Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice
Abstract
:1. Introduction
2. Results
2.1. Behavioral Testing
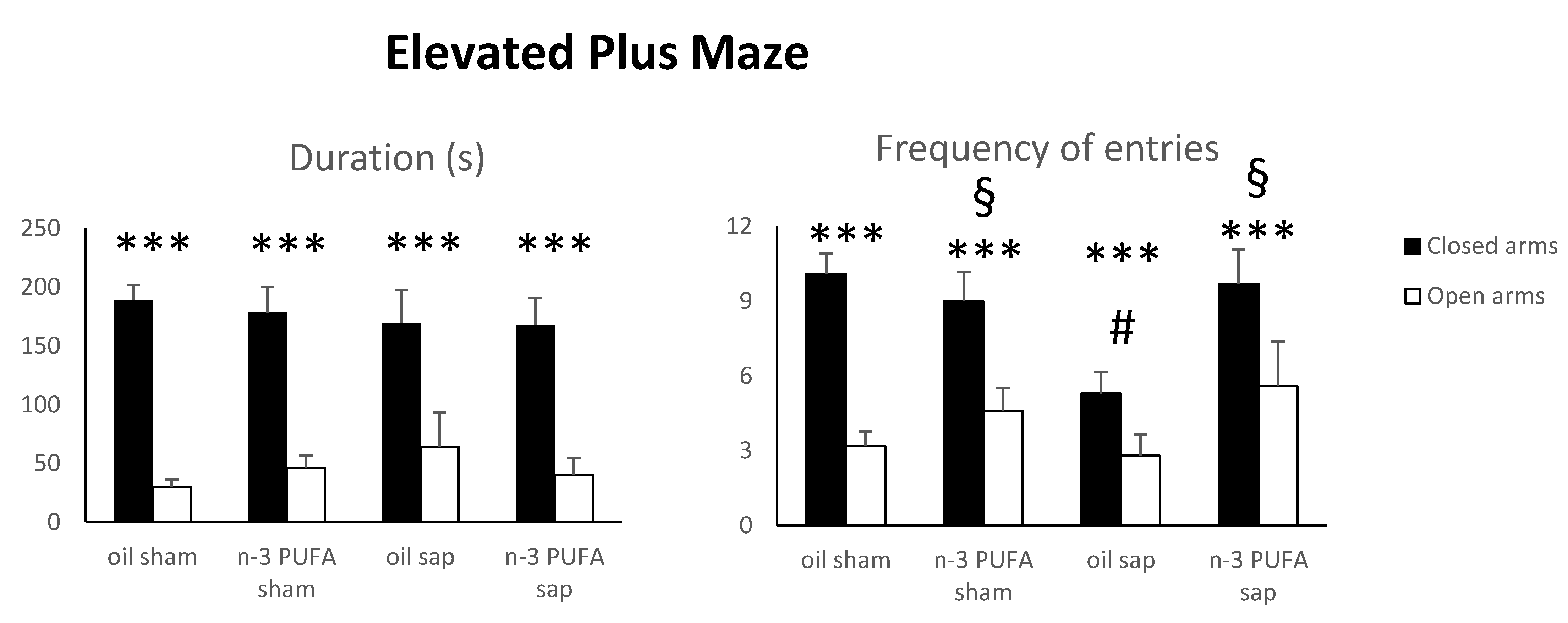
2.1.1. Elevated Plus Maze (EPM)
2.1.2. Splash Test (ST)
2.1.3. Social Interactions (SI)
2.1.4. Hidden Food Test (HFT)
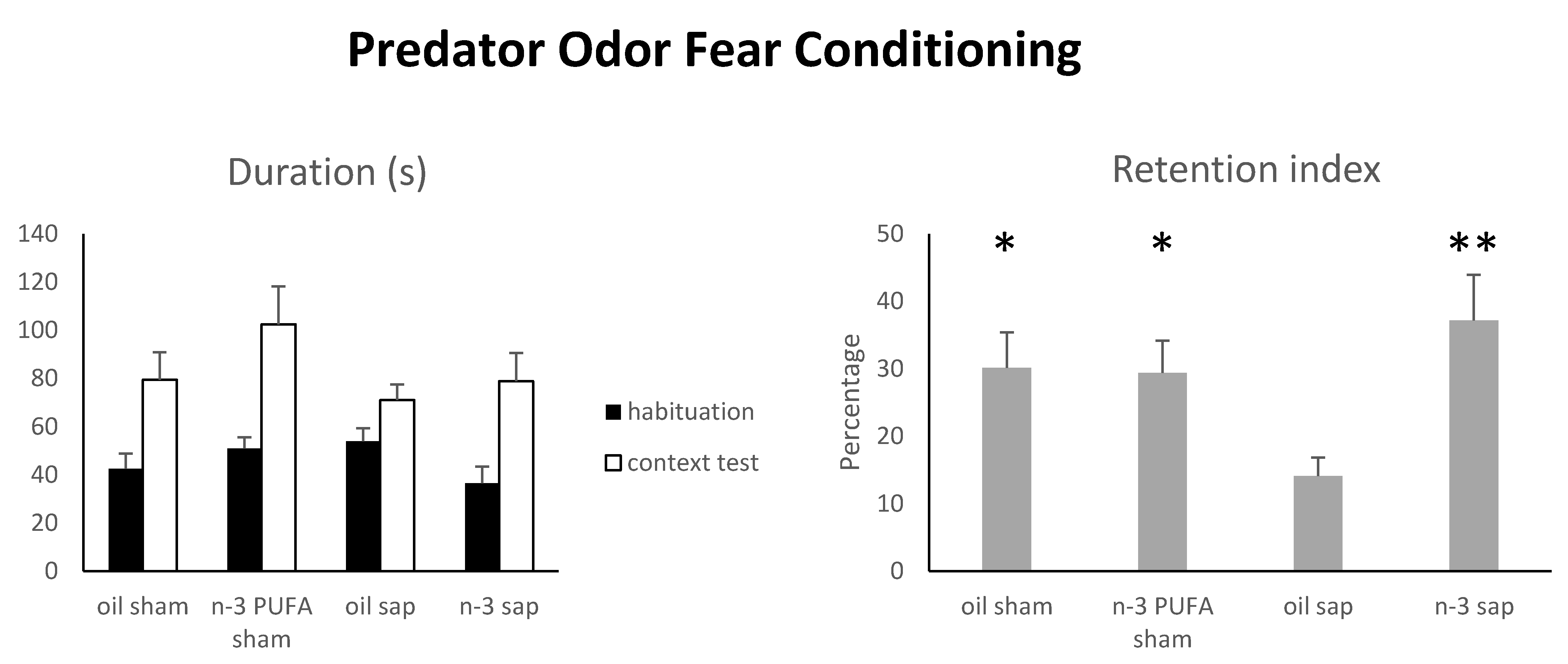
2.1.5. Predator Odor Fear Conditioning (POFC)
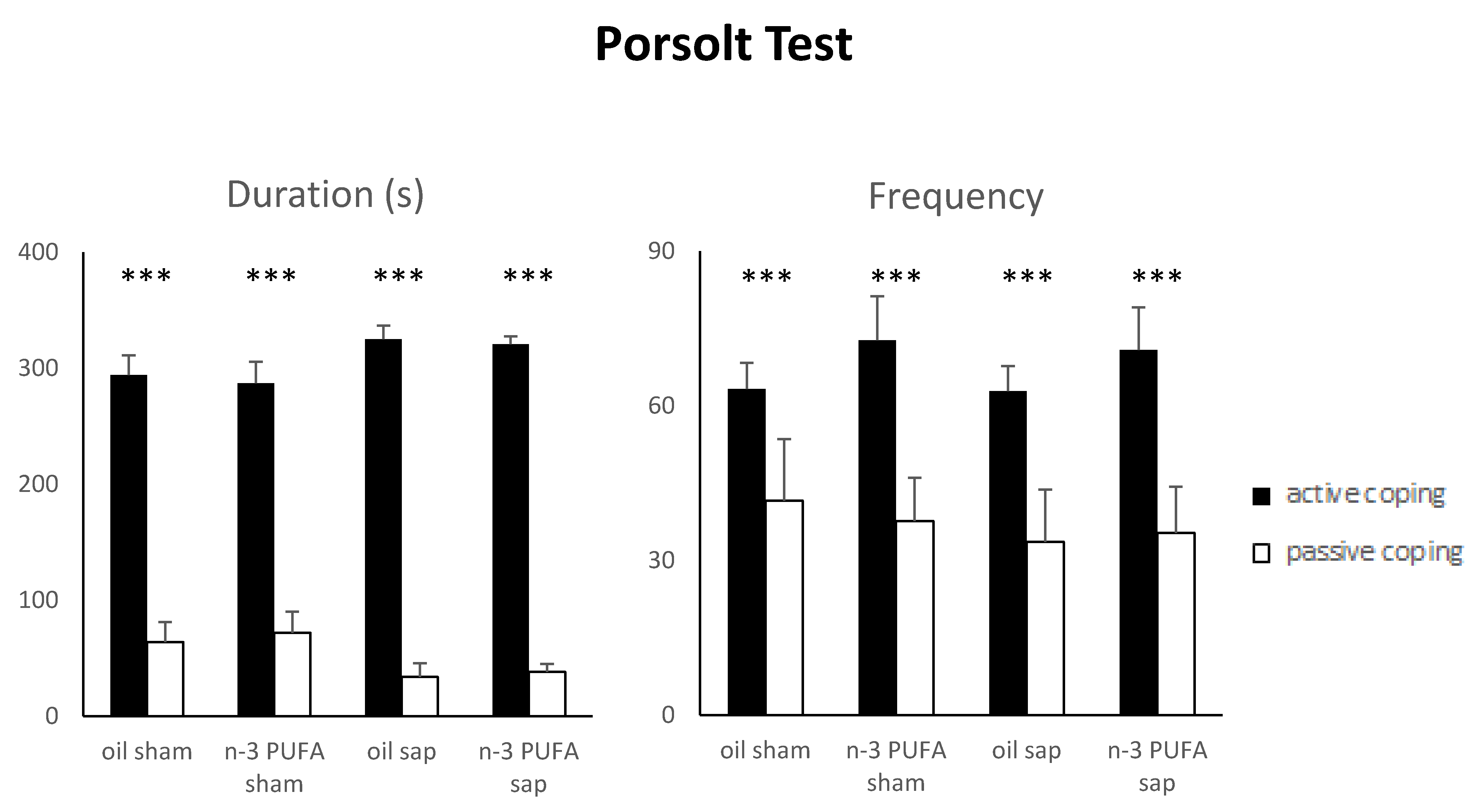
2.1.6. Porsolt Test (PT)
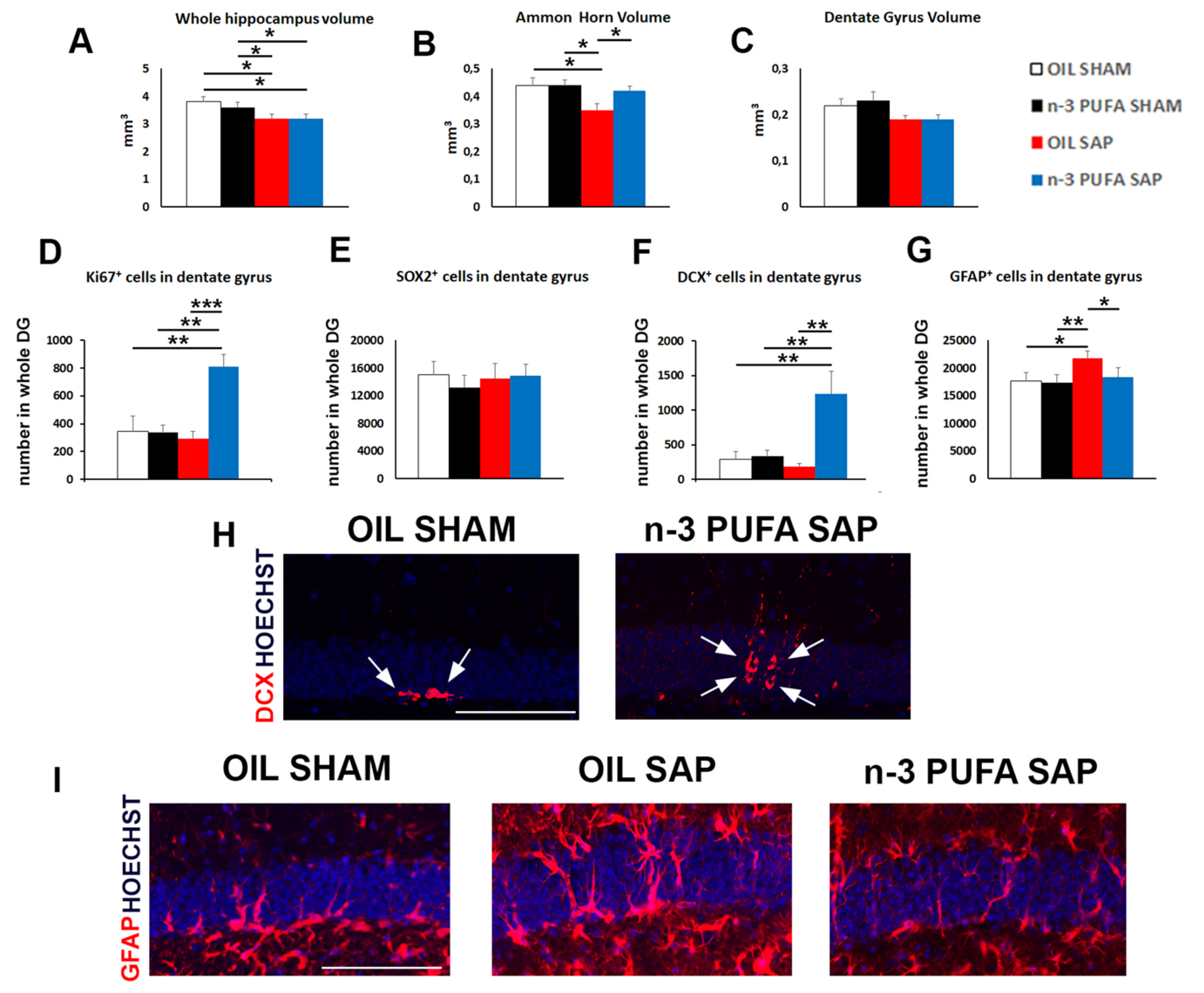
2.2. Morphological Analyses
2.3. Hippocampal Neurogenesis and Astrogliosis
2.3.1. Neural Stem Cells and Proliferation
2.3.2. Hippocampal Astrogliosis
2.3.3. Hippocampal Choline Acetyltransferase (ChAT) and Vesicular Acetylcholine Transporter (VAChT) Expression
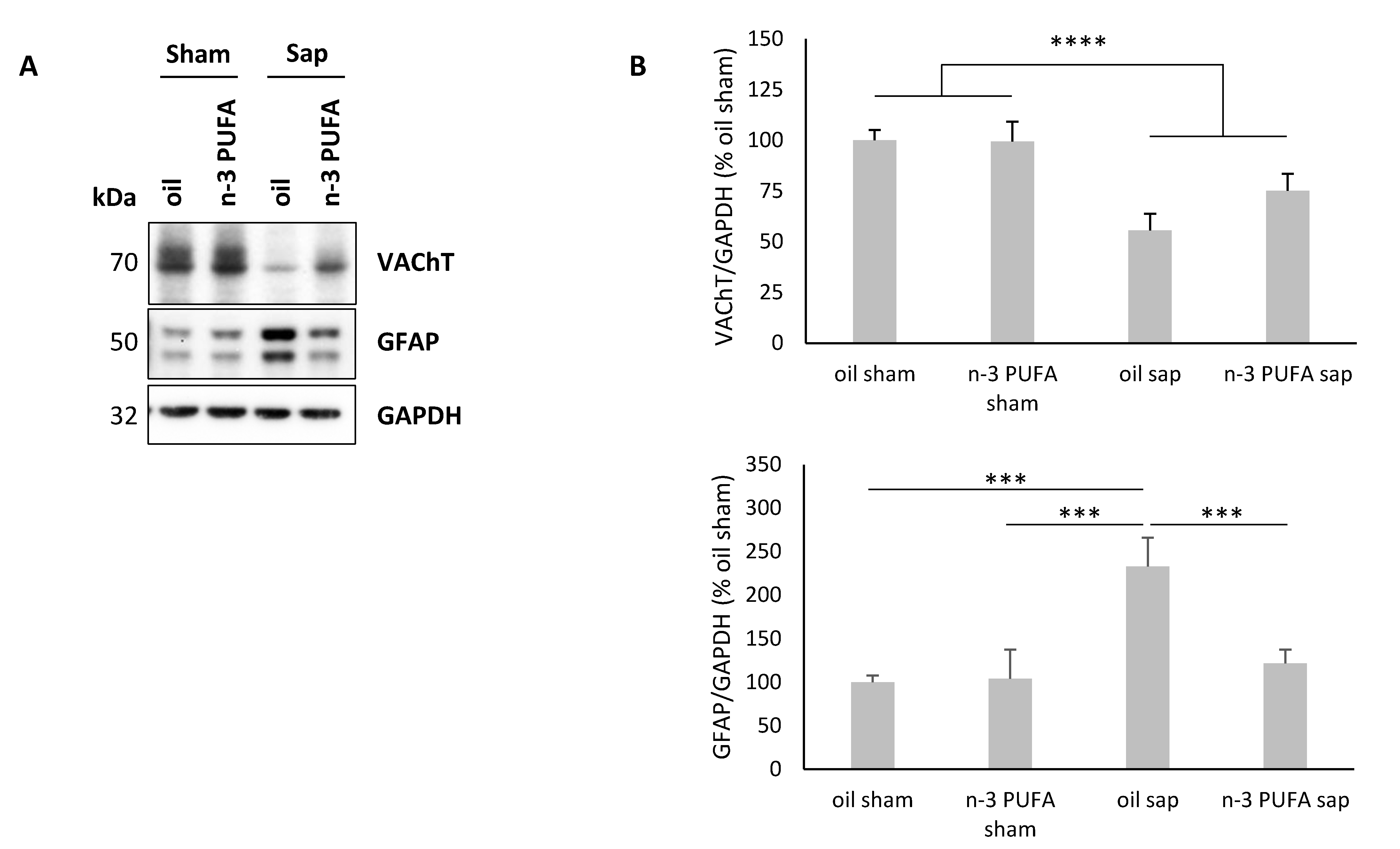
2.4. Western Blot Analysis of Hippocampal VAChT and GFAP Levels
3. Discussion
4. Materials and Methods
4.1. Animals
- -
- sham-lesioned aged mice pre-treated with olive oil (oil sham, n = 12);
- -
- sham-lesioned aged mice pre-treated with n-3 PUFA (n-3 PUFA sham, n = 10);
- -
- mu-p75-saporin-lesioned aged mice pre-treated with olive oil (oil sap, n = 10);
- -
- mu-p75-saporin-lesioned aged mice pre-treated with n-3 PUFA (n-3 PUFA sap, n = 10).
4.1.1. Dietary Manipulations
4.1.2. Lesioning Procedure
4.1.3. Behavioral Testing
4.1.4. Elevated Plus Maze
4.1.5. Splash Test
4.1.6. Social Interactions
4.1.7. Hidden Food Test
4.1.8. Predator Odor Fear Conditioning
4.1.9. Porsolt Test
- -
- passive behaviors: immobility (total absence of movement); paddling (small movements of one of the posterior paws not producing displacement);
- -
- active behaviors: swimming (large and horizontal movements of the paws leading to displacement of the body around the cylinder); climbing (vigorous vertical movements of the forepaws, directed against the wall of the tank, leading to displacement of the body around the cylinder).
4.1.10. Morphological and Biochemical Analyses
4.2. Histology
Morphological Analyses
4.3. Western Blot Analysis
4.3.1. Total Protein Extraction
4.3.2. Immunoblotting Analysis
4.3.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Denis, I.; Potier, B.; Vancassel, S.; Heberden, C.; Lavialle, M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Res. Rev. 2013, 12, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Maruszak, A.; Pilarski, A.; Murphy, T.; Branch, N.; Thuret, S. Hippocampal neurogenesis in Alzheimers disease: Is there a role for dietary modulation? J. Alzheimers Dis. 2014, 38, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S. The macroeconomics of dementia—Will the world economy get Alzheimer’s disease? Arch. Med. Res. 2012, 43, 705–709. [Google Scholar] [CrossRef]
- Sengoku, R. Aging and Alzheimer’s disease pathology. Neuropathology 2019. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 3 January 2020).
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., III; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.H.; Cosquer, B.; Jeltsch, H.; Cassel, J.C.; Mathis, C. Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus 2008, 18, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Nag, N.; Baxter, M.G.; Berger-Sweeney, J.E. Efficacy of a murine-p75-saporin immunotoxin for selective lesions of basal forebrain cholinergic neurons in mice. Neurosci. Lett. 2009, 452, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Petrosini, L.; De Bartolo, P.; Cutuli, D.; Gelfo, F. Perinatal 192 IgG-Saporin as neuroteratogen. Curr. Top. Behav. Neurosci. 2016, 29, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Leanza, G.; Nilsson, O.G.; Nikkhah, G.; Wiley, R.G.; Björklund, A. Effects of neonatal lesions of the basal forebrain cholinergic system by 192 immunoglobulin G-saporin: Biochemical, behavioural and morphological characterization. Neuroscience 1996, 74, 119–141. [Google Scholar] [CrossRef]
- Berger-Sweeney, J.; Stearns, N.A.; Murg, S.L.; Floerke-Nashner, L.R.; Lappi, D.A.; Baxter, M.G. Selective immunolesions of cholinergic neurons in mice: Effects on neuroanatomy, neurochemistry, and behavior. J. Neurosci. 2001, 21, 8164–8173. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Denis, I.; Potier, B.; Heberden, C.; Vancassel, S. Omega-3 polyunsaturated fatty acids and brain aging. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Calder, P.C.; Reed, S.C.; Simpson, M.J.A. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 2005, 18, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Appleton, K.M.; Rogers, P.J.; Ness, A.R. Is there a role for n-3 long-chain polyunsaturated fatty acids in the regulation of mood and behaviour? A review of the evidence to date from epidemiological studies, clinical studies and intervention trials. Nutr. Res. Rev. 2008, 21, 13–41. [Google Scholar] [CrossRef] [Green Version]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutuli, D. Functional and structural benefits induced by omega-3 polyunsaturated fatty acids during aging. Curr. Neuropharmacol. 2017, 15, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujiguchi, H.; Thi Thu Nguyen, T.; Goto, D.; Miyagi, S.; Kambayashi, Y.; Hara, A.; Yamada, Y.; Nakamura, H.; Shimizu, Y.; Hori, D.; et al. Relationship between the intake of n-3 polyunsaturated fatty acids and depressive symptoms in elderly Japanese people: Differences according to sex and weight status. Nutrients 2019, 11, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J. Nutr. Biochem. 2010, 21, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehuda, S. Polyunsaturated fatty acids as putative cognitive enhancers. Med. Hypotheses 2012, 79, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bertoni-Freddari, C.; Fattoretti, P.; Casoli, T.; Caselli, U.; Meier-Ruge, W. Deterioration threshold of synaptic morphology in aging and senile dementia of Alzheimer’s type. Anal. Quant. Cytol. Histol. 1996, 18, 209–213. [Google Scholar]
- Geinisman, Y.; de Toledo-Morrell, L.; Morrell, F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res. 1986, 398, 266–275. [Google Scholar] [CrossRef]
- Geinisman, Y.; de Toledo-Morrell, L.; Morrell, F. Loss of perforated synapses in the dentate gyrus: Morphological substrate of memory deficit in aged rats. Proc. Natl. Acad. Sci. USA 1986, 83, 3027–3031. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, I.; Howard, S.R.; Stone, J.C.; Monfils, M.H.; Tomanek, B.; Brooks, W.M.; Sutherland, R.J. The aging hippocampus: A multi-level analysis in the rat. Neuroscience 2006, 139, 1173–1185. [Google Scholar] [CrossRef] [Green Version]
- Lynch, A.M.; Loane, D.J.; Minogue, A.M.; Clarke, R.M.; Kilroy, D.; Nally, R.E.; Roche, O.J.; O’Connell, F.; Lynch, M.A. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol. Aging 2007, 28, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.; Grehan, B.; Chiesa, A.D.; O’Mara, S.M.; Downer, E.; Sahyoun, G.; Massey, K.A.; Nicolaou, A.; Lynch, M.A. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol. Aging 2011, 32, 2318. [Google Scholar] [CrossRef]
- O’Donnell, E.; Vereker, E.; Lynch, M.A. Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: Analysis of underlying mechanisms. Eur. J. Neurosci. 2000, 12, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.; Nally, R.; Nolan, Y.; McCartney, Y.; Linden, J.; Lynch, M.A. The age-related attenuation in long-term potentiation is associated with microglial activation. J. Neurochem. 2006, 99, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Minogue, A.M.; Schmid, A.W.; Fogarty, M.P.; Moore, A.C.; Campbell, V.A.; Herron, C.E.; Lynch, M.A. Activation of the c-Jun Nterminal kinase signaling cascade mediates the effect of amyloid-beta on long term potentiation and cell death in hippocampus: A role for interleukin-1beta? J. Biol. Chem. 2003, 278, 27971–27980. [Google Scholar] [CrossRef] [Green Version]
- Fedorova, I.; Salem, N., Jr. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 271–289. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Pasker-de Jong, P.C.; de Vries, R.B.; Ritskes-Hoitinga, M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimers pathology in animal models of Alzheimers disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2012, 28, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Cutuli, D.; De Bartolo, P.; Caporali, P.; Laricchiuta, D.; Foti, F.; Ronci, M.; Rossi, C.; Neri, C.; Spalletta, G.; Caltagirone, C.; et al. N-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front. Aging Neurosci. 2014, 6, 220. [Google Scholar] [CrossRef] [Green Version]
- Cutuli, D.; Pagani, M.; Caporali, P.; Galbusera, A.; Laricchiuta, D.; Foti, F.; Neri, C.; Spalletta, G.; Caltagirone, C.; Petrosini, L.; et al. Effects of omega-3 fatty acid supplementation on cognitive functions and neural substrates: A voxel-based morphometry study in aged mice. Front. Aging Neurosci. 2016, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Jernigan, T.L.; Archibald, S.L.; Fennema-Notestine, C.; Gamst, A.C.; Stout, J.C.; Bonner, J.; Hesselink, J.R. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 2001, 22, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Masliah, E.; Crews, L.; Hansen, L. Synaptic remodeling during aging and in Alzheimers disease. J. Alzheimers Dis. 2006, 9 (Suppl. 3), 91–99. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35 (Suppl. 2), S20–S28. [Google Scholar] [CrossRef] [Green Version]
- Conklin, S.M.; Gianaros, P.J.; Brown, S.M.; Yao, J.K.; Hariri, A.R.; Manuck, S.B.; Muldoon, M.F. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci. Lett. 2007, 421, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Féart, C.; Proust-Lima, C.; Peuchant, E.; Dartigues, J.F.; Amieva, H.; Barberger-Gateau, P. ω-3 fatty acids and cognitive decline: Modulation by ApoEε4 allele and depression. Neurobiol. Aging 2011, 32, 2317. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Maillard, P.; Crivello, F.; Proust-Lima, C.; Peuchant, E.; Helmer, C.; Amieva, H.; Allard, M.; Dartigues, J.F.; Cunnane, S.C.; et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 2012, 79, 642–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.S.; Harris, W.S.; Beiser, A.S.; Au, R.; Himali, J.J.; Debette, S.; Pikula, A.; Decarli, C.; Wolf, P.A.; Vasan, R.S.; et al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 2012, 78, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Titova, O.E.; Sjögren, P.; Brooks, S.J.; Kullberg, J.; Ax, E.; Kilander, L.; Riserus, U.; Cederholm, T.; Larsson, E.M.; Johansson, L.; et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr) 2013, 35, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Pottala, J.V.; Yaffe, K.; Robinson, J.G.; Espeland, M.A.; Wallace, R.; Harris, W.S. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology 2014, 82, 435–442. [Google Scholar] [CrossRef]
- Bowman, G.L.; Silbert, L.C.; Howieson, D.; Dodge, H.H.; Traber, M.G.; Frei, B.; Kaye, J.A.; Shannon, J.; Quinn, J.F. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 2012, 78, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.K.; Siscovick, D.S.; Lemaitre, R.N.; Longstreth, W.T.; Spiegelman, D.; Rimm, E.B.; King, I.B.; Mozaffarian, D. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: The cardiovascular health study. J. Am. Heart Assoc. 2013, 2, e000305. [Google Scholar] [CrossRef] [Green Version]
- Yurko-Mauro, K. Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr. Alzheimer Res. 2010, 7, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 2014, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iolascon, G.; Gimigliano, R.; Bianco, M.; De Sire, A.; Moretti, A.; Giusti, A.; Malavolta, N.; Migliaccio, S.; Migliore, A.; Napoli, N.; et al. Are dietary supplements and nutraceuticals effective for musculoskeletal health and cognitive function? A scoping review. J. Nutr. Health Aging 2017, 21, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Woodbury-Fariña, M.A.; Shad, M.U.; Husni, M.; Copen, J.; Bureau, Y.; Cernovsky, Z.; Hou, J.J.; Raheb, H.; Terpstra, K.; et al. The role of nutrient-based epigenetic changes in buffering against stress, aging, and Alzheimer’s disease. Psychiatr. Clin. N. Am. 2014, 37, 591–623. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the Chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Agosti, P.; Lozupone, M.; Custodero, C.; Schilardi, A.; Valiani, V.; Santamato, A.; Sardone, R.; Dibello, V.; Di Lena, L.; et al. Nutritional interventions and cognitive-related outcomes in patients with late-life cognitive disorders: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 480–498. [Google Scholar] [CrossRef]
- Martí Del Moral, A.; Fortique, F. Omega-3 fatty acids and cognitive decline: A systematic review. Nutr. Hosp. 2019, 36, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef]
- Mosconi, L.; Murray, J.; Tsui, W.H.; Li, Y.; Davies, M.; Williams, S.; Pirraglia, E.; Spector, N.; Osorio, R.S.; Glodzik, L.; et al. Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer’s disease. J. Prev. Alzheimers Dis. 2014, 1, 23–32. [Google Scholar]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feart, C.; Samieri, C.; Barberger-Gateau, P. Mediterranean diet and cognitive health: An update of available knowledge. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Masana, M.F.; Koyanagi, A.; Haro, J.M.; Tyrovolas, S. N-3 fatty acids, Mediterranean diet and cognitive function in normal aging: A systematic review. Exp. Gerontol. 2017, 91, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Walters, M.; Sterling, J.; Quinn, C.; McHugh, P.; Andrews, R.E.; Matthews, D.C.; Ganzer, C.; Osorio, R.S.; Isaacson, R.S.; et al. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer’s disease: A cross-sectional study of middle-aged adults from the broader New York city area. BMJ Open 2018, 8, e019362. [Google Scholar] [CrossRef]
- Gorina, Y.V.; Komleva, Y.K.; Lopatina, O.L.; Volkova, V.V.; Chernykh, A.I.; Shabalova, A.A.; Olovyannikova, R.Y.; Salmina, A.B. The battery of tests for behavioral phenotyping of aging animals in the experiment. Adv. Gerontol. 2017, 30, 49–55. [Google Scholar]
- Roni, M.A.; Rahman, S. Neuronal nicotinic receptor antagonist reduces anxiety-like behavior in mice. Neurosci. Lett. 2011, 504, 237–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.Y.; Shao, S.; Zhang, C.; Liu, F.Y.; Wan, Y.; Yi, M. Inhibiting medial septal cholinergic neurons with DREADD alleviated anxiety-like behaviors in mice. Neurosci. Lett. 2017, 638, 139–144. [Google Scholar]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Ruehle, S.; Remmers, F.; Romo-Parra, H.; Massa, F.; Wickert, M.; Wörtge, S.; Häring, M.; Kaiser, N.; Marsicano, G.; Pape, H.C.; et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: Distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J. Neurosci. 2013, 33, 10264–10277. [Google Scholar] [CrossRef] [Green Version]
- Isingrini, E.; Camus, V.; Le Guisquet, A.M.; Pingaud, M.; Devers, S.; Belzung, C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: A model of fluoxetine resistance in mice. PLoS ONE 2010, 5, e10404. [Google Scholar] [CrossRef]
- Pothion, S.; Bizot, J.C.; Trovero, F.; Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 2004, 155, 135–146. [Google Scholar] [CrossRef]
- Griebel, G.; Stemmelin, J.; Scatton, B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol. Psychiatry 2005, 57, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Garau, A.; Marti, M.A.; Sala, J.; Balada, F. Age effects on the social interaction test in early adulthood male rats. Depress. Anxiety 2000, 12, 226–231. [Google Scholar] [CrossRef]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 48, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.C.; Larson, J. Impaired olfactory discrimination learning and decreased olfactory sensitivity in aged C57Bl/6 mice. Neurobiol. Aging 2009, 30, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. Rev. 2004, 3, 215–232. [Google Scholar] [CrossRef]
- Takahashi, L.K.; Chan, M.M.; Pilar, M.L. Predator odor fear conditioning: Current perspectives and new directions. Neurosci. Biobehav. Rev. 2008, 32, 1218–1227. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, L.K. Olfactory systems and neural circuits that modulate predator odor fear. Front. Behav. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.E.; Wann, E.G.; Yuan, R.K.; Álvarez, M.M.R.; Stead, S.M.; Muzzio, I.A. Long-term stabilization of place cell remapping produced by a fearful experience. J. Neurosci. 2012, 32, 15802–15814. [Google Scholar] [CrossRef]
- Hamann, S.; Monarch, E.S.; Goldstein, F.C. Impaired fear conditioning in Alzheimer’s disease. Neuropsychologia 2002, 40, 1187–1195. [Google Scholar] [CrossRef]
- Hoefer, M.; Allison, S.C.; Schauer, G.F.; Neuhaus, J.M.; Hall, J.; Dang, J.N.; Weiner, M.W.; Miller, B.L.; Rosen, H.J. Fear conditioning in frontotemporal lobar degeneration and Alzheimer’s disease. Brain 2008, 131, 1646–1657. [Google Scholar] [CrossRef]
- Keers, R.; Pedroso, I.; Breen, G.; Aitchison, K.J.; Nolan, P.M.; Cichon, S.; Nothen, M.M.; Rietschel, M.; Schalkwyk, L.C.; Fernandes, C. Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PLoS ONE 2012, 7, e38263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.P.; Vieira, C.; Bohner, L.O.; Silva, C.F.; Santos, E.C.; De Lima, T.C.; Lino-de-Oliveira, C. A proposal for refining the forced swim test in Swiss mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, R.; Coccurello, R.; Andolina, D.; Latagliata, E.C.; Zanettini, C.; Lampis, V.; Battaglia, M.; D’Amato, F.R.; Moles, A. Postnatal aversive experience impairs sensitivity to natural rewards and increases susceptibility to negative events in adult life. Cereb. Cortex 2013, 23, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.J.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørkløf, G.H.; Engedal, K.; Selbæk, G.; Kouwenhoven, S.E.; Helvik, A. Coping and depression in old age: A literature review. Dement. Geriatr. Cogn. Disord. 2013, 35, 121–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, N.B.; Singh, S.; Subramanyam, A.A.; Pinto, C.; Kamath, R.M.; Shanker, S. Study of loneliness, depression and coping mechanisms in elderly. J. Geriatr. Ment. Health 2014, 1, 20–27. [Google Scholar] [CrossRef]
- Maeng, S.; Oh, H.; Song, M.; Kim, Y.; Cha, S.; Bae, J.; Shin, J.; Sohn, N. Changes in coping strategy with age. Innov. Aging. 2017, 1 (Suppl. 1), 897. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; Delon, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and beyond. Curr. Alzheimer Res. 2009, 6, 86–96. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Trichopoulou, A.; Corbi, G.; De Vivo, I.; Scapagnini, G. The potential nutrigeroprotective role of Mediterranean diet and its functional components on telomere length dynamics. Ageing Res. Rev. 2019, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish intake, genetic predisposition to Alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef]
- Zhou, M.M.; Ding, L.; Wen, M.; Che, H.X.; Huang, J.Q.; Zhang, T.T.; Xue, C.H.; Mao, X.Z.; Wang, Y.M. Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Araya-Quintanilla, F.; Gutiérrez-Espinoza, H.; Sánchez-Montoya, U.; Muñoz-Yañez, M.J.; Baeza-Vergara, A.; Petersen-Yanjarí, M.; Fernández-Lecaros, L. Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: A systematic review and meta-analysis. Neurologia 2017. [Google Scholar] [CrossRef]
- Dobryakova, Y.V.; Volobueva, M.N.; Manolova, A.O.; Medvedeva, T.M.; Kvichansky, A.A.; Gulyaeva, N.V.; Markevich, V.A.; Stepanichev, M.Y.; Bolshakov, A.P. Cholinergic deficit induced by central administration of 192 IgG-saporin is associated with activation of microglia and cell loss in the dorsal hippocampus of rats. Front. Neurosci. 2019, 13, 146. [Google Scholar] [CrossRef] [Green Version]
- Šimić, G.; Kostović, I.; Winblad, B.; Bogdanović, N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J. Comp. Neurol. 1997, 379, 482–494. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Mavroudis, I.; Fotiou, D.; Stavros, B. Hippocampal neuronal loss in the CA1 and CA3 areas of AD patients. Psychiatr. Danub. 2012, 24, 152–158. [Google Scholar]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef]
- Fillit, H.; Ding, W.; Buee, L.; Kalman, J.; Altstiel, L.; Lawlor, B.; Wolf-Klein, G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 1991, 129, 318–320. [Google Scholar] [CrossRef]
- Strauss, S.; Bauer, J.; Ganter, U.; Jonas, U.; Berger, M.; Volk, B. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab. Investig. 1992, 66, 223–230. [Google Scholar] [PubMed]
- Ho, N.F.; Han, S.P.; Dawe, G.S. Effect of voluntary running on adult hippocampal neurogenesis in cholinergic lesioned mice. BMC Neurosci. 2009, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprea, M.R.; Cardenas, F.P.; Silveira, R.; Walsh, T.J.; Morato, S. Effects of septal cholinergic lesion on rat exploratory behavior in an open-field. Braz. J. Med. Biol. Res. 2003, 36, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.L.; Quintero, E.M.; Gilstrap, L.; Bhat, N.R.; Granholm, A.C. Minocycline protects basal forebrain cholinergic neurons from mu p75-saporin immunotoxic lesioning. Eur. J. Neurosci. 2004, 19, 3305–3316. [Google Scholar] [CrossRef]
- Matchynski, J.J.; Lowrance, S.A.; Pappas, C.; Rossignol, J.; Puckett, N.; Sandstrom, M.; Dunbar, G.L. Combinatorial treatment of tart cherry extract and essential fatty acids reduces cognitive impairments and inflammation in the mu-p75 saporin-induced mouse model of Alzheimer’s disease. J. Med. Food 2013, 16, 288–295. [Google Scholar] [CrossRef]
- Lamprea, M.R.; Cardenas, F.P.; Silveira, R.; Morato, S.; Walsh, T.J. Dissociation of memory and anxiety in a repeated elevated plus maze paradigm: Forebrain cholinergic mechanisms. Behav. Brain Res. 2000, 117, 97–105. [Google Scholar] [CrossRef]
- McHugh, S.B.; Francis, A.; McAuley, J.D.; Stewart, A.L.; Baxter, M.G.; Bannerman, D.M. Hippocampal acetylcholine depletion has no effect on anxiety, spatial novelty preference, or differential reward for low rates of responding (DRL) performance in rats. Behav. Neurosci. 2015, 129, 491–501. [Google Scholar] [CrossRef]
- Dautan, D.; Bay, H.H.; Bolam, J.P.; Gerdjikov, T.V.; Mena-Segovia, J. Extrinsic sources of cholinergic innervation of the striatal complex: A whole-brain mapping analysis. Front. Neuroanat. 2016, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Nizari, S.; Carare, R.O.; Romero, I.A.; Hawkes, C.A. 3D Reconstruction of the neurovascular unit reveals differential loss of cholinergic innervation in the cortex and hippocampus of the adult mouse brain. Front. Aging Neurosci. 2019, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.K. DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J. Nutr. 2010, 140, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, A.L.; Ousman, S.S. Astrocytes and aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: A focus on Alzheimer’s disease. BioMed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [Green Version]
- Wurtman, R.J. A nutrient combination that can affect synapse formation. Nutrients 2014, 6, 1701–1710. [Google Scholar] [CrossRef] [Green Version]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Dash, P.K.; Mach, S.A.; Moore, A.N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001, 63, 313–319. [Google Scholar] [CrossRef]
- Sakurai, A.; Tamvacakis, A.N.; Katz, P.S. Recruitment of polysynaptic connections underlies functional recovery of a neural circuit after lesion. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Solway, K.; Messing, R.O.; Sharp, F.R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998, 18, 7768–7778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.; Minami, M.; Lan, J.Q.; Mao, X.O.; Batteur, S.; Simon, R.P.; Greenberg, D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 2001, 98, 4710–4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.; Galvan, V.; Xie, L.; Mao, X.O.; Gorostiza, O.F.; Bredesen, D.E.; Greenberg, D.A. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc. Natl. Acad. Sci. USA 2004, 101, 13363–13367. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Peel, A.L.; Mao, X.O.; Xie, L.; Cottrell, B.A.; Henshall, D.C.; Greenberg, D.A. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G.; Gast, D.; Gage, F.H. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 2002, 52, 135–143. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Högyes, E.; Kitajka, K.; Puskás, L.G.; Zvara, A.; Hackler, L., Jr.; Nyakas, C.; Penke, Z.; Farkas, T. Modification by docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. Proc. Natl. Acad. Sci. USA 2003, 100, 11321–11326. [Google Scholar] [CrossRef] [Green Version]
- Willis, L.M.; Shukitt-Hale, B.; Joseph, J.A. Dietary polyunsaturated fatty acids improve cholinergic transmission in the aged brain. Genes Nutr. 2009, 4, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, N.; Okano, H.; Sawamoto, K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 2006, 11, 1145–1159. [Google Scholar] [CrossRef]
- Mohapel, P.; Leanza, G.; Kokaia, M.; Lindvall, O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 2005, 26, 939–946. [Google Scholar] [CrossRef]
- Campbell, N.R.; Fernandes, C.C.; Halff, A.W.; Berg, D.K. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J. Neurosci. 2010, 30, 8734–8744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazic, S.E. Modeling hippocampal neurogenesis across the lifespan in seven species. Neurobiol. Aging 2012, 33, 1664–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, B.; Mørk, A.; Plath, N.; Kristiansen, U.; Bastlund, J.F. Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice. Behav. Brain Res. 2013, 240, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Bohren, Y.; Waltisperger, E.; Sage-Ciocca, D.; Yin, J.C.; Freund-Mercier, M.J.; Barrot, M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry 2011, 70, 946–953. [Google Scholar] [CrossRef]
- Moretti, M.; Neis, V.B.; Matheus, F.C.; Cunha, M.P.; Rosa, P.B.; Ribeiro, C.M.; Prediger, R.D. Effects of agmatine on depressive-like behavior induced by intracerebroventricular administration of 1-methyl-4-phenylpyridinium (MPP+). Neurotox. Res. 2015, 28, 222–231. [Google Scholar] [CrossRef]
- Borges Filho, C.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes de Gomes, M.G.; Goes, A.T.R.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI). Behav. Brain Res. 2005, 160, 1–10. [Google Scholar] [CrossRef]
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Belzung, C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry 2008, 64, 293–301. [Google Scholar] [CrossRef]
- Petit, A.C.; Quesseveur, G.; Gressier, F.; Colle, R.; David, D.J.; Gardier, A.M.; Guiard, B.P. Converging translational evidence for the involvement of the serotonin 2A receptor gene in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 76–82. [Google Scholar] [CrossRef]
- Kimchi, T.; Xu, J.; Dulac, C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 2007, 448, 1009–1114. [Google Scholar] [CrossRef]
- Oddi, D.; Subashi, E.; Middei, S.; Bellocchio, L.; Lemaire-Mayo, V.; Guzmán, M.; Pietropaolo, S. Early social enrichment rescues adult behavioral and brain abnormalities in a mouse model of fragile X syndrome. Neuropsychopharmacology 2015, 40, 1113–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondoh, K.; Lu, Z.; Ye, X.; Olson, D.P.; Lowell, B.B.; Buck, L.B. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature 2016, 532, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Andolina, D.; Maran, D.; Valzania, A.; Conversi, D.; Puglisi-Allegra, S. Prefrontal/amygdalar system determines stress coping behavior through 5-HT/GABA connection. Neuropsychopharmacology 2013, 38, 2057–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessberger, S.; Romer, B.; Babu, H.; Kempermann, G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 2005, 196, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Teixeira, C.M.; Wang, A.H.; Frankland, P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007, 10, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Farioli-Vecchioli, S.; Saraulli, D.; Costanzi, M.; Pacioni, S.; Cinà, I.; Aceti, M.; Micheli, L.; Bacci, A.; Cestari, V.; Tirone, F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol. 2008, 6, e246. [Google Scholar] [CrossRef] [PubMed]
- Pakkenberg, B.; Gundersen, H.J. Neocortical neuron number in humans: Effect of sex and age. J. Comp. Neurol. 1997, 384, 312–320. [Google Scholar] [CrossRef]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Lo Iacono, L.; Valzania, A.; Visco-Comandini, F.; Viscomi, M.T.; Felsani, A.; Puglisi-Allegra, S.; Carola, V. Regulation of nucleus accumbens transcript levels in mice by early-life social stress and cocaine. Neuropharmacology 2016, 103, 183–194. [Google Scholar] [CrossRef]
- Nobili, A.; Krashia, P.; Cordella, A.; La Barbera, L.; Dell’Acqua, M.C.; Caruso, A.; Pignataro, A.; Marino, R.; Sciarra, F.; Biamonte, F.; et al. Ambra1 shapes hippocampal inhibition/excitation balance: Role in neurodevelopmental disorders. Mol. Neurobiol. 2018, 55, 7921–7940. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutuli, D.; Landolfo, E.; Decandia, D.; Nobili, A.; Viscomi, M.T.; La Barbera, L.; Sacchetti, S.; De Bartolo, P.; Curci, A.; D’Amelio, M.; et al. Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. Int. J. Mol. Sci. 2020, 21, 1741. https://doi.org/10.3390/ijms21051741
Cutuli D, Landolfo E, Decandia D, Nobili A, Viscomi MT, La Barbera L, Sacchetti S, De Bartolo P, Curci A, D’Amelio M, et al. Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. International Journal of Molecular Sciences. 2020; 21(5):1741. https://doi.org/10.3390/ijms21051741
Chicago/Turabian StyleCutuli, Debora, Eugenia Landolfo, Davide Decandia, Annalisa Nobili, Maria Teresa Viscomi, Livia La Barbera, Stefano Sacchetti, Paola De Bartolo, Annacarmen Curci, Marcello D’Amelio, and et al. 2020. "Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice" International Journal of Molecular Sciences 21, no. 5: 1741. https://doi.org/10.3390/ijms21051741
APA StyleCutuli, D., Landolfo, E., Decandia, D., Nobili, A., Viscomi, M. T., La Barbera, L., Sacchetti, S., De Bartolo, P., Curci, A., D’Amelio, M., Farioli-Vecchioli, S., & Petrosini, L. (2020). Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. International Journal of Molecular Sciences, 21(5), 1741. https://doi.org/10.3390/ijms21051741




