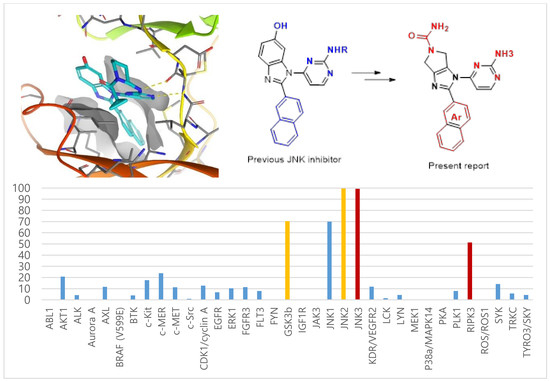
3.1. Chemistry
3.1.1. General Chemical Methods
All chemicals were of reagent grade and were purchased from Sigma-Aldrich, Inc. (Seoul, Korea). Separation of the compounds by column chromatography was carried out with silica gel 60 (200–300 mesh ASTM, E. Merck, Darmstadt, Germany). The quantity of silica gel used was 50–100 times the weight charged on the column. Thin layer chromatography (TLC) was run on silica gel-coated aluminum sheets (silica gel 60 GF254, E. Merck, Darmstadt, Germany) and visualized under ultraviolet (UV) light (254 nm). Both 1H Nuclear Magnetic Resonance (NMR )and 13C NMR spectra were recorded on a Bruker model digital AVANCE III 400 MHz spectrometer (Billerica, MA, USA) at 25 °C using tetramethylsilane (TMS) as an internal standard. High-resolution Mass Spectra (HR/MS) experiments were conducted with a Finnigan LTQ Orbitrap mass spectrometer (Thermo Fisher Scientific Inc., New York, NY, USA) operated in positive-ion electrospray mode.
3.1.2. General Syntheses of tert-Butyl 2, 5-Dihydro-1H-Pyrrole-1-Carboxylate (2)
Compound 1 (4.65 mmol) was dissolved in dimethyl formamide (5 mL), and then sodium hydride (10.23 mmol) was added at 0 °C and stirred for 10 min. Compound 1 was added to the mixture and stirred at room temperature for 15 min, followed by stirring at 65 °C for 4 to 6 h. It was then cooled to ambient temperature, extracted with an organic layer (Ethylacetate:n-Hexane (EA:HEX) = 1:4), and washed with water. This was followed by drying with anhydrous magnesium sulfate and evaporation of the solvent to obtain compound 3 as a yellow oil. (45%); 1H NMR (400 MHz, CDCl3): δ 5.76 (s, 2H), 4.11 (s, 4H), 1.47 (s, 9H); HRMS m/z calcd for C9H15NO2 169.2240; found 170.0130 (M+H+).
3.1.3. General Syntheses of Tert-Butyl (3R, 4S)-3, 4-Dihydroxypyrrolidine-1-Carboxylate (3)
Compound 2 (6.3 mmol) was dissolved in tetrahydrofuran (15.8 mL) and we then slowly dropped the mixture of osmium tetroxide (0.113 mmol) and N-methylmorpholine-N-oxide (8.29 mmol) in 15.8 mL of water. The mixture was stirred at room temperature for 3 to 5 h. It was then concentrated in vacuo, extracted with ethyl acetate, and washed with water. This was followed by drying with anhydrous magnesium sulfate, evaporation of the solvent, and then purification of the product by column chromatography on silica gel using a mobile phase of EA: HEX (3: 1) to obtain compound 3 as a yellow oil. (59%); 1H NMR (400 MHz, CDCl3): δ 4.25 (qd, J = 4.4, 2.1 Hz, 2H), 3.59 (dd, J = 11.4, 5.7 Hz, 2H), 3.34 (dd, J = 11.3, 3.8 Hz, 2H), 1.45 (s, 9H); HRMS m/z calcd for C9H17NO4 203.2380; found 204.4411 (M+H+).
3.1.4. General Syntheses of Tert-Butyl (3R, 4S)-3, 4-Bis((Methylsulfonyl)Oxy)Pyrrolidine-1-Carboxylate (4)
Compound 3 (0.96 mmol) was dissolved in dichloromethane (4.8 mL), and then methanesulfonyl chloride (2.11 mmol) and triethylamine (2.11 mmol) were added and stirred at room temperature for 30 min to 1 h. The reaction mixture was washed with water and brine. This was followed by drying with anhydrous magnesium sulfate and evaporation of the solvent to obtain compound 4 as a white solid. (90%); 1H NMR (400 MHz, CDCl3): δ 5.17 (t, J = 4.0 Hz, 2H), 3.84–3.74 (m, 2H), 3.68–3.59 (m, 2H), 3.14 (d, J = 5.6 Hz, 6H), 1.46 (s, 9H); HRMS m/z calcd for C11H21NO8S2 359.4080; found 360.0501 (M+H+).
3.1.5. General Syntheses of Tert-Butyl (3S, 4R)-3,4-Diazidopyrrolidine-1-Carboxylate (5)
Compound 4 (3.3 mmol) was dissolved in dimethylformamide (33 mL), and then sodium azide (33 mmol) was added and the mixture was stirred at 90 °C for 24 h. The mixture was then cooled to ambient temperature, extracted with ethyl acetate, and washed with brine. After drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo, the product was purified by column chromatography on silica gel using a mobile phase of EA:HEX (1:4) to obtain compound 5 as a yellow oil. (79%); 1H NMR (400 MHz, CDCl3): δ 4.08 (d, J = 3.3 Hz, 2H), 3.63 (dd, J = 8.8, 5.5 Hz, 2H), 3.44 (ddd, J = 16.3, 10.8, 4.1 Hz, 2H), 1.46 (s, 9H); HRMS m/z calcd for C9H15N7O2 253.2660; found 254.3558 (M+H+).
3.1.6. General Synthesis of Tert-Butyl (3S, 4R)-3,4-Diaminopyrrolidine-1-Carboxylate (6)
Compound 5 (2.6 mmol) was dissolved in methanol (10.4 mL), and then palladium hydroxide on carbon (0.52 mmol) was added. It was stirred for 4 h at room temperature under hydrogen gas. The mixture was filtered through a celitepad, and the filtrate was concentrated to obtain compound 6 as a yellow oil. (98%); 1H NMR (400 MHz, DMSO-d6): δ 3.26 (dd, J = 10.9, 5.9 Hz, 3H), 3.14 (dq, J = 9.6, 4.8 Hz, 2H), 2.95 (dd, J = 10.6, 4.9 Hz, 2H), 1.38 (s, 9H). HRMS m/z calcd for C9H19N3O2 201.2700; found 202.3284 (M+H+).
3.1.7. General Syntheses of Compounds 7a-d
Tert-Butyl (3aS,6aR)-2-(3,4-Dichlorophenyl)-3a,4,6,6a-Tetrahydropyrrolo-Imidazole-5(1H)-Carboxylate (7a)
Compound 6 (2.69 mmol) and aryl-substituted imidate (2.5 mmol) were dissolved in ethanol (13.4 mL) and stirred at 80 °C for 1 to 2 h. The mixture was cooled to ambient temperature and then concentrated in vacuo. The concentrated mixture was extracted with ethyl acetate and washed with brine. This was followed by drying with anhydrous magnesium sulfate, and the solvent was purified by column chromatography on silica gel using a mobile phase of EA:HEX (3:1) to obtain compound 7a as a white solid. (47%); 1H NMR (400 MHz, MeOD): δ 7.96 (d, J = 2.0 Hz, 1H), 7.71 (dd, J = 8.4, 2.0 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 4.65 (s, 2H), 3.71 (d, J = 12.0 Hz, 2H), 3.55–3.44 (m, 2H), 1.43 (s, 9H); HRMS m/z calcd for C16H19Cl2N3O2 356.2470; found 357.4684 (M+H+).
Tert-Butyl (3aS,6aR)-2-(Naphthalen-2-yl)-3a,4,6,6a-Tetrahydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (7b)
Compound 7b was obtained as a white solid by the same procedure as above. (crude yield: 95%); 1H NMR (400 MHz, DMSO-d6): δ 8.77 (d, J = 1.4 Hz, 1H), 8.18 (d, J = 8.7 Hz, 1H), 8.10–8.03 (m, 3H), 7.74 (ddd, J = 14.4, 7.9, 1.2 Hz, 2H), 4.94 (d, J = 1.4 Hz, 2H), 3.82 (d, J = 12.5 Hz, 2H), 3.45 (d, J = 12.4 Hz, 2H), 3.32 (s, 1H), 1.32 (s, 9H); HRMS m/z calcd for C20H23N3O2 337.4230; found 338.3165 (M+H+).
Tert-Butyl (3aS,6aR)-2-(Benzo[d][1,3]Dioxol-5-yl)-3a,4,6,6a-Tetrahydro Pyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (7c)
Compound 7c was obtained as a white solid by the same procedure as above. (crude yield: 98%); 1H NMR (400 MHz, DMSO-d6): δ 7.68 (d, J = 11.4 Hz, 2H), 7.21 (d, J = 8.1 Hz, 1H), 6.21 (s, 2H), 4.85 (s, 2H), 3.76 (d, J = 12.5 Hz, 2H), 3.39 (d, J = 12.1 Hz, 2H), 3.32 (s, 1H), 1.34 (s, 9H); HRMS m/z calcd for C17H21N3O4 331.3720; found 332.3018 (M+H+).
Tert-Butyl (3aS,6aR)-2-(2,3-Dihydrobenzofuran-5-yl)-3a,4,6,6a-Tetrahyd Ropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (7d)
Compound 7d was obtained as a white solid by the same procedure as above. (crude yield: 98%); 1H NMR (400 MHz, CDCl3): δ 8.15 (s, 1H), 8.08 (d, J = 8.5 Hz, 1H), 6.74 (d, J = 8.5 Hz, 1H), 4.85 (s, 2H), 4.62 (t, J = 8.8 Hz, 2H), 4.01 (d, J = 12.5 Hz, 2H), 3.44 (d, J = 12.1 Hz, 2H), 3.18 (t, J = 8.7 Hz, 2H), 1.39 (s, 9H); HRMS m/z calcd for C18H23N3O3 329.4000; found 330.3653 (M+H+).
3.1.8. General Syntheses of Compounds 8a-d
Tert-Butyl 2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5 (1H)-Carboxylate (8a)
Oxalyl chloride (0.59 mmol) and dimethyl sulfoxide (1.18 mmol) were dissolved in 7 mL of dichloromethane, stirred at −78 °C for 10 min, and then slowly added to compound 7a (0.59 mmol) dissolved in 5 mL of dichloromethane at −78 °C for 30 min. Then, triethylamine (5.9 mmol) was added slowly and stirred at room temperature for 1 h 30 min. The reaction mixture was washed with water and brine, followed by drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo to obtain compound 8a as a white solid. (50%); 1H NMR (400 MHz, Dimethyl sulfoxide-d6 (DMSO-d6): δ 12.82 (d, J = 24.4 Hz, 1H), 8.41 (s, 1H), 8.07 (d, J = 8.6 Hz, 1H), 7.99–7.88 (m, 3H), 7.59–7.48 (m, 2H), 4.50 (s, 2H), 4.33 (d, J = 10.1 Hz, 2H), 1.47 (s, 9H); HRMS m/z calcd for C16H17Cl2N3O2 354.2310; found 355.4510 (M+H+).
Tert-Butyl 2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (8b)
Compound 8b was obtained as a white solid by the same procedure as above. (51%); 1H NMR (400 MHz, DMSO): δ 12.82 (d, J = 24.4 Hz, 1H), 8.41 (s, 1H), 8.07 (d, J = 8.6 Hz, 1H), 7.99–7.88 (m, 3H), 7.59–7.48 (m, 2H), 4.50 (s, 2H), 4.33 (d, J = 10.1 Hz, 2H), 1.47 (s, 9H); HRMS m/z calcd for C20H21N3O2 335.4070; found 336.2996 (M+H+).
Tert-Butyl 2-(Benzo[d][1,3]Dioxol-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (8c)
Compound 8c was obtained as a white solid by the same procedure as above. (50%); 1H NMR (400 MHz, CDCl3): δ 7.32 (dd, J = 10.6, 1.6 Hz, 2H), 6.81 (d, J = 8.0 Hz, 1H), 5.99 (s, 2H), 4.49–4.39 (m, 4H), 1.51 (s, 9H); HRMS m/z calcd for C17H19N3O4 329.3560; found 330.3209 (M+H+).
Tert-Butyl 2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (8d)
Compound 8d was obtained as a white solid by the same procedure as above. (50%); 1H NMR (400 MHz, CDCl3): δ 7.75 (s, 1H), 7.55 (dd, J = 8.3, 1.9 Hz, 1H), 6.78 (d, J = 8.3 Hz, 1H), 4.61 (t, J = 8.8 Hz, 2H), 4.50–4.41 (m, 4H), 3.22 (t, J = 8.7 Hz, 2H), 1.51 (s, 9H); HRMS m/z calcd for C18H21N3O3 327.3840; found 328.3040 (M+H+).
3.1.9. General Syntheses of Compounds 9a–d
Tert-Butyl 2-(3,4-Dichlorophenyl)-1-(2-(Methylthio)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (9a)
Compound 8a (0.75 mol), 4-chloro-2-(methylthio)pyrimidine (0.75 mmol) and cesium carbonate (0.9 mmol) were dissolved in 7.5 mL of N, N-dimethylformamide and stirred for 2 h at 100 °C in a microwave reactor. After 2 h, the reaction mixture was extracted with ethyl acetate and washed with water and brine. After drying over anhydrous magnesium sulfate and concentration, the product was purified by column chromatography on silica gel using a mobile phase of EA:HEX (1:2) to obtain compound 9a as a yellow solid. (50%); 1H NMR (400 MHz, CDCl3) δ 8.28 (dd, J = 7.6, 5.5 Hz, 1H), 8.13 (d, J = 16.5 Hz, 1H), 7.88 (t, J = 8.7 Hz, 3H), 7.62–7.53 (m, 3H), 6.42 (d, J = 5.5 Hz, 1H), 4.86–4.79 (m, 2H), 4.59 (s, 2H), 2.55 (d, J = 3.4 Hz, 3H), 1.54 (s, 9H).; HRMS m/z calcd for C21H21Cl2N5O2S 477.3920, Found 478.4457 (M+H+).
Tert-Butyl 1-(2-(Methylthio)Pyrimidin-4-yl)-2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo [3,4-d]Imidazole-5(1H)-Carboxylate (9b)
Compound 9b was obtained as a yellow solid by the same procedure as above. (22%); 1H NMR (400 MHz, CDCl3): δ 8.28 (dd, J = 7.6, 5.5 Hz, 1H), 8.13 (d, J = 16.5 Hz, 1H), 7.89 (d, J = 9.0 Hz, 3H), 7.62–7.53 (m, 2H), 7.53–7.47 (m, 1H), 6.42 (d, J = 5.5 Hz, 1H), 4.86–4.79 (m, 2H), 4.59 (s, 2H), 2.55 (d, J = 3.4 Hz, 3H), 1.54 (s, 9H); HRMS m/z calcd for C25H25N5O2S 459.5680; found 460.4038 (M+H).
Tert-Butyl 2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-(Methylthio)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (9c)
Compound 9c was obtained as a yellow solid by the same procedure as above. (47%); 1H NMR (400 MHz, CDCl3): δ 8.35 (dd, J = 5.5, 1.1 Hz, 1H), 7.00–6.93 (m, 2H), 6.85 (d, J = 7.9 Hz, 1H), 6.46 (t, J = 5.6 Hz, 1H), 6.04 (s, 2H), 4.83–4.72 (m, 2H), 4.54–4.45 (m, 2H), 2.56 (d, J = 2.1 Hz, 3H), 1.52 (d, J = 1.8 Hz, 9H); HRMS m/z calcd for C22H23N5O4S 453.5170; found 454.3178 (M+H+).
Tert-Butyl 2-(2,3-Dihydrobenzofuran-5-yl)-1-(2-(Methylthio)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (9d)
Compound 9d was obtained as a yellow solid by the same procedure as above. (52%); 1H NMR (400 MHz, CDCl3): δ 8.32 (d, J = 5.5 Hz, 1H), 7.38 (s, 1H), 7.19 (d, J = 8.3 Hz, 1H), 6.80 (d, J = 8.3 Hz, 1H), 6.46 (t, J = 5.5 Hz, 1H), 4.82–4.74 (m, 2H), 4.65 (t, J = 8.8 Hz, 2H), 4.51 (dd, J = 12.7, 9.5 Hz, 2H), 3.24 (t, J = 8.8 Hz, 2H), 2.57 (d, J = 1.3 Hz, 3H), 1.53 (d, J = 1.7 Hz, 9H); HRMS m/z calcd for C23H25N5O3S 454.5450; found 452.6696 (M+H+).
3.1.10. General Syntheses of Compounds 11a–d
Tert-Butyl (S)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (11a)
Compound 9a (0.16 mmol) was dissolved in 2 mL of methanol, and then potassium peroxomonosulfate (0.8 mmol) dissolved in 2 mL of water was added, followed by stirring at ambient temperature for 2 h. Methanol was concentrated and extracted with ethyl acetate and washed with water and brine, followed by drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo to obtain compound 10a as a white solid. Next, (S)-(3-aminopiperidin-1-yl)(cyclopropyl)methanone (0.32 mmol) was dissolved in 1 mL of dimethylformamide, and 44.5 µL (0.32 mmol) of triethylamine were added. Then, compound 10a (0.14 mmol) dissolved in 3 mL of tetrahydrofuran was added and stirred at 80 °C for 24 h. Tetrahydrofuran was concentrated in vacuo, extracted with ethyl acetate, and washed with water and brine. After drying over anhydrous magnesium sulfate and concentration, the product was purified by column chromatography on silica gel using a mobile phase of EA:HEX (5:1) to obtain compound 11a as a yellow solid. (48%); 1H NMR (400 MHz, CDCl3): δ 8.20 (s, 1H), 7.66 (s, 1H), 7.47 (d, J = 8.3 Hz, 1H), 7.35–7.27 (m, 1H), 6.13 (d, J = 5.4 Hz, 1H), 4.71 (d, J = 33.9 Hz, 2H), 4.49 (d, J = 25.9 Hz, 2H), 3.75 (d, J = 31.6 Hz, 5H), 1.79–1.59 (m, 5H), 1.52 (d, J = 2.8 Hz, 9H), 1.01 (s, 2H), 0.77 (s, 2H); HRMS m/z calcd for C29H33Cl2N7O3 598.5290; found 599.5036 (M+H+).
Tert-Butyl (S)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (11b)
Compound 11b was obtained as a yellow solid by the same procedure as above. (38%); 1H NMR (400 MHz, MeOD): δ 8.34–8.26 (m, 1H), 7.76–7.70 (m, 1H), 7.62 (dd, J = 8.3, 1.1 Hz, 1H), 7.41 (ddd, J = 8.2, 4.0, 2.0 Hz, 1H), 6.31 (d, J = 5.2 Hz, 1H), 4.77 (d, J = 12.9 Hz, 2H), 4.48 (d, J = 2.6 Hz, 2H), 3.96–3.54 (m, 4H), 2.32–2.03 (m, 4H), 1.56 (d, J = 2.5 Hz, 9H), 0.94–0.85 (m, 4H); HRMS m/z calcd for C33H37N7O3 579.2958; found 580.5351 (M+H).
Tert-Butyl (S)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (11c)
Compound 11c was obtained as a yellow solid by the same procedure as above. (52%); 1H NMR (400 MHz, CDCl3): δ 8.11 (s, 1H), 6.98–6.88 (m, 2H), 6.81 (d, J = 8.0 Hz, 1H), 6.02 (d, J = 8.7 Hz, 3H), 4.69 (d, J = 27.0 Hz, 2H), 4.46 (d, J = 26.1 Hz, 2H), 3.97–3.52 (m, 5H), 1.82–1.56 (m, 5H), 1.50 (d, J = 2.4 Hz, 9H), 1.02–0.92 (m, 2H), 0.76 (d, J = 3.2 Hz, 2H); HRMS m/z calcd for C30H35N7O5 573.6540; found 574.4855 (M+H).
Tert-Butyl (S)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (11d)
Compound 11d was obtained as a yellow solid by the same procedure as above. (52%); 1H NMR (400 MHz, CDCl3): δ 8.09 (s, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.17 (s, 1H), 6.76 (d, J = 8.3 Hz, 1H), 6.07 (s, 1H), 4.71 (d, J = 29.1 Hz, 2H), 4.62 (t, J = 8.8 Hz, 2H), 4.47 (d, J = 26.7 Hz, 2H), 3.70 (dd, J = 91.3, 72.0 Hz, 6H), 3.22 (t, J = 8.7 Hz, 2H), 1.80–1.56 (m, 5H), 1.51 (d, J = 2.5 Hz, 9H), 1.00 (s, 2H), 0.88 (d, J = 6.5 Hz, 2H); HRMS m/z calcd for C31H37N7O4 571.6820; found 572.2907 (M+H).
3.1.11. General Syntheses of Compounds 12a–d
Tert-Butyl (R)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (12a)
Compound 9a (0.16 mmol) was dissolved in 2 mL of methanol, and then potassium peroxomonosulfate (0.8 mmol) dissolved in 2 mL of water was added, followed by stirring at ambient temperature for 2 h. Methanol was concentrated and extracted with ethyl acetate and washed with water and brine. This was followed by drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo to obtain compound 10a as white solid. Next, (R)-(3-aminopirrolidine-1-yl)(cyclopropyl)methanone (0.32 mmol) was dissolved in 1 mL of dimethylformamide, and 44.5 µl (0.32 mmol) of triethylamine were added. Then, compound 10a (0.14 mmol) dissolved in 3 mL of tetrahydrofuran was added and stirred at 80 °C for 24 h. Tetrahydrofuran was concentrated in vacuo, extracted with ethyl acetate, and washed with water and brine. After drying over anhydrous magnesium sulfate and concentration, the product was purified by column chromatography on silica gel using a mobile phase of EA: HEX (5: 1) to obtain compound 12a as a yellow solid. (38%); 1H NMR (400 MHz, MeOD): δ 8.34–8.26 (m, 1H), 7.76–7.70 (m, 1H), 7.62 (dd, J = 8.3, 1.1 Hz, 1H), 7.41 (ddd, J = 8.2, 4.0, 2.0 Hz, 1H), 6.31 (d, J = 5.2 Hz, 1H), 4.77 (d, J = 12.9 Hz, 2H), 4.48 (d, J = 2.6 Hz, 2H), 3.96–3.54 (m, 4H), 2.32–2.03 (m, 4H), 1.56 (d, J = 2.5 Hz, 9H), 0.94–0.85 (m, 4H); HRMS m/z calcd for C28H31Cl2N7O3 584.5020; found 585.3686 (M+H).
Tert-Butyl (R)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (12b)
Compound 12b was obtained as a yellow solid by the same procedure as above. (30%); 1H NMR (400 MHz, CDCl3): δ 8.14–8.00 (m, 2H), 7.85 (d, J = 7.9 Hz, 3H), 7.58–7.49 (m, 3H), 6.16–6.03 (m, 1H), 4.77 (d, J = 16.4 Hz, 2H), 4.53 (d, J = 26.8 Hz, 2H), 3.79–3.50 (m, 4H), 2.18 (d, J = 13.6 Hz, 4H), 1.53 (s, 9H), 1.00 (s, 2H), 0.78 (d, J = 5.1 Hz, 2H); HRMS m/z calcd for C32H35N7O3 565.6780; found 566.2753 (M+H).
Tert-Butyl (R)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclopropanecarbonyl) Pyrrolidine-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (12c)
Compound 12c was obtained as a yellow solid by the same procedure as above. (44%); 1H NMR (400 MHz, CDCl3): δ 8.11 (dd, J = 11.3, 5.3 Hz, 1H), 6.98–6.92 (m, 2H), 6.81 (dd, J = 8.0, 5.6 Hz, 1H), 6.06–6.00 (m, 3H), 4.71 (dd, J = 23.3, 9.4 Hz, 2H), 4.43 (s, 2H), 3.80–3.58 (m, 4H), 2.37–1.95 (m, 4H), 1.52–1.48 (m, 9H), 0.98 (d, J = 4.8 Hz, 2H), 0.78–0.74 (m, 2H); HRMS m/z calcd for C29H33N7O5 559.6270; found 560.4778 (M+H).
Tert-Butyl (R)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (12d)
Compound 12d was obtained as a yellow solid by the same procedure as above. (48%); 1H NMR (400 MHz, CDCl3): δ 8.09 (dd, J = 12.1, 5.2 Hz, 1H), 7.35 (d, J = 10.3 Hz, 1H), 7.21–7.14 (m, 1H), 6.77 (dd, J = 8.2, 3.7 Hz, 1H), 6.10 (ddd, J = 15.8, 12.2, 5.4 Hz, 1H), 4.82–4.68 (m, 2H), 4.63 (d, J = 8.8 Hz, 2H), 4.43 (s, 2H), 3.99–3.53 (m, 5H), 3.22 (t, J = 8.7 Hz, 2H), 2.39–2.05 (m, 2H), 1.92 (dd, J = 12.5, 6.2 Hz, 1H), 1.53–1.48 (m, 9H), 0.77 (dd, J = 7.8, 4.7 Hz, 4H); HRMS m/z calcd for C30H35N7O4 557.6550; found 558.2751 (M+H).
3.1.12. General Syntheses of Compounds 13a–d
Tert-Butyl (S)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (13a)
Compound 9a (0.16 mmol) was dissolved in 2 mL of methanol, and then potassium peroxomonosulfate (0.8 mmol) dissolved in 2 mL of water was added, followed by stirring at ambient temperature for 2 h. Methanol was concentrated and extracted with ethyl acetate and washed with water and brine, followed by drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo to obtain compound 10a as white solid. (S)-(3-aminopirrolidine-1-yl)(cyclopropyl)methanone (0.32 mmol) was dissolved in 1 mL of dimethylformamide, and 44.5 µL (0.32 mmol) of triethylamine were added. Then, compound 10a (0.14 mmol) dissolved in 3 mL of tetrahydrofuran was added and stirred at 80 °C for 24 h. Tetrahydrofuran was concentrated in vacuo, extracted with ethyl acetate, and washed with water and brine. After drying over anhydrous magnesium sulfate and concentration, the product was purified by column chromatography on silica gel using a mobile phase of EA: HEX (5: 1) to obtain compound 13a as a yellow solid. (32%); 1H NMR (400 MHz, MeOD): δ 8.34–8.26 (m, 1H), 7.76–7.70 (m, 1H), 7.62 (dd, J = 8.3, 1.1 Hz, 1H), 7.41 (ddd, J = 8.2, 4.0, 2.0 Hz, 1H), 6.31 (d, J = 5.2 Hz, 1H), 4.77 (d, J = 12.9 Hz, 2H), 4.48 (d, J = 2.6 Hz, 2H), 3.89–3.48 (m, 4H), 2.25–2.02 (m, 4H), 1.56 (d, J = 2.5 Hz, 9H), 0.96–0.84 (m, 4H); HRMS m/z calcd for C28H31Cl2N7O3 584.5020; found 585.3209 (M+H).
Tert-Butyl (S)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (13b)
Compound 13b was obtained as a yellow solid by the same procedure as above. (30%); 1H NMR (400 MHz, CDCl3): δ 8.10–8.02 (m, 2H), 7.85 (d, J = 7.2 Hz, 3H), 7.56–7.48 (m, 3H), 6.18–6.02 (m, 1H), 4.77 (d, J = 16.3 Hz, 2H), 4.53 (d, J = 27.3 Hz, 2H), 3.76–3.55 (m, 4H), 2.28–2.03 (m, 4H), 1.53 (s, 9H), 1.00–0.95 (m, 2H), 0.77 (d, J = 4.3 Hz, 2H); HRMS m/z calcd for C32H35N7O3 565.6780; found 566.5274 (M+H).
Tert-Butyl (S)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclopropanecarbonyl) Pyrrolidine-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (13c)
Compound 13c was obtained as a yellow solid by the same procedure as above. (26%); 1H NMR (400 MHz, CDCl3): δ 8.18–8.09 (m, 1H), 6.97 (ddd, J = 8.7, 5.3, 1.5 Hz, 2H), 6.83 (d, J = 8.0 Hz, 1H), 6.19–6.06 (m, 1H), 6.02 (d, J = 1.1 Hz, 2H), 4.83–4.64 (m, 2H), 4.57–4.42 (m, 2H), 4.03–3.52 (m, 5H), 2.44–2.07 (m, 2H), 1.99–1.81 (m, 1H), 1.52–1.43 (m, 9H), 1.01 (d, J = 2.4 Hz, 2H), 0.78 (dd, J = 7.5, 4.3 Hz, 2H); HRMS m/z calcd for C29H33N7O5 559.6270; found 560.2543 (M+H).
Tert-Butyl (S)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (13d)
Compound 13d was obtained as a yellow solid by the same procedure as above. (52%); 1H NMR (400 MHz, CDCl3) δ 8.14–8.03 (m, 1H), 7.35 (d, J = 11.0 Hz, 1H), 7.18 (d, J = 7.2 Hz, 1H), 6.78 (dd, J = 8.2, 3.9 Hz, 1H), 6.10 (ddd, J = 15.7, 12.5, 5.5 Hz, 1H), 4.75 (d, J = 1.9 Hz, 2H), 4.62 (d, J = 8.8 Hz, 2H), 4.44 (s, 2H), 3.85–3.53 (m, 5H), 3.21 (d, J = 8.7 Hz, 2H), 2.37–2.07 (m, 2H), 1.93 (dd, J = 12.4, 6.0 Hz, 1H), 1.54–1.49 (m, 9H), 1.00 (d, J = 2.3 Hz, 2H), 0.79–0.74 (m, 2H); HRMS m/z calcd for C30H35N7O4 557.6550; found 558.1865 (M+H).
3.1.13. General Syntheses of Compounds 14a and 14c
Tert-Butyl (S)-1-(2-((1-(Cyclobutanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (14a)
Compound 9a (0.16 mmol) was dissolved in 2 mL of methanol, and then potassium peroxomonosulfate (0.8 mmol) dissolved in 2 mL of water was added, followed by stirring at ambient temperature for 2 h. Methanol was concentrated and extracted with ethyl acetate and washed with water and brine, followed by drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo to obtain compound 10a as white solid. (S)-(3-aminopiperidin-1-yl)(cyclobutyl)methanone (0.32 mmol) was dissolved in 1 mL of dimethylformamide, and 44.5 µl (0.32 mmol) of triethylamine were added. Then, compound 10a (0.14 mmol) dissolved in 3 mL of tetrahydrofuran was added and stirred at 80 °C for 24 h. Tetrahydrofuran was concentrated in vacuo, extracted with ethyl acetate, and washed with water and brine. After drying over anhydrous magnesium sulfate and concentration, the product was purified by column chromatography on silica gel using a mobile phase of EA: HEX (5: 1) to obtain compound 14a as a yellow solid. (49%); 1H NMR (400 MHz, CDCl3): δ 8.25–8.05 (m, 1H), 7.71–7.62 (m, 1H), 7.47 (dd, J = 10.5, 4.8 Hz, 1H), 7.29 (d, J = 7.6 Hz, 1H), 6.16 (d, J = 5.0 Hz, 1H), 4.70 (dd, J = 31.8, 22.8 Hz, 2H), 4.46 (t, J = 17.4 Hz, 2H), 3.97–3.54 (m, 3H), 3.42–3.13 (m, 3H), 2.39–2.09 (m, 4H), 2.00–1.62 (m, 6H), 1.50 (d, J = 2.4 Hz, 9H); HRMS m/z calcd for C30H35Cl2N7O3 612.5560; found 613.3670 (M+H).
Tert-Butyl (S)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclobutanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (14c)
Compound 14c was obtained as a yellow solid by the same procedure as above. (20%); 1H NMR (400 MHz, MeOD): δ 8.28–8.14 (m, 1H), 7.03–6.83 (m, 3H), 6.28 (s, 1H), 6.04 (d, J = 2.2 Hz, 2H), 4.80 (s, 2H), 4.47 (d, J = 12.3 Hz, 2H), 4.06 (d, J = 81.6 Hz, 2H), 3.12–3.02 (m, 1H), 2.94 (s, 1H), 2.15–1.43 (m, 12H); HRMS m/z calcd for C31H37N7O5 587.6810; found 588.5726 (M+H).
3.1.14. General Syntheses of Tert-Butyl (S)-1-(2-((1-(Cyclopentanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxylate (15a)
Compound 9a (0.16 mmol) was dissolved in 2 mL of methanol, and then potassium peroxomonosulfate (0.8 mmol) dissolved in 2 mL of water was added, followed by stirring at ambient temperature for 2 h. Methanol was concentrated and extracted with ethyl acetate and washed with water and brine, followed by drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo to obtain compound 10a as white solid. (S)-(3-aminopiperidin-1-yl)(cyclopentyl)methanone (0.32 mmol) was dissolved in 1 mL of dimethylformamide, and 44.5 µl (0.32 mmol) of triethylamine were added. Then, compound 10a (0.14 mmol) dissolved in 3 mL of tetrahydrofuran was added and stirred at 80 °C for 24 h. Tetrahydrofuran was concentrated in vacuo, extracted with ethyl acetate, and washed with water and brine. After drying over anhydrous magnesium sulfate and concentration, the product was purified by column chromatography on silica gel using a mobile phase of EA: HEX (5: 1) to obtain compound 15a as a yellow solid. (8%); 1H NMR (400 MHz, CDCl3): δ 8.19 (t, J = 30.4 Hz, 1H), 7.68 (s, 1H), 7.51 (dd, J = 13.1, 6.2 Hz, 1H), 7.35–7.28 (m, 1H), 6.22 (s, 1H), 4.85–4.66 (m, 2H), 4.49 (d, J = 20.4 Hz, 2H), 3.80–3.50 (m, 4H), 2.92 (s, 2H), 1.75 (d, J = 38.6 Hz, 12H), 1.51 (d, J = 2.5 Hz, 9H); HRMS m/z calcd for C31H37Cl2N7O3 626.5830; found 627.3383 (M+H).
3.1.15. General Syntheses of Compounds 17a–d, 18a–d, 19a–d, 20a, 20c, and 21a
(S)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (17a)
Compound 11a (0.05 mol) was dissolved in 0.5 mL of 1,4-dioxane, and 0.25 mL of 4 M HCl in 1,4-dioxane was added. The mixture was stirred at ambient temperature for 1 h 30 min. After concentrating 1,4-dioxane in vacuo, compound 16 was obtained. Compound 16 (0.048 mol) was dissolved in 0.48 mL of 1,4-dioxane, and then phenyl carbamate (0.048 mmol) and triethylamine (0.048 mmol) were added, and the mixture was stirred at ambient temperature for 24 h. 1,4-Dioxane was concentrated in vacuo, extracted with ethyl acetate, and washed with water and brine. After drying with anhydrous magnesium sulfate and concentration of the solvent in vacuo, the product was purified by column chromatography on silica gel using a mobile phase of MC:MeOH (10:1) to obtain compound 17a as a white solid. (31%); 1H NMR (400 MHz, MeOD): δ 8.36–8.23 (m, 1H), 7.69 (s, 1H), 7.59 (d, J = 8.2 Hz, 1H), 7.37 (t, J = 6.3 Hz, 1H), 6.33 (d, J = 75.5 Hz, 1H), 4.76 (s, 2H), 4.49 (dd, J = 11.6, 8.5 Hz, 2H), 4.06 (d, J = 60.6 Hz, 2H), 3.40–3.05 (m, 5H), 2.10–1.40 (m, 5H), 0.96–0.53 (m, 4H); HRMS m/z calcd for C25H26Cl2N8O2 541.4370; found 542.4286 (M+H).
(S)-1-(2-((1-(Cyclopropanecarbonyl)piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (17b)
Compound 17b was obtained as a white solid by the same procedure as above. (31%); 1H NMR (400 MHz, MeOD): δ 8.29–8.05 (m, 2H), 7.92 (s, 3H), 7.65–7.39 (m, 3H), 6.26 (d, J = 102.0 Hz, 1H), 4.69 (d, J = 17.1 Hz, 2H), 4.63–4.48 (m, 2H), 4.19–3.72 (m, 2H), 3.29–2.31 (m, 3H), 2.17–1.43 (m, 5H), 0.90 (dt, J = 73.7, 30.3 Hz, 4H); HRMS m/z calcd for C29H30N8O2 522.6130; found 523.4595 (M+H).
(S)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (17c)
Compound 17c was obtained as a white solid by the same procedure as above. (47%); 1H NMR (400 MHz, MeOD): δ 8.28–8.11 (m, 1H), 6.92 (dt, J = 20.5, 4.7 Hz, 3H), 6.34–5.99 (m, 3H), 4.76 (s, 2H), 4.47 (s, 2H), 3.98 (ddd, J = 139.3, 60.2, 35.9 Hz, 3H), 2.14–1.45 (m, 7H), 0.92–0.68 (m, 4H); HRMS m/z calcd for C26H28N8O4 516.5620; found 517.4097 (M+H).
(S)-1-(2-((1-(Cyclopropanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (17d)
Compound 17d was obtained as a white solid by the same procedure as above. (40%); 1H NMR (400 MHz, MeOD): δ 8.24–8.09 (m, 1H), 7.32 (s, 1H), 7.19 (d, J = 7.8 Hz, 1H), 6.78 (d, J = 8.1 Hz, 1H), 6.17 (d, J = 77.2 Hz, 1H), 4.77 (s, 2H), 4.61 (t, J = 8.8 Hz, 2H), 4.48 (d, J = 2.8 Hz, 2H), 4.26–3.52 (m, 3H), 3.23 (dd, J = 10.2, 8.0 Hz, 2H), 2.19–1.43 (m, 6H), 1.31 (dd, J = 12.7, 5.3 Hz, 1H), 0.81 (ddd, J = 94.9, 52.5, 36.9 Hz, 4H); HRMS m/z calcd for C27H30N8O3 514.5900; found 515.4652 (M+H).
(R)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (18a)
Compound 18a was obtained as a white solid by the same procedure as above. (21%); 1H NMR (400 MHz, MeOD): δ 8.29 (dd, J = 10.5, 5.3 Hz, 1H), 7.72 (t, J = 1.8 Hz, 1H), 7.61 (dd, J = 8.3, 2.0 Hz, 1H), 7.42–7.38 (m, 1H), 6.30 (s, 1H), 4.78 (d, J = 3.0 Hz, 2H), 4.50 (s, 2H), 3.90–3.42 (m, 4H), 2.30–1.71 (m, 4H), 0.87 (ddd, J = 10.2, 7.3, 3.4 Hz, 4H); HRMS m/z calcd for C24H24Cl2N8O2 527.4100; found 528.3486 (M+H).
(R)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(Naphthalen-2-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (18b)
Compound 18b was obtained as a white solid by the same procedure as above. (37%); 1H NMR (400 MHz, MeOD): δ 8.26–8.12 (m, 1H), 8.06 (d, J = 3.9 Hz, 1H), 7.96–7.85 (m, 3H), 7.62–7.46 (m, 3H), 6.24 (s, 1H), 4.82 (d, J = 2.4 Hz, 2H), 4.53 (t, J = 3.0 Hz, 2H), 3.99–3.41 (m, 4H), 1.86 (dt, J = 102.9, 33.3 Hz, 4H), 0.96–0.70 (m, 4H); HRMS m/z calcd for C28H28N8O2 508.5860; found 509.4154 (M+H).
(R)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (18c)
Compound 18c was obtained as a white solid by the same procedure as above. (13%); 1H NMR (400 MHz, MeOD): δ 8.21 (dd, J = 11.0, 5.4 Hz, 1H), 6.97 (tt, J = 3.1, 1.4 Hz, 2H), 6.90 (d, J = 8.5 Hz, 1H), 6.18 (s, 1H), 6.04 (dd, J = 2.5, 1.3 Hz, 2H), 4.78 (s, 2H), 4.49 (d, J = 3.0 Hz, 2H), 3.93–3.39 (m, 5H), 2.40–1.73 (m, 3H), 0.87 (dddd, J = 12.5, 9.8, 5.8, 2.2 Hz, 4H); HRMS m/z calcd for C25H26N8O4 502.5350; found 503.4016 (M+H).
(R)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (18d)
Compound 18d was obtained as a white solid by the same procedure as above. (40%); 1H NMR (400 MHz, MeOD): δ 8.17 (dd, J = 12.3, 5.4 Hz, 1H), 7.33 (dd, J = 5.0, 1.3 Hz, 1H), 7.19 (d, J = 8.3 Hz, 1H), 6.79 (dd, J = 8.3, 1.6 Hz, 1H), 6.13 (s, 1H), 4.77 (s, 2H), 4.61 (td, J = 8.8, 2.0 Hz, 2H), 4.48 (d, J = 2.8 Hz, 2H), 4.37 (s, 1H), 3.83 (dddd, J = 29.1, 23.2, 18.4, 16.3 Hz, 2H), 3.68–3.38 (m, 2H), 3.23 (t, J = 8.7 Hz, 2H), 2.36–1.95 (m, 2H), 1.87–1.71 (m, 1H), 0.97–0.76 (m, 4H); HRMS m/z calcd for C26H28N8O3 500.5630; found 501.4210 (M+H).
(S)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (19a)
Compound 19a was obtained as a white solid by the same procedure as above. (35%); 1H NMR (400 MHz, MeOD): δ 8.29 (dd, J = 10.6, 5.3 Hz, 1H), 7.72 (t, J = 1.8 Hz, 1H), 7.61 (dd, J = 8.3, 2.0 Hz, 1H), 7.42–7.37 (m, 1H), 6.31 (s, 1H), 4.77 (d, J = 2.9 Hz, 2H), 4.50 (s, 2H), 3.92–3.46 (m, 4H), 2.30–1.68 (m, 4H), 0.93–0.81 (m, 4H); HRMS m/z calcd for C24H24Cl2N8O2 527.4100; found 528.3846 (M+H).
(S)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (19b)
Compound 19b was obtained as a white solid by the same procedure as above. (27%); 1H NMR (400 MHz, MeOD): δ 8.25–8.13 (m, 1H), 8.09–8.04 (m, 1H), 7.97–7.86 (m, 3H), 7.64–7.47 (m, 3H), 6.24 (s, 1H), 4.82 (d, J = 2.4 Hz, 2H), 4.53 (t, J = 2.9 Hz, 2H), 3.91–3.40 (m, 4H), 2.14–1.50 (m, 4H), 0.93–0.76 (m, 4H); HRMS m/z calcd for C28H28N8O2 508.5860; found 509.4514 (M+H).
(S)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (19c)
Compound 19c was obtained as a white solid by the same procedure as above. (34%); 1H NMR (400 MHz, MeOD): δ 8.21 (dd, J = 11.0, 5.4 Hz, 1H), 7.01–6.95 (m, 2H), 6.90 (d, J = 8.5 Hz, 1H), 6.21 (d, J = 29.0 Hz, 1H), 6.07–6.02 (m, 2H), 4.78 (s, 2H), 4.48 (t, J = 3.0 Hz, 2H), 4.35 (s, 1H), 4.02–3.73 (m, 2H), 3.61–3.41 (m, 2H), 2.36–2.05 (m, 2H), 1.80 (dddd, J = 28.7, 12.7, 7.9, 4.7 Hz, 1H), 0.92–0.77 (m, 4H); HRMS m/z calcd for C25H26N8O4 502.5350; found 503.4160 (M+H).
(S)-1-(2-((1-(Cyclopropanecarbonyl)Pyrrolidin-3-yl)Amino)Pyrimidin-4-yl)-2-(2,3-Dihydrobenzofuran-5-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (19d)
Compound 19d was obtained as a white solid by the same procedure as above. (30%); 1H NMR (400 MHz, MeOD): δ 8.18 (dd, J = 12.2, 5.4 Hz, 1H), 7.34 (d, J = 3.8 Hz, 1H), 7.20 (d, J = 8.3 Hz, 1H), 6.79 (dd, J = 8.3, 1.6 Hz, 1H), 6.13 (s, 1H), 4.78 (s, 2H), 4.61 (td, J = 8.8, 2.0 Hz, 2H), 4.48 (s, 2H), 4.38 (s, 1H), 4.07–3.69 (m, 2H), 3.65–3.38 (m, 2H), 3.23 (t, J = 8.7 Hz, 2H), 2.36–1.95 (m, 2H), 1.79 (dddd, J = 29.6, 12.7, 7.9, 4.7 Hz, 1H), 0.96–0.77 (m, 4H); HRMS m/z calcd for C26H28N8O3 500.5630; found 501.2041 (M+H).
(S)-1-(2-((1-(Cyclobutanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (20a)
Compound 20a was obtained as a white solid by the same procedure as above. (24%); 1H NMR (400 MHz, MeOD): δ 8.40–8.22 (m, 1H), 7.71 (d, J = 15.5 Hz, 1H), 7.61 (dd, J = 8.3, 4.8 Hz, 1H), 7.43–7.35 (m, 1H), 6.39 (d, J = 97.2 Hz, 1H), 4.81 (s, 2H), 4.50 (dd, J = 12.3, 2.9 Hz, 2H), 3.68 (s, 1H), 3.53–3.39 (m, 1H), 3.03 (d, J = 16.4 Hz, 1H), 2.91 (dd, J = 18.5, 8.5 Hz, 1H), 2.42–2.17 (m, 4H), 2.12–1.98 (m, 2H), 1.91–1.71 (m, 3H), 1.66–1.41 (m, 3H).; HRMS m/z calcd for C26H28Cl2N8O2 555.4640; found 556.4531 (M+H).
(S)-2-(Benzo[d][1,3]Dioxol-5-yl)-1-(2-((1-(Cyclobutanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (20c)
Compound 20c was obtained as a white solid by the same procedure as above. (29%); 1H NMR (400 MHz, MeOD): δ 8.28–8.14 (m, 1H), 7.03–6.83 (m, 3H), 6.20 (d, J = 61.5 Hz, 3H), 4.80 (s, 2H), 4.48 (s, 2H), 4.06 (d, J = 81.6 Hz, 2H), 3.13–3.02 (m, 1H), 2.94 (s, 1H), 2.15–1.43 (m, 12H); HRMS m/z calcd for C27H30N8O4 530.5890; found 531.2490 (M+H).
(S)-1-(2-((1-(Cyclopentanecarbonyl)Piperidin-3-yl)Amino)Pyrimidin-4-yl)-2-(3,4-Dichlorophenyl)-4,6-Dihydropyrrolo[3,4-d]Imidazole-5(1H)-Carboxamide (21a)
Compound 21a was obtained as a white solid by the same procedure as above. (68%); 1H NMR (400 MHz, MeOD): δ 8.38–8.24 (m, 1H), 7.71 (d, J = 10.9 Hz, 1H), 7.60 (d, J = 8.3 Hz, 1H), 7.42–7.34 (m, 1H), 6.36 (d, J = 86.1 Hz, 1H), 4.82 (s, 2H), 4.52 (d, J = 9.3 Hz, 2H), 4.21 (s, 1H), 3.97 (d, J = 35.3 Hz, 1H), 3.26–3.04 (m, 2H), 2.87 (s, 2H), 2.06–1.56 (m, 12H); HRMS m/z calcd for C27H30Cl2N8O2 569.4910; found 570.3722 (M+H).
3.1.16. General Synthesis of (S)-(1-(2-((1-(Cyclopropanecarbonyl)piperidin-3-yl)amino)pyrimidin-4-yl)-2-(3,4-dichlorophenyl)-4,6-dihydropyrrolo[3,4-d]Imidazol-5(1H)-yl)(4-hydroxypiperidin-1-yl)methanone (22a)
Compound 11a (0.05 mol) was dissolved in 0.5 mL of 1,4-dioxane, and 0.25 mL of 4 M HCl in 1,4-dioxane was added. The mixture was stirred at ambient temperature for 1 h 30 min. After concentrating 1,4-dioxane in vacuo, compound 16 was obtained. Compound 16 (0.044 mmol) was dissolved in 0.44 mL of 1,4-dioxane and treated with 4-nitrophenyl chloroformate (0.044 mmol) and dimethylformamide (0.22 mL) and stirred at ambient temperature for 1 h. 4-Piperidinol (0.22 mmol) was added and stirred at ambient temperature for 48 h. We concentrated the 1,4-dioxane in vacuo, extracted with ethyl acetate, and washed with water and brine. Drying over anhydrous magnesium sulfate, the solvent was concentrated in vacuo and the product purified using column chromatography on silica gel using a mobile phase of MC: MeOH (10: 1) to afford compound 22a as a white solid. (16%); 1H NMR (400 MHz, MeOD): δ 8.27 (dd, J = 17.3, 5.0 Hz, 1H), 7.70 (d, J = 1.9 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.38 (s, 1H), 6.29 (s, 1H), 4.95 (s, 2H), 4.58 (t, J = 11.4 Hz, 2H), 4.35 (s, 1H), 4.10 (dd, J = 14.3, 7.1 Hz, 1H), 3.82 (ddd, J = 12.9, 8.6, 3.9 Hz, 1H), 3.71 (d, J = 13.7 Hz, 2H), 3.45 (d, J = 28.8 Hz, 1H), 3.00 (dd, J = 66.0, 53.2 Hz, 4H), 1.90 (dd, J = 42.4, 32.4 Hz, 5H), 1.55 (td, J = 12.9, 3.6 Hz, 4H), 0.83 (dd, J = 32.6, 25.9 Hz, 4H); HRMS m/z calcd for C30H34Cl2N8O3 625.5550; found 626.4741 (M+H).