Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases
Abstract
:1. Introduction
1.1. Dendritic Cells
1.2. DCs of the Oral Mucosal Tissues
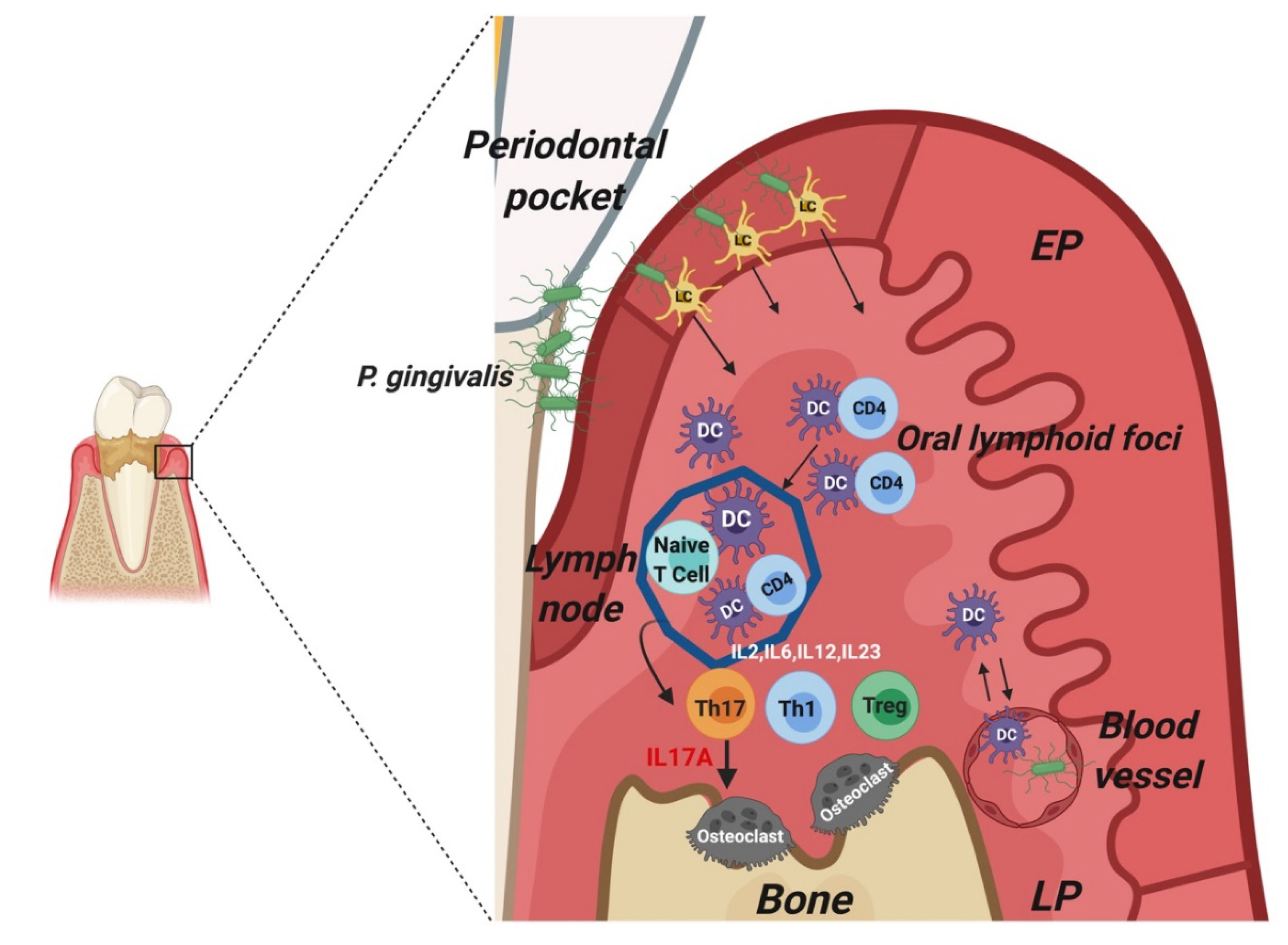
1.3. Periodontitis and Dendritic Cells
1.4. Apoptosis
1.5. Defective Apoptosis and Risk of Autoimmunity and Microbial Dissemination
1.6. Autophagy
1.7. Autophagosome Maturation
1.8. Autophagy Regulation
1.9. Autophagy in Infection and Immunity
1.10. Autophagy, a Tool for Resistance or Susceptibility to Infection
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| P. gingivalis | Porphyromonas gingivalis |
| DCs | Dendritic cells |
| PD | Periodontitis |
| APCs | Antigen-presenting cells |
| PBMCs | Peripheral blood mononuclear cells |
| MHC | Major histocompatibility complex |
| pDC | Plasmacytoid dendritic cells |
| PRR | Pattern recognition receptors |
| PAMP | Pathogen-associated molecular patterns |
| LPS | Lipopolysaccharide |
| TLRs | Toll-like receptors |
| CTLs | C-type lectin receptors |
| DC-SIGN | Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin |
| LCs | Langerhans cells |
| OLF | Oral Lymphoid Foci |
| PMNs | Polymorphonuclear leukocytes |
| Treg | T regulatory cells |
| ATGs | Autophagy-related proteins |
| mTOR | Mechanistic target of rapamycin |
References
- Steinman, R.M. Dendritic cells and the control of immunity: Enhancing the efficiency of antigen presentation. Mt. Sinai J. Med. 2001, 68, 160–166. [Google Scholar] [PubMed]
- O’Doherty, U.; Peng, M.; Gezelter, S.; Swiggard, W.J.; Betjes, M.; Bhardwaj, N.; Steinman, R.M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 1994, 82, 487–493. [Google Scholar] [PubMed]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindstedt, M.; Lundberg, K.; Borrebaeck, C.A. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J. Immunol. 2005, 175, 4839–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, K.P.; Munster, D.J.; Clark, G.J.; Dzionek, A.; Schmitz, J.; Hart, D.N. Characterization of human blood dendritic cell subsets. Blood 2002, 100, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Breton, G.; Oliveira, T.Y.; Zhou, Y.J.; Aljoufi, A.; Puhr, S.; Cameron, M.J.; Sékaly, R.P.; Nussenzweig, M.C.; Liu, K. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J. Exp. Med. 2015, 212, 385–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton, G.; Lee, J.; Zhou, Y.J.; Schreiber, J.J.; Keler, T.; Puhr, S.; Anandasabapathy, N.; Schlesinger, S.; Caskey, M.; Liu, K.; et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J. Exp. Med. 2015, 212, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Evans, V.A.; Lal, L.; Akkina, R.; Solomon, A.; Wright, E.; Lewin, S.R.; Cameron, P.U. Thymic plasmacytoid dendritic cells are susceptible to productive HIV-1 infection and efficiently transfer R5 HIV-1 to thymocytes in vitro. Retrovirology 2011, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Zhang, Y.L.; Chen, L.J.; Zhou, T.; Huang, W.K.; Zhou, X.; Shao, L.Q. The role of Toll-Like Receptors in Periodontitis. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/odi.12468 (accessed on 30 December 2019).
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Curtis, B.M.; Scharnowske, S.; Watson, A.J. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 1992, 89, 8356–8360. [Google Scholar] [CrossRef] [Green Version]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Appelmelk, B.J.; van Die, I.; van Vliet, S.J.; Vandenbroucke-Grauls, C.M.; Geijtenbeek, T.B.; van Kooyk, Y. Cutting edge: Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003, 170, 1635–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivatsan, J.; Smith, D.F.; Cummings, R.D. The human blood fluke Schistosoma mansoni synthesizes glycoproteins containing the Lewis X antigen. J. Biol. Chem. 1992, 267, 20196–20203. [Google Scholar] [PubMed]
- Zeituni, A.E.; McCaig, W.; Scisci, E.; Thanassi, D.G.; Cutler, C.W. The native 67-kilodalton minor fimbria of Porphyromonas gingivalis is a novel glycoprotein with DC-SIGN-targeting motifs. J. Bacteriol. 2010, 192, 4103–4110. [Google Scholar] [CrossRef] [Green Version]
- van Gisbergen, K.P.; Ludwig, I.S.; Geijtenbeek, T.B.; van Kooyk, Y. Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 2005, 579, 6159–6168. [Google Scholar] [CrossRef] [Green Version]
- Martinez, O.; Brackenridge, S.; El-Idrissi, M.-A.; Prabhakar, B.S. DC-SIGN, but not sDC-SIGN, can modulate IL-2 production from PMA- and anti-CD3-stimulated primary human CD4 T. cells. Int. Immunol. 2005, 17, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Sprokholt, J.K.; Heineke, M.H.; Kaptein, T.M.; van Hamme, J.L.; Geijtenbeek, T.B.H. DCs facilitate B cell responses against microbial DNA via DC-SIGN. PLoS ONE 2017, 12, e0185580. [Google Scholar] [CrossRef] [Green Version]
- Simmons, G.; Reeves, J.D.; Grogan, C.C.; Vandenberghe, L.H.; Baribaud, F.; Whitbeck, J.C.; Burke, E.; Buchmeier, M.J.; Soilleux, E.J.; Riley, J.L.; et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 2003, 305, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Pohlmann, S.; Zhang, J.; Baribaud, F.; Chen, Z.; Leslie, G.J.; Lin, G.; Granelli-Piperno, A.; Doms, R.W.; Rice, C.M.; McKeating, J.A. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 2003, 77, 4070–4080. [Google Scholar] [CrossRef] [Green Version]
- Halary, F.; Amara, A.; Lortat-Jacob, H.; Messerle, M.; Delaunay, T.; Houlès, C.; Fieschi, F.; Arenzana-Seisdedos, F.; Moreau, J.F.; Déchanet-Merville, J. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 2002, 17, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Geijtenbeek, T.B.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000, 100, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Geijtenbeek, T.B.; Van Vliet, S.J.; Koppel, E.A.; Sanchez-Hernandez, M.; Vandenbroucke-Grauls, C.M.; Appelmelk, B.; Van Kooyk, Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003, 197, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.P.; Engering, A.; Smits, H.H.; van Vliet, S.J.; van Bodegraven, A.A.; Wirth, H.P.; Kapsenberg, M.L.; Vandenbroucke-Grauls, C.M.; van Kooyk, Y.; Appelmelk, B.J. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 2004, 200, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Koppel, E.A.; Saeland, E.; de Cooker, D.J.; van Kooyk, Y.; Geijtenbeek, T.B. DC-SIGN specifically recognizes Streptococcus pneumoniae serotypes 3 and 14. Immunobiology 2005, 210, 203–210. [Google Scholar] [CrossRef]
- Caparros, E.; Serrano, D.; Puig-Kröger, A.; Riol, L.; Lasala, F.; Martinez, I.; Vidal-Vanaclocha, F.; Delgado, R.; Rodríguez-Fernández, J.L.; Rivas, L.; et al. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology 2005, 210, 185–193. [Google Scholar] [CrossRef]
- van Gisbergen, K.P.; Aarnoudse, C.A.; Meijer, G.A.; Geijtenbeek, T.B.; van Kooyk, Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005, 65, 5935–5944. [Google Scholar] [CrossRef] [Green Version]
- Cameron, P.U.; Freudenthal, P.S.; Barker, J.M.; Gezelter, S.; Inaba, K.; Steinman, R.M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 1992, 257, 383–387. [Google Scholar] [CrossRef]
- Melki, M.T.; Saïdi, H.; Dufour, A.; Olivo-Marin, J.C.; Gougeon, M.L. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathog. 2010, 6, e1000862. [Google Scholar] [CrossRef]
- Cutler, C.W.; Jotwani, R. Dendritic cells at the oral mucosal interface. J. Dent. Res. 2006, 85, 678–689. [Google Scholar] [CrossRef]
- Jotwani, R.; Cutler, C.W. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J. Dent. Res. 2003, 82, 736–741. [Google Scholar] [CrossRef]
- Jotwani, R.; Muthukuru, M.; Cutler, C.W. Increase in HIV receptors/co-receptors/alpha-defensins in inflamed human gingiva. J. Dent. Res. 2004, 83, 371–377. [Google Scholar]
- Jotwani, R.; Palucka, A.K.; Al-Quotub, M.; Nouri-Shirazi, M.; Kim, J.; Bell, D.; Banchereau, J.; Cutler, C.W. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: In situ, in vivo, and in vitro studies. J. Immunol. 2001, 167, 4693–4700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, B.; Zakhary, I.; El-Awady, A.; Scisci, E.; Carrion, J.; O’Neill, J.C.; Rawlings, A.; Stern, J.k.; Susin, C.; Cutler, C.W. Secondary lymphoid organ homing phenotype of human myeloid dendritic cells disrupted by an intracellular oral pathogen. Infect. Immun. 2014, 82, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Bittner-Eddy, P.D.; Fischer, L.A.; Kaplan, D.H. Mucosal Langerhans Cells Promote Differentiation of Th17 Cells in a Murine Model of Periodontitis but Are Not Required for Porphyromonas gingivalis-Driven Alveolar Bone Destruction. J. Immunol. 2016, 15, 1435–1446. [Google Scholar] [CrossRef] [Green Version]
- Romagnani, S. T-cell Subsets (Th1 Versus Th2). (1081-1206 (Print)). Available online: https://www.sciencedirect.com/science/article/abs/pii/S108112061062426X (accessed on 30 December 2019).
- Romagnani, S. Th1/Th2 Cells. (1078-0998 (Print)). Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ibd.3780050410 (accessed on 30 December 2019).
- Yun, P.L.; Decarlo, A.A.; Collyer, C.; Hunter, N. Hydrolysis of Interleukin-12 by Porphyromonas gingivalis Major Cysteine Proteinases may Affect Local Gamma Interferon Accumulation and the Th1 or Th2 T-Cell Phenotype in Periodontitis. (0019-9567 (Print)). Available online: https://iai.asm.org/content/69/9/5650.long (accessed on 30 December 2019).
- Garlet, G.P.; Cardoso, C.R.; Mariano, F.S.; Claudino, M.; de Assis, G.F.; Campanelli, A.P.; Avila-Campos, M.J.; Silva, J.S. Regulatory T Cells Attenuate Experimental Periodontitis Progression in Mice. (1600-051X (Electronic)). Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-051X.2010.01586.x (accessed on 30 December 2019).
- Baker, P.J.; Dixon, M.; Evans, R.T.; Dufour, L.; Johnson, E.; Roopenian, D.C. CD4(+) T Cells and the Proinflammatory Cytokines Gamma Interferon and Interleukin-6 Contribute to Alveolar Bone Loss in Mice. (0019-9567 (Print)). Available online: https://europepmc.org/article/PMC/96585 (accessed on 30 December 2019).
- Carrion, J.; Scisci, E.; Miles, B.; Sabino, G.J.; Zeituni, A.E.; Gu, Y.; Bear, A.; Genco, C.A.; Brown, D.L.; Cutler, C.W. Microbial Carriage State of Peripheral Blood Dendritic Cells (DCs) in Chronic Periodontitis Influences DC Differentiation, Atherogenic Potential. J. Immunol. 2012, 189, 3178–3187. [Google Scholar] [CrossRef] [Green Version]
- Samaranayake, L.P. Essential Microbiology for Dentistry, 2nd ed.; Elsevier: Frisco, CO, USA, 2002. [Google Scholar]
- Cutler, C.W.; Kalmar, J.R.; Genco, C.A. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995, 3, 45–51. [Google Scholar] [CrossRef]
- Ezzo, P.J.; Cutler, C.W. Microorganisms as risk indicators for periodontal disease. Periodontol. 2000 2003, 32, 24–35. [Google Scholar] [CrossRef]
- Zeituni, A.E.; Jotwani, R.; Carrion, J.; Cutler, C.W. Targeting of DC-SIGN on human dendritic cells by minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. J. Immunol. 2009, 183, 5694–5704. [Google Scholar] [CrossRef] [Green Version]
- Holt, S.C.; Kesavalu, L.; Walker, S.; Genco, C.A. Virulence factors of Porphyromonas gingivalis. Periodontol. 2000 1999, 20, 168–238. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Sojar, H.T.; Cho, M.I.; Genco, R.J. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect. Immun. 1996, 64, 4788–4794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Lamont, R.J. Promoter architecture of the Porphyromonas gingivalis fimbrillin gene. Infect. Immun. 1999, 67, 3227–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, B.; Scisci, E.; Carrion, J.; Sabino, G.J.; Genco, C.A.; Cutler, C.W. Noncanonical dendritic cell differentiation and survival driven by a bacteremic pathogen. J. Leukoc. Biol. 2013, 94, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Patterson, S.D.; Spahr, C.S.; Daugas, E.; Susin, S.A.; Irinopoulou, T.; Koehler, C.; Kroemer, G. Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ. 2000, 7, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Chinnaiyan, A.M.; O’ Rourke, K.; Tewari, M.; Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995, 81, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef]
- Muzio, M.; Chinnaiyan, A.M.; Kischkel, F.C.; O’Rourke, K.; Shevchenko, A.; Ni, J.; Scaffidi, C.; Bretz, J.D.; Zhang, M.; Gentz, R.; et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell 1996, 85, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Cremer, I.; Dieu-Nosjean, M.C.; Maréchal, S.; Dezutter-Dambuyant, C.; Goddard, S.; Adams, D.; Winter, N.; Menetrier-Caux, C.; Sautès-Fridman, C.; Fridman, W.H.; et al. Long-lived immature dendritic cells mediated by TRANCE-RANK interaction. Blood 2002, 100, 3646–3655. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, Y.H.; Wang, Y.; Huang, L.; Sandova, H.; Liu, Y.J.; Wang, J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 2006, 311, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, N.; Riol-Blanco, L.; de la Rosa, G.; Puig-Kröger, A.; García-Bordas, J.; Martín, D.; Longo, N.; Cuadrado, A.; Cabañas, C.; Corbí, A.L.; et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 2004, 104, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe-Fukunaga, R.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992, 356, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tanaka, M.; Brannan, C.I.; Jenkins, N.A.; Copeland, N.G.; Suda, T.; Nagata, S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 1994, 76, 969–976. [Google Scholar] [CrossRef]
- Madkaikar, M.; Mhatre, S.; Gupta, M.; Ghosh, K. Advances in autoimmune lymphoproliferative syndromes. Eur. J. Haematol. 2011, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.H. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 1995, 81, 935–946. [Google Scholar] [CrossRef] [Green Version]
- Rieux-Laucat, F.; Le Deist, F.; Hivroz, C.; Roberts, I.A.; Debatin, K.M.; Fischer, A.; de Villartay, J.P. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995, 268, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, J.C.; Cooke, M.P.; Ho, W.Y.; Grein, J.; Townsend, S.E.; Davis, M.M.; Goodnow, C.C. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature 1995, 376, 181–184. [Google Scholar] [CrossRef]
- Bouillet, P.; Metcalf, D.; Huang, D.C.; Tarlinton, D.M.; Kay, T.W.; Köntgen, F.; Adams, J.M.; Strasser, A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999, 286, 1735–1738. [Google Scholar] [CrossRef]
- Kotzin, J.J.; Spencer, S.P.; McCright, S.J.; Kumar, D.B.U.; Collet, M.A.; Mowel, W.K.; Elliott, E.N.; Uyar, A.; Makiya, M.A.; Dunagin, M.C.; et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 2016, 537, 239–243. [Google Scholar] [CrossRef]
- Meghil, M.M.; Tawfik, O.K.; Elashiry, M.; Rajendran, M.; Arce, R.M.; Fulton, D.J.; Schoenlein, P.V.; Cutler, C.W. Disruption of Immune Homeostasis in Human Dendritic Cells via Regulation of Autophagy and Apoptosis by Porphyromonas gingivalis. (1664-3224 (Electronic)). Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2019.02286/full (accessed on 30 December 2019).
- Xiao, W.; Dong, G.; Pacios, S.; Alnammary, M.; Barger, L.A.; Wang, Y.; Wu, Y.; Graves, D.T. FOXO1 Deletion Reduces Dendritic Cell Function and Enhances Susceptibility to Periodontitis. (1525-2191 (Electronic)). Available online: https://ajp.amjpathol.org/article/S0002-944000013-9/fulltext (accessed on 30 December 2019).
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008, 9, 908–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.L.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimbeni, A.C.; Giordano, F.; Dupont, N.; Grasso, D.; Vaccaro, M.I.; Codogno, P.; Morel, E. ER-plasma membrane contact sites contribute to autophagosome biogenesis by regulation of local PI3P synthesis. Embo J. 2017, 36, 2018–2033. [Google Scholar] [CrossRef]
- Abada, A.; Elazar, Z. Getting ready for building: Signaling and autophagosome biogenesis. EMBO Rep. 2014, 15, 839–852. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Tamura, N.; Kono, N.; Shimanaka, Y.; Arai, H.; Yamamoto, H.; Mizushima, N. Autophagosome formation is initiated at phosphatidylinositol synthase-enriched ER subdomains. Embo J. 2017, 36, 1719–1735. [Google Scholar] [CrossRef]
- Chan, E.Y.; Longatti, A.; McKnight, N.C.; Tooze, S.A. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol. Cell Biol. 2009, 29, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Karanasios, E.; Stapleton, E.; Manifava, M.; Kaizuka, T.; Mizushima, N.; Walker, S.A.; Ktistakis, N.T. Dynamic association of the ULK1 complex with omegasomes during autophagy induction. J. Cell Sci. 2013, 126, 5224–5238. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Morita, E.; Saitoh, T.; Akira, S.; Ktistakis, N.T.; Izumi, T.; Noda, T.; Yoshimori, T. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J. Cell Biol. 2010, 190, 511–521. [Google Scholar] [CrossRef]
- Molejon, M.I.; Ropolo, A.; Re, A.L.; Boggio, V.; Vaccaro, M.I. The VMP1-Beclin 1 interaction regulates autophagy induction. Sci. Rep. 2013, 3, 1055. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Itoh, T.; Omori, H.; Fukuda, M.; Noda, T.; Yoshimori, T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 2008, 19, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Slobodkin, M.R.; Elazar, Z. The Atg8 family: Multifunctional ubiquitin-like key regulators of autophagy. Essays Biochem. 2013, 55, 51–64. [Google Scholar] [PubMed] [Green Version]
- Weidberg, H.; Shpilka, T.; Shvets, E.; Abada, A.; Shimron, F.; Elazar, Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell 2011, 20, 444–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsvik, H.L.; Lamark, T.; Takagi, K.; Larsen, K.B.; Evjen, G.; Øvervatn, A.; Mizushima, T.; Johansen, T. FYCO1 Contains a C-terminally Extended, LC3A/B-preferring LC3-interacting Region (LIR) Motif Required for Efficient Maturation of Autophagosomes during Basal Autophagy. J. Biol. Chem. 2015, 290, 29361–29374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwan, D.G.; Popovic, D.; Gubas, A.; Terawaki, S.; Suzuki, H.; Stadel, D.; Coxon, F.P.; Miranda de Stegmann, D.; Bhogaraju, S.; Maddi, K.; et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell 2015, 57, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. Embo J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 24, 400–406. [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurumurthy, S.; Xie, S.Z.; Alagesan, B.; Kim, J.; Yusuf, R.Z.; Saez, B.; Tzatsosm, A.; Ozsolak, F.; Milos, P.; Ferrari, F.; et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature 2010, 468, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.N.; Chowdhury, R.; Trudel, L.J.; Tee, A.R.; Slack, R.S.; Walker, C.L.; Wogan, G.N. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc. Natl. Acad. Sci. USA 2013, 110, E2950–E2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.C..; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012, 338, 956–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Zou, Z.; Becker, N.; Anderson, M.; Sumpter, R.; Xiao, G.; Kinch, L.; Koduru, P.; Christudass, C.S.; Veltri, R.W.; et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 2013, 154, 1269–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.M.; Seo, M.; Jung, C.H.; Grunwald, D.; Stone, M.; Otto, N.M.; Toso, E.; Ahn, Y.; Kybam, M.; Griffin, T.J.; et al. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy 2018, 14, 584–597. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; An, Z.; Zou, Z.; Sumpter, R.; Su, M.; Zang, X.; Sinha, S.; Gaestel, M.; Levine, B. The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. Elife 2015, 4. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Sun, X.; Xu, D.; Wang, C.; Zhang, Q.; Wang, H.; Luo, W.; Chen, Y1,2.; Chen, H.; et al. AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy 2016, 12, 1447–1459. [Google Scholar] [CrossRef] [Green Version]
- Zalckvar, E.; Berissi, H.; Mizrachy, L.; Idelchuk, Y.; Koren, I.; Eisenstein, M.; Sabanay, H.; Pinkas-Kramarski, R.; Kimchi, A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009, 10, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell. 2009, 20, 1981–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.H.; Jun, C.B.; Ro, S.H.; Kim, Y.M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell. 2009, 20, 1992–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Awady, A.R.; Miles, B.; Scisci, E.; Kurago, Z.B.; Palani, C.D.; Arce, R.M.; Waller, J.L.; Genco, C.A.; Slocum, C.; Manning, M.; et al. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 2015, 10, e1004647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011, 333, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Paludan, C.; Schmid, D.; Landthaler, M.; Vockerodt, M.; Kube, D.; Tuschl, T.; Münz, C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005, 307, 593–596. [Google Scholar] [CrossRef]
- Loi, M.; Müller, A1.; Steinbach, K2.; Niven, J3.; Barreira da Silva, R1.; Paul, P1.; Ligeon, L.A1.; Caruso, A3.; Albrecht, R.A.; Becker, A.C.; et al. Macroautophagy Proteins Control MHC Class I Levels on Dendritic Cells and Shape Anti-viral CD8(+) T Cell Responses. Cell Rep. 2016, 15, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Long, L.; Yang, K.; Guy, C.; Shrestha, S.; Chen, Z.; Wu, C.; Vogel, P.; Neale, G.; Green, D.R.; et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 2016, 17, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoffm, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Cosio, G.; Grinstein, S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 2009, 7, 355–366. [Google Scholar] [CrossRef]
- Xu, Y.; Jagannath, C.; Liu, X.D.; Sharafkhaneh, A.; Kolodziejska, K.E.; Eissa, N.T. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007, 27, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponpuak, M.; Davis, A.S.; Roberts, E.A.; Delgado, M.A.; Dinkins, C.; Zhao, Z.; Virgin, H.W. 4th; Kyei, G.B.; Johansen, T.; Vergne, I.; et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010, 32, 329–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alissafi, T.; Banos, A.; Boon, L.; Sparwasser, T.; Ghigo, A.; Wing, K.; Vassilopoulos, D.; Boumpas, D.; Chavakis, T.; Cadwell, K.; et al. Tregs Restrain Dendritic Cell Autophagy to Ameliorate Autoimmunity. (1558-8238 (Electronic)). Available online: https://www.ncbi.nlm.nih.gov/pubmed/28581446 (accessed on 30 December 2019).
- Weindel, C.G.; Richey, L.J.; Mehta, A.J.; Shah, M.; Huber, B.T. Autophagy in Dendritic Cells and B Cells Is Critical for the Inflammatory State of TLR7-Mediated Autoimmunity. (1550-6606 (Electronic)). Available online: https://www.ncbi.nlm.nih.gov/pubmed/28031336 (accessed on 30 December 2019).
- Shelly, S.; Lukinova, N.; Bambina, S.; Berman, A.; Cherry, S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 2009, 30, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Fux, B.; Goodwin, M.; Dunay, I.R.; Strongm, D.; Miller, B.C.; Cadwell, K.; Delgado, M.A.; Ponpuak, M.; Green, K.G.; et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008, 4, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orvedahl, A.; MacPherson, S.; Sumpter, R., Jr.; Tallóczy, Z.; Zou., Z.; Levine, B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe. 2010, 7, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lian, H.; Zhao, Y.; Kauss, M.A.; Spindel, S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J. Biol. Chem. 2008, 283, 25596–25605. [Google Scholar] [CrossRef] [Green Version]
- Hoyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Elling, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009, 6, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Guillet, A.; Brocard, A.; Bach Ngohou, K.; Graveline, N.; Leloup, A.G.; Ali, D.; Nguyen, J.M.; Loirat, M.J.; Chevalier, C.; Khammari, A.; et al. Verneuil’s disease, innate immunity and vitamin D: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1347–1353. [Google Scholar] [CrossRef]
- Meghil, M.M..; Hutchens, L.; Raed, A.; Multani, N.A.; Rajendran, M.; Zhu, H.; Looney, S.; Elashiry, M.; Arce, R.M.; Peacock, M.E.; et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]

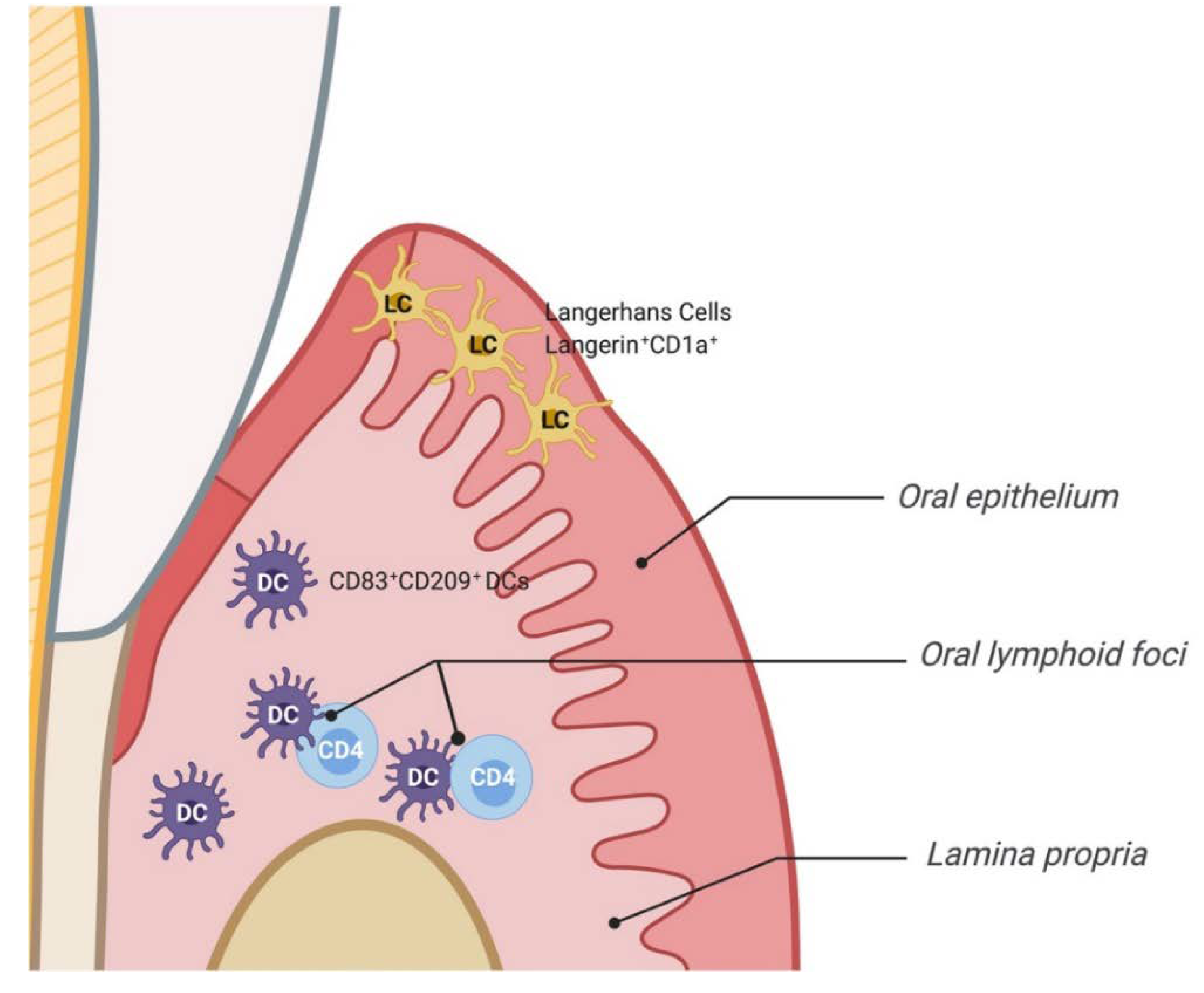
| Mice | Oral Epithelium DCs | Langerin+ -CD11c+ -MHC class II+ -Ep-CAM+ Langerhans cells |
| Lamina propria DCs | D11c+-CD11b+- MHC class II+ interstitial DCs | |
| CD11c+-CD11b+- MHC class II+-CD103+ | ||
| CD11c+-CD11b+- MHC class II+-CD103+-langerin+ CD11c−-CD11b+- MHC class II+ | ||
| CD11c−-CD11b+- MHC class II+ | ||
| Humans | Oral epithelium DCs | Langerin+-CD1a+ Langerhans cells |
| Lamina propria DCs | CD83+-CD209+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meghil, M.M.; Cutler, C.W. Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 1643. https://doi.org/10.3390/ijms21051643
Meghil MM, Cutler CW. Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases. International Journal of Molecular Sciences. 2020; 21(5):1643. https://doi.org/10.3390/ijms21051643
Chicago/Turabian StyleMeghil, Mohamed M., and Christopher W. Cutler. 2020. "Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases" International Journal of Molecular Sciences 21, no. 5: 1643. https://doi.org/10.3390/ijms21051643
APA StyleMeghil, M. M., & Cutler, C. W. (2020). Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases. International Journal of Molecular Sciences, 21(5), 1643. https://doi.org/10.3390/ijms21051643





