Beta3-Tubulin Is Critical for Microtubule Dynamics, Cell Cycle Regulation, and Spontaneous Release of Microvesicles in Human Malignant Melanoma Cells (A375)
Abstract
1. Introduction
2. Results
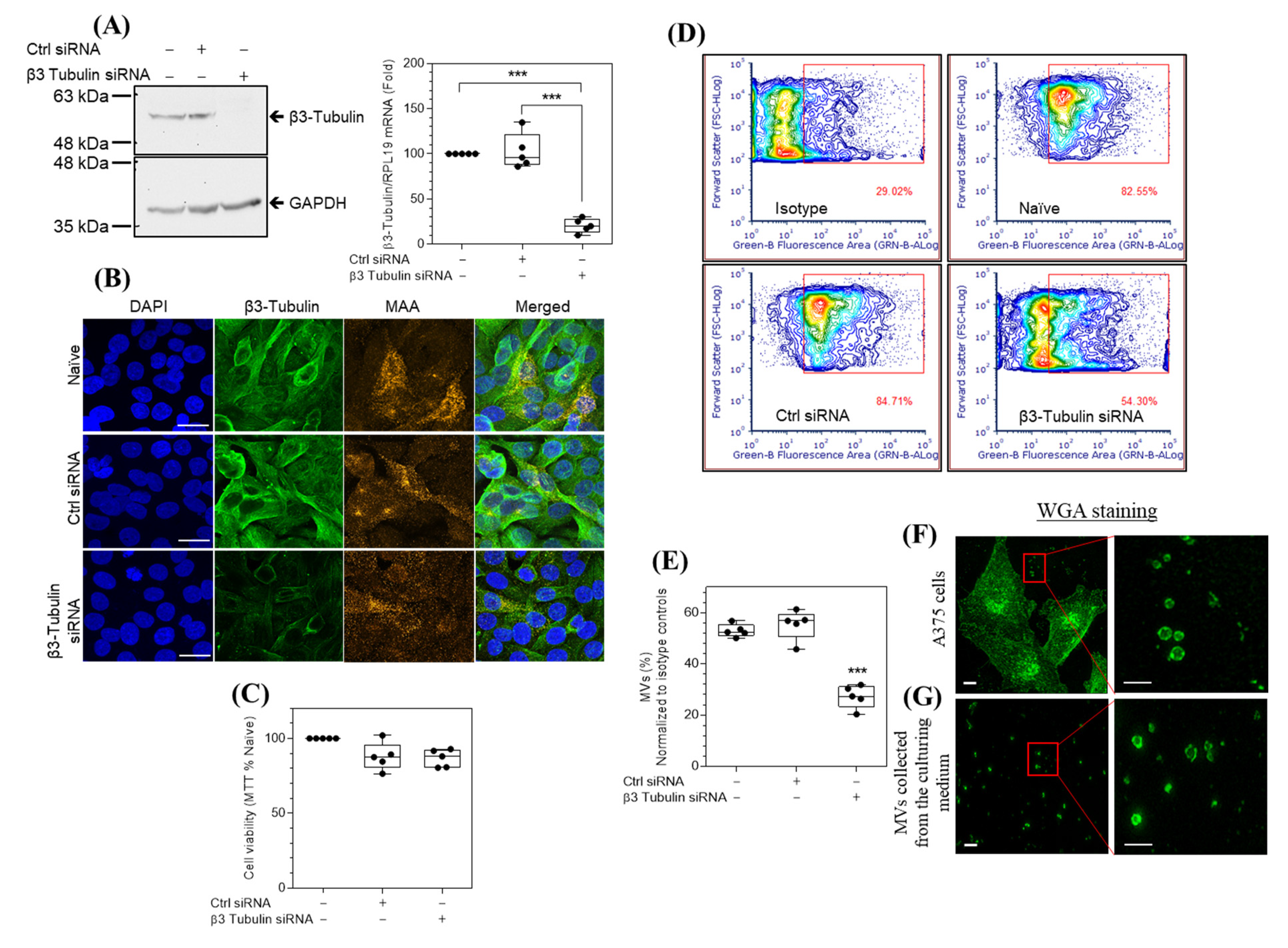
2.1. A375 Cells Express β3-Tubulin mRNA and Protein
2.2. siRNA Knockdown of β3-Tubulin Reduces the Spontaneous Release of MVs by A375 Cells
2.3. siRNA Knockdown of β3-Tubulin Suppresses MTs Dynamics and Induces G2/M Cell Cycle Arrest
3. Discussion
4. Materials and Methods
4.1. Cell Culture EGFP-MAP-4 Transfection and siRNA Silencing
4.2. RNA Isolation, cDNA Synthesis, and qPCR
4.3. Electrophoresis, Western Analysis, and Fluorescent Microscopy
4.4. MVs Purification and Flowcytometric Quantification
4.5. Analysis of Microtubules Dynamics
4.6. Propidium Iodide (PI)-Staining and Cell Cycle Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTs | Microtubules |
| MVs | Microvesicles |
| WGA | Wheat germ agglutinin |
| MAA | Melanoma associated antigen |
References
- Katsetos, C.D.; Herman, M.M.; Mork, S.J. Class III beta-tubulin in human development and cancer. Cell Motil. Cytoskelet. 2003, 55, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Kueh, H.Y.; Mitchison, T.J. Structural plasticity in actin and tubulin polymer dynamics. Science 2009, 325, 960–963. [Google Scholar] [CrossRef]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Karp, G. Cell and Molecular Biology: Concepts and Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 355. [Google Scholar]
- Sugiyama, T.; Pramanik, M.K.; Yumura, S. Microtubule-Mediated Inositol Lipid Signaling Plays Critical Roles in Regulation of Blebbing. PLoS ONE 2015, 10, e0137032. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Yang, H.; Sharma, R.; Patel, K.D.; Cabral, F. The role of microtubules and their dynamics in cell migration. J. Biol. Chem. 2012, 287, 43359–43369. [Google Scholar] [CrossRef] [PubMed]
- Knabbe, J.; Nassal, J.P.; Verhage, M.; Kuner, T. Secretory vesicle trafficking in awake and anaesthetized mice: Differential speeds in axons versus synapses. J. Physiol. 2018, 596, 3759–3773. [Google Scholar] [CrossRef]
- Kobayashi, T.; Okamoto, H.; Yamada, J.-I.; Setaka, M.; Kwan, T. Vesiculation of platelet plasma membranes. Dilauroylglycerophosphocholine-induced shedding of a platelet plasma membrane fraction enriched in acetylcholinesterase activity. Biochim. Biophys. Acta 1984, 778, 210–218. [Google Scholar]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Kunder, C.; John, A.L.S.; Li, G.; Leong, K.W.; Berwin, B.; Staats, H.F.; Abraham, S.N. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 2009, 206, 2455–2467. [Google Scholar] [CrossRef]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef]
- Leroyer, A.; Anfosso, F.; Lacroix, R.; Sabatier, F.; Simoncini, S.; Njock, M.-S.; Jourde, N.; Brunet, P.; Camoin-Jau, L.; Sampol, J.; et al. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb. Haemost. 2010, 104, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.G.; Chammas, R.; Monteiro, R.Q.; Moreira, M.E.C.; Barcinski, M.A. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009, 283, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Laresche, C.; Pelletier, F.; Garnache-Ottou, F.; Lihoreau, T.; Biichlé, S.; Mourey, G.; Saas, P.; Humbert, P.; Seilles, E.; Aubin, F. Increased levels of circulating microparticles are associated with increased procoagulant activity in patients with cutaneous malignant melanoma. J. Investig. Dermatol. 2014, 134, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.; Whiteside, T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Muhsin-Sharafaldine, M.-R.; Saunderson, S.; Dunn, A.C.; Faed, J.M.; Kleffmann, T.; McLellan, A. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget 2016, 7, 56279–56294. [Google Scholar] [CrossRef]
- Orfanidis, K.; Wäster, P.; Lundmark, K.; Rosdahl, I.; Öllinger, K. Evaluation of tubulin beta-3 as a novel senescence-associated gene in melanocytic malignant transformation. Pigment Cell Melanoma Res. 2017, 30, 243–254. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Molina, A.; Nahmias, C. Microtubule-associated tumor suppressors as prognostic biomarkers in breast cancer. Breast Cancer Res. Treat. 2019, 179, 267–273. [Google Scholar] [CrossRef]
- Cirillo, L.; Gotta, M.; Meraldi, P. The Elephant in the Room: The Role of Microtubules in Cancer. Adv. Exp. Med. Biol. 2017, 1002, 93–124. [Google Scholar]
- Dumontet, C.; Duran, G.E.; Steger, K.A.; Murphy, G.L.; Sussman, H.H.; Sikic, B.I. Differential expression of tubulin isotypes during the cell cycle. Cell Motil. Cytoskelet. 1996, 35, 49–58. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Cicchillitti, L.; Penci, R.; Di Michele, M.; Filippetti, F.; Rotilio, D.; Donati, M.B.; Scambia, G.; Ferlini, C. Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol. Cancer 2008, 7, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Yang, H.; Cabral, F. Class III beta-tubulin counteracts the ability of paclitaxel to inhibit cell migration. Oncotarget 2011, 2, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Kamath, K. How do microtubule-targeted drugs work? An overview. Curr. Cancer Drug Targets 2007, 7, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Cabral, F. A ubiquitous beta-tubulin disrupts microtubule assembly and inhibits cell proliferation. Mol. Biol. Cell 2004, 15, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.A.; Kavallaris, M.; Band Horwitz, S. The role of beta-tubulin isotypes in resistance to antimitotic drugs. Biochim. Biophys. Acta 2001, 1471, O1–O9. [Google Scholar]
- Priebe, M.K.; Dewert, N.; Amschler, K.; Erpenbeck, L.; Heinzerling, L.; Schön, M.P.; Seitz, C.S.; Lorenz, V.N. c-Rel is a cell cycle modulator in human melanoma cells. Exp. Dermatol. 2019, 28, 121–128. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, G.; Mao, P.; Zhang, J.; Zhang, L.; Liu, L.; Wang, J.; Owusu, L.; Ren, B.; Tang, Y.; et al. Down-regulation of GADD45A enhances chemosensitivity in melanoma. Sci. Rep. 2018, 8, 4111. [Google Scholar] [CrossRef]
- Shibazaki, M.; Maesawa, C.; Akasaka, K.; Kasai, S.; Yasuhira, S.; Kanno, K.; Nakayama, I.; Sugiyama, T.; Wakabayasi, G.; Masuda, T.; et al. Transcriptional and post-transcriptional regulation of betaIII-tubulin protein expression in relation with cell cycle-dependent regulation of tumor cells. Int. J. Oncol. 2012, 40, 695–702. [Google Scholar]
- Gan, P.P.; McCarroll, J.A.; Po’Uha, S.T.; Kamath, K.; Jordan, M.A.; Kavallaris, M. Microtubule dynamics, mitotic arrest, and apoptosis: Drug-induced differential effects of betaIII-tubulin. Mol. Cancer 2010, 9, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Castellana, D.; Toti, F.; Freyssinet, J.M. Membrane microvesicles: Macromessengers in cancer disease and progression. Thromb. Res. 2010, 125 (Suppl. 2), S84–S88. [Google Scholar] [CrossRef]
- Pelletier, F.; Garnache-Ottou, F.; Angelot, F.; Biichlé, S.; Vidal, C.; Humbert, P.; Saas, P.; Seilles, E.; Aubin, F. Increased levels of circulating endothelial-derived microparticles and small-size platelet-derived microparticles in psoriasis. J. Investig. Dermatol. 2011, 131, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Liepins, A. Possible role of microtubules in tumor cell surface membrane shedding, permeability, and lympholysis. Cell. Immunol. 1983, 76, 120–128. [Google Scholar] [CrossRef]
- Downing, K.H.; Nogales, E. New insights into microtubule structure and function from the atomic model of tubulin. Eur. Biophys. J. 1998, 27, 431–436. [Google Scholar] [CrossRef]
- Nogales, E. Structural insights into microtubule function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef]
- Gierke, S.; Kumar, P.; Wittmann, T. Analysis of microtubule polymerization dynamics in live cells. Methods Cell Biol. 2010, 97, 15–33. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altonsy, M.O.; Ganguly, A.; Amrein, M.; Surmanowicz, P.; Li, S.S.; Lauzon, G.J.; Mydlarski, P.R. Beta3-Tubulin Is Critical for Microtubule Dynamics, Cell Cycle Regulation, and Spontaneous Release of Microvesicles in Human Malignant Melanoma Cells (A375). Int. J. Mol. Sci. 2020, 21, 1656. https://doi.org/10.3390/ijms21051656
Altonsy MO, Ganguly A, Amrein M, Surmanowicz P, Li SS, Lauzon GJ, Mydlarski PR. Beta3-Tubulin Is Critical for Microtubule Dynamics, Cell Cycle Regulation, and Spontaneous Release of Microvesicles in Human Malignant Melanoma Cells (A375). International Journal of Molecular Sciences. 2020; 21(5):1656. https://doi.org/10.3390/ijms21051656
Chicago/Turabian StyleAltonsy, Mohammed O., Anutosh Ganguly, Matthias Amrein, Philip Surmanowicz, Shu Shun Li, Gilles J. Lauzon, and P. Régine Mydlarski. 2020. "Beta3-Tubulin Is Critical for Microtubule Dynamics, Cell Cycle Regulation, and Spontaneous Release of Microvesicles in Human Malignant Melanoma Cells (A375)" International Journal of Molecular Sciences 21, no. 5: 1656. https://doi.org/10.3390/ijms21051656
APA StyleAltonsy, M. O., Ganguly, A., Amrein, M., Surmanowicz, P., Li, S. S., Lauzon, G. J., & Mydlarski, P. R. (2020). Beta3-Tubulin Is Critical for Microtubule Dynamics, Cell Cycle Regulation, and Spontaneous Release of Microvesicles in Human Malignant Melanoma Cells (A375). International Journal of Molecular Sciences, 21(5), 1656. https://doi.org/10.3390/ijms21051656




