.; Lotti, T.; Malpei, M.F.; et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019, 151, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; Evli, S.; Dabanca, M.B.; Basbulbul, G.; Uygun, M.; Uygun, D.A. Bacteria killer enzyme attached magnetic nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef]
| Concentration (mg/mL) | P. aeruginosa | K. pneumoniae | E. coli | S. aureus |
|---|---|---|---|---|
| CS-NPs | >10 | 10 | 10 | 10 |
| Free lysozyme | >10 | 10 | 10/4 | 10/8 |
| Free lysozyme + CS-NPs | 10/2 | 10/2 | 10/16 | 10/32 |
| Lys-CS-NPs | 10/4 | 10/8 | 10/16 | 10/64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, S.; Jin, M.; Han, Q.; Liu, S.; Chen, X.; Han, Y. Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles. Int. J. Mol. Sci. 2020, 21, 1635. https://doi.org/10.3390/ijms21051635
Wang Y, Li S, Jin M, Han Q, Liu S, Chen X, Han Y. Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles. International Journal of Molecular Sciences. 2020; 21(5):1635. https://doi.org/10.3390/ijms21051635
Chicago/Turabian StyleWang, Yanan, Shangyong Li, Mengfei Jin, Qi Han, Songshen Liu, Xuehong Chen, and Yantao Han. 2020. "Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles" International Journal of Molecular Sciences 21, no. 5: 1635. https://doi.org/10.3390/ijms21051635
APA StyleWang, Y., Li, S., Jin, M., Han, Q., Liu, S., Chen, X., & Han, Y. (2020). Enhancing the Thermo-Stability and Anti-Bacterium Activity of Lysozyme by Immobilization on Chitosan Nanoparticles. International Journal of Molecular Sciences, 21(5), 1635. https://doi.org/10.3390/ijms21051635




