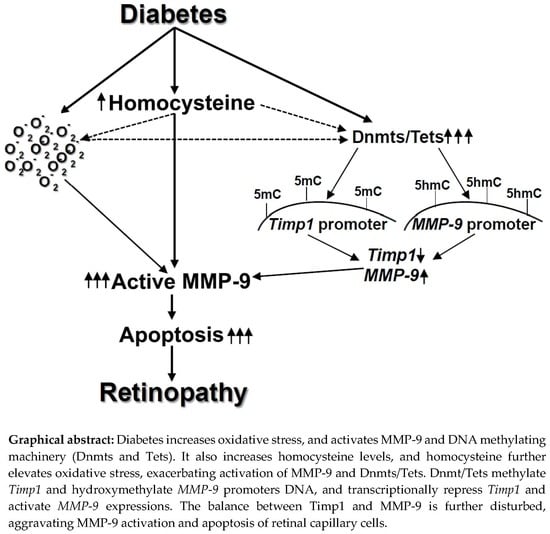
Homocysteine Disrupts Balance between MMP-9 and Its Tissue Inhibitor in Diabetic Retinopathy: The Role of DNA Methylation
Abstract
:1. Introduction
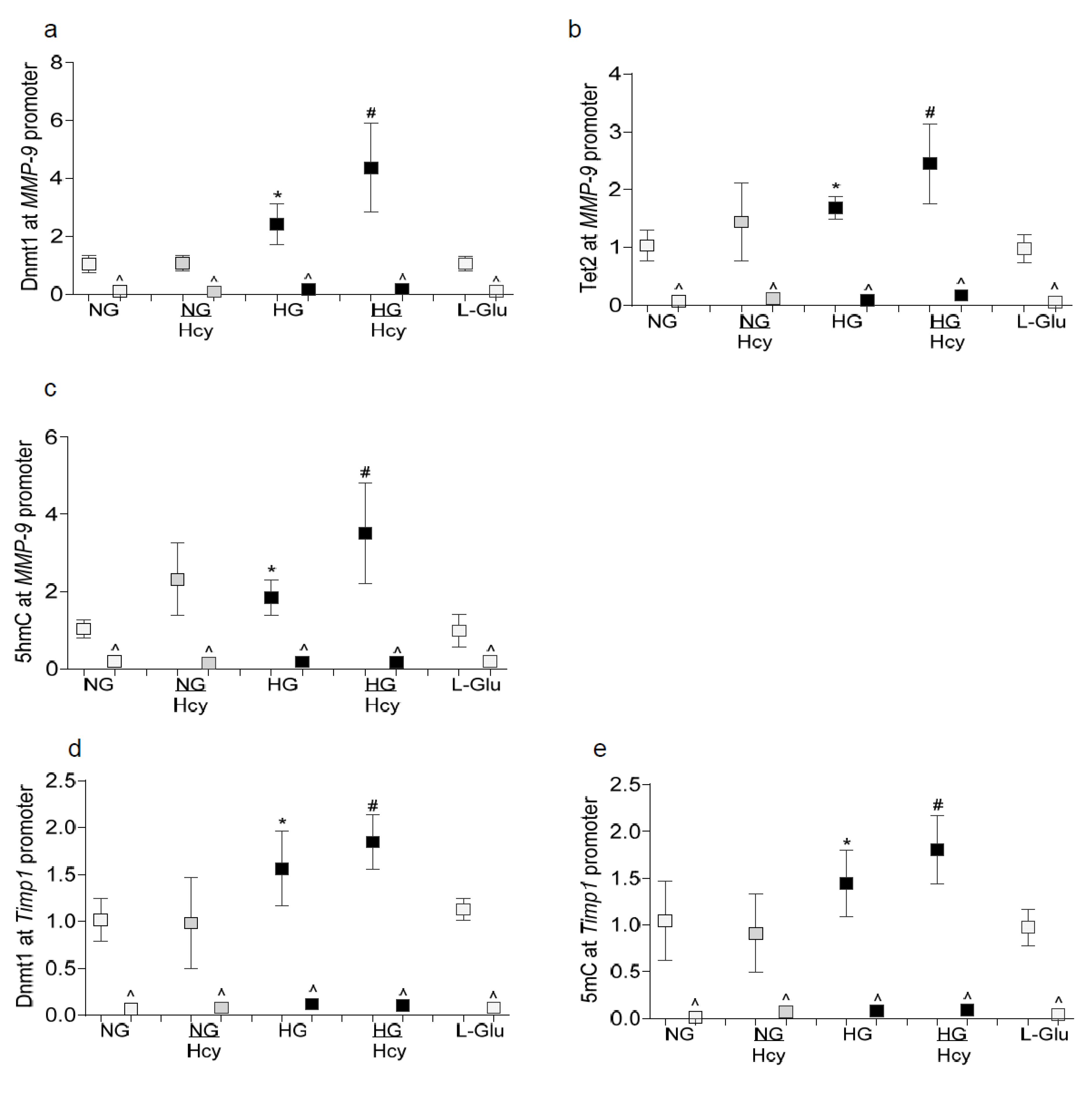
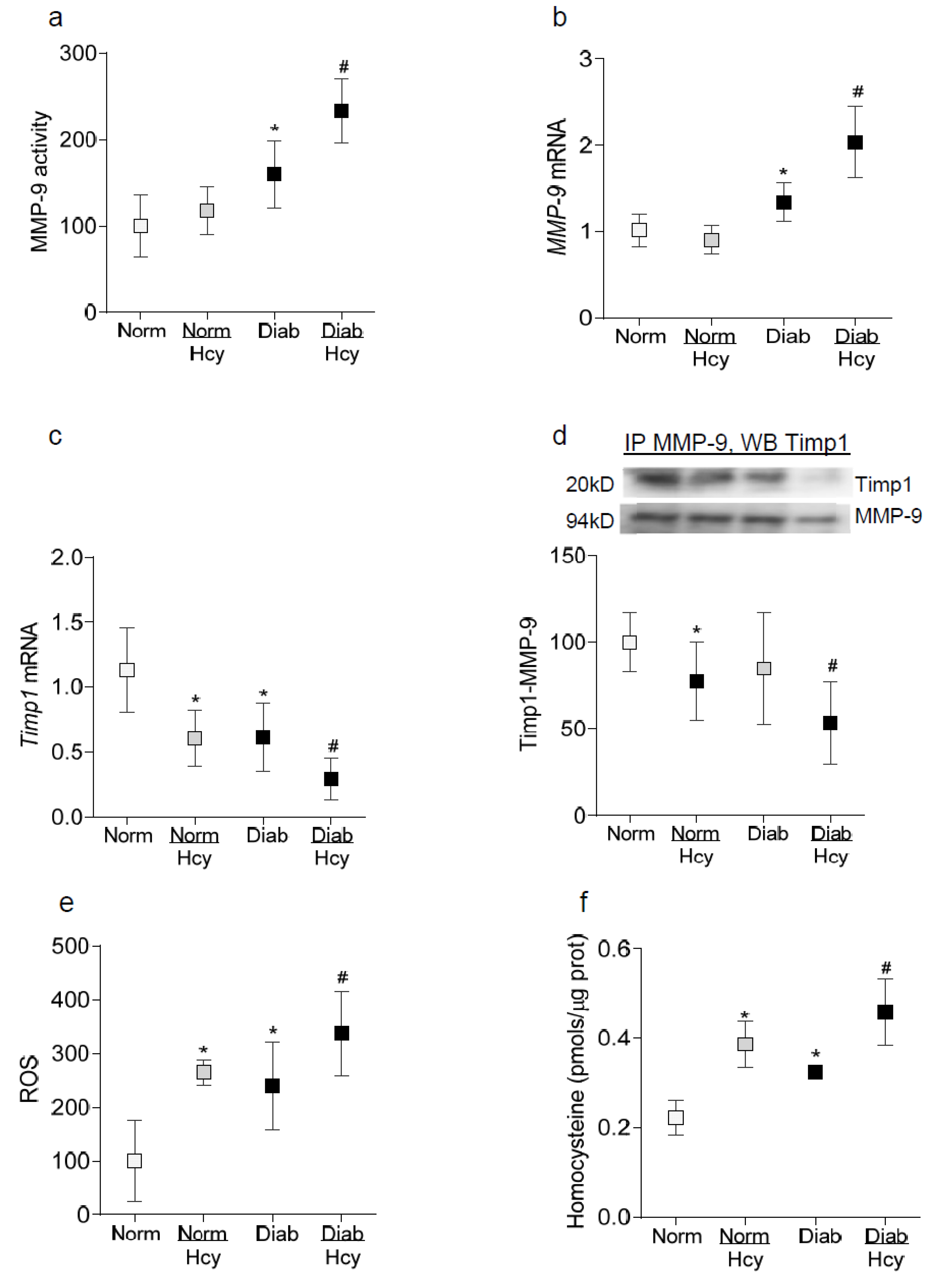
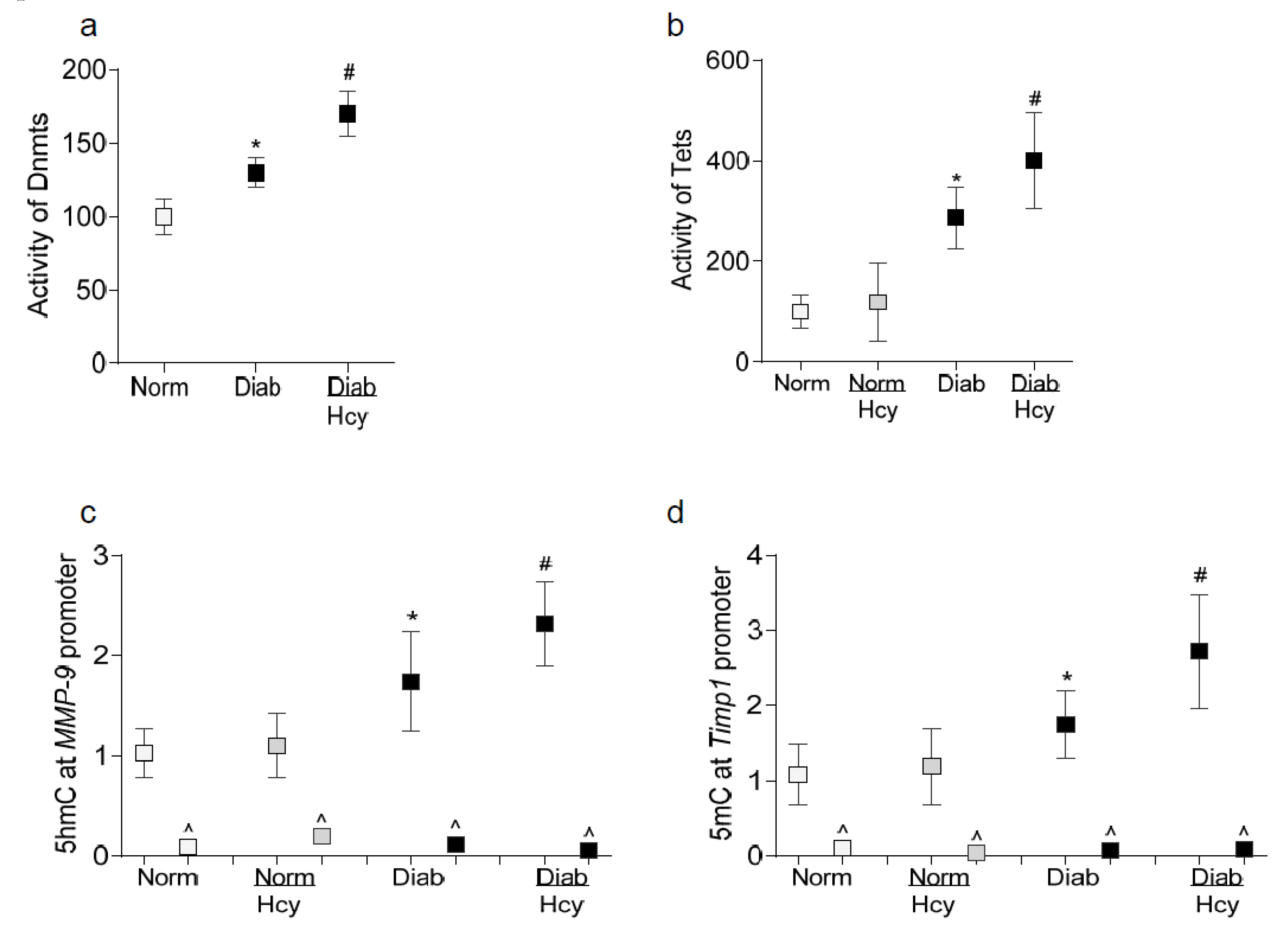
2. Results
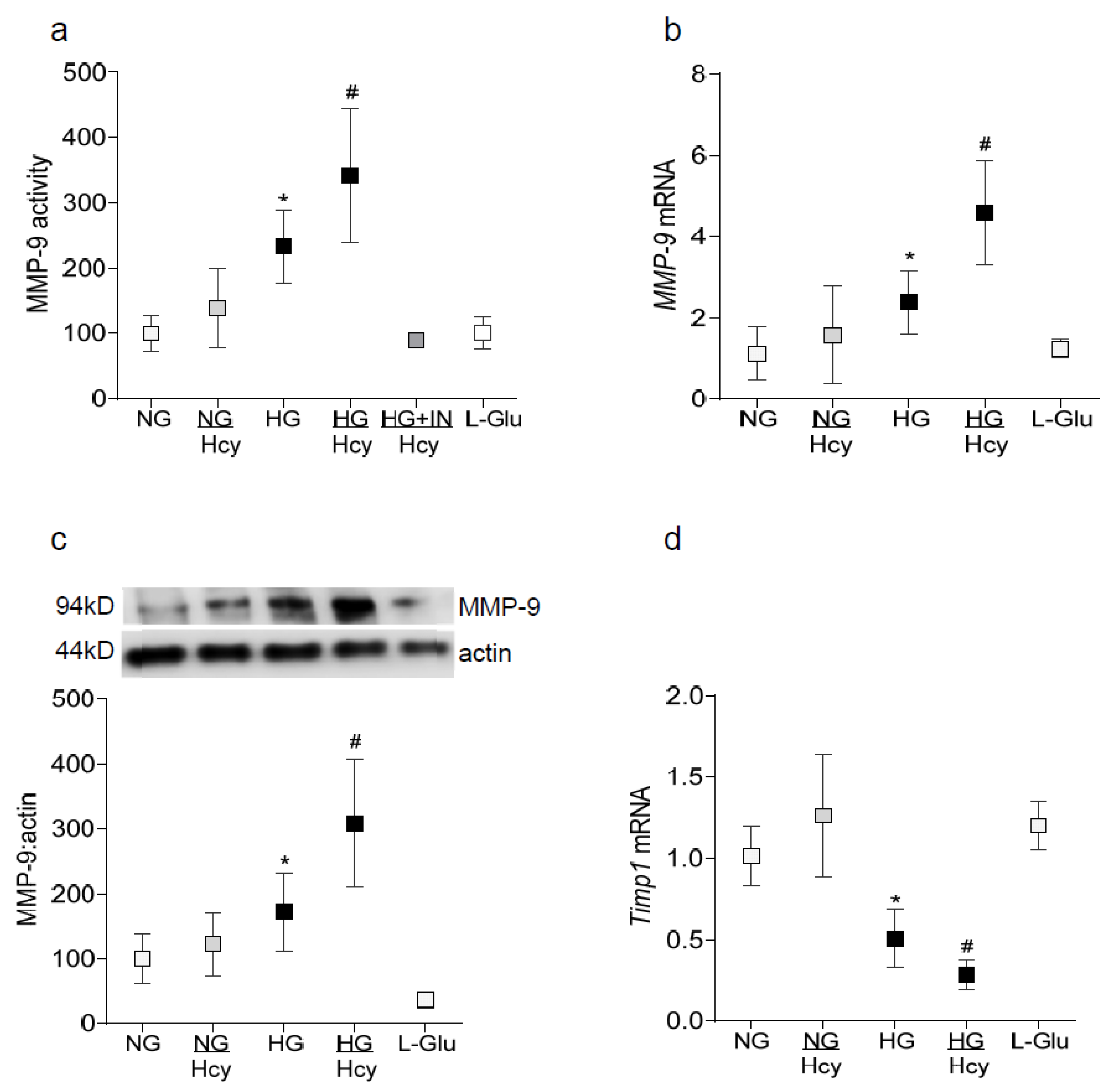
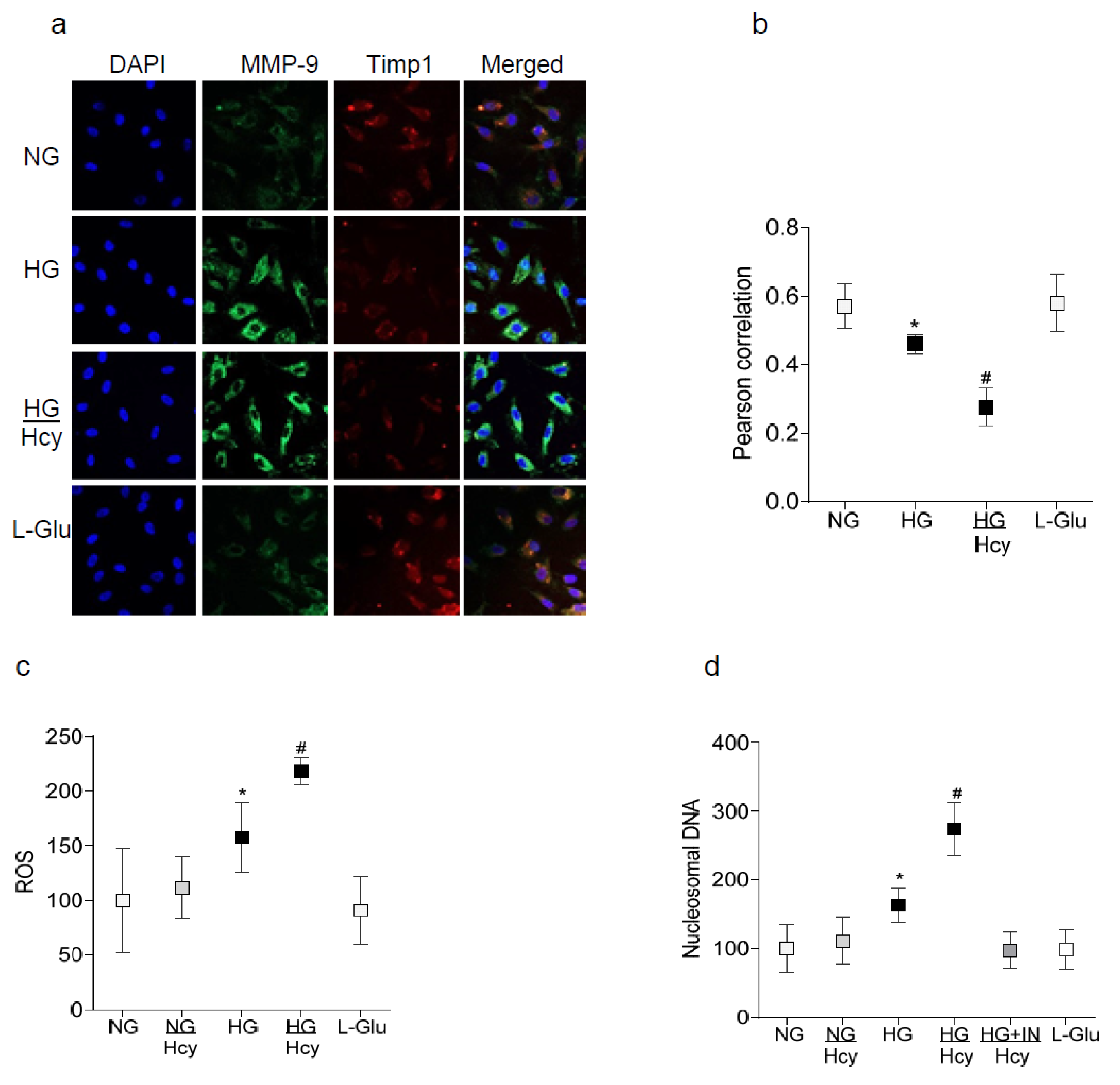
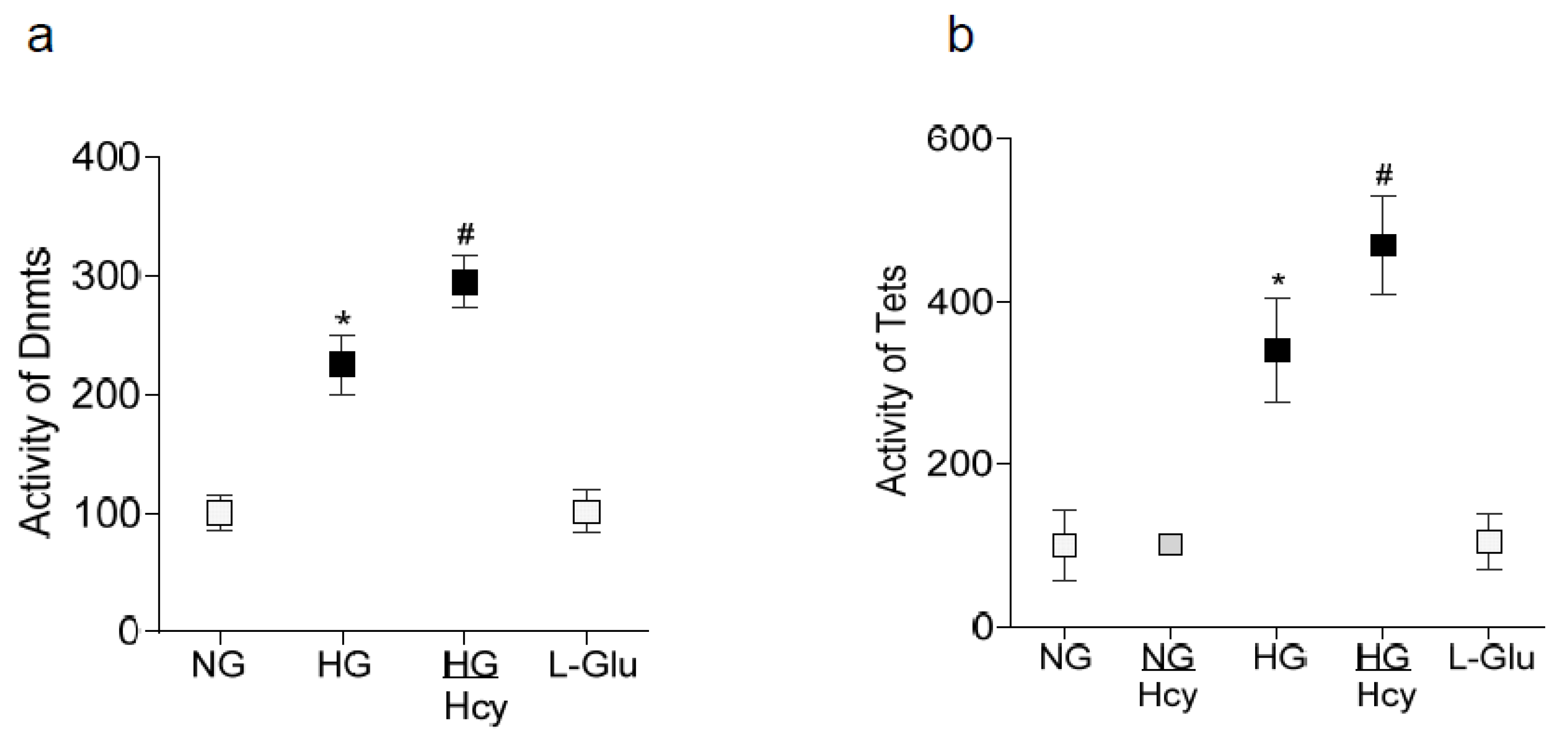
2.1. In Vitro: Retinal Endothelial Cells in Culture
2.2. In Vivo Model: Rat Retinal Microvasculature
3. Discussion
4. Materials and Methods
4.1. Diabetic Rats
4.2. Retinal Microvessels
4.3. Homocysteine Levels
4.4. Gene Transcripts
4.5. Western Blotting
4.6. Activities of Enzymes
4.7. Immunofluorescence
4.8. Co-Immunoprecipitation
4.9. Reactive Oxygen Species
4.10. Apoptosis of Retinal Endothelial Cells
4.11. Methylated/Hydroxymethylated Cytosine
4.12. Chromatin Immunoprecipitation (ChIP)
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, R.N. Diabetic retinopathy. New Eng. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, A.; Veluthakal, R.; Mohammad, G.; Syed, I.; Santos, J.M.; Mishra, M. Tiam1-Rac1 signaling axis-mediated activation of nadph oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 2014, 57, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Ret. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Tewari, S.; Lin, J.Y.; Kowluru, R.A. Interrelationship between activation of matrix metalloproteinases and mitochondrial dysfunction in the development of diabetic retinopathy. Biochem. Biophys. Res. Commun. 2013, 438, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and timps. Cardiovas. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Matrix metalloproteinases in diabetic retinopathy: Potential role of MMP-9. Expert Opin Investig. Drugs 2012, 21, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, G.; Gagliano, C.; Giordano, M.; Salomone, S.; Vacante, M.; Bucolo, C.; Caraci, F.; Reibaldi, M.; Drago, F.; Avitabile, T.; et al. Homocysteine serum levels in diabetic patients with non-proliferative, proliferative and without retinopathy. BioMed Res. Int. 2014, 2014, 191497. [Google Scholar] [CrossRef]
- Aydin, E.; Demir, H.D.; Ozyurt, H.; Etikan, I. Association of plasma homocysteine and macular edema in type 2 diabetes mellitus. Eur. J. Ophthalmol. 2008, 18, 226–232. [Google Scholar] [CrossRef]
- Srivastav, K.; Saxena, S.; Mahdi, A.A.; Shukla, R.K.; Meyer, C.H.; Akduman, L.; Khanna, V.K. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol. Vis. 2016, 22, 1352–1360. [Google Scholar]
- Lei, X.; Zeng, G.; Zhang, Y.; Li, Q.; Zhang, J.; Bai, Z.; Yang, K. Association between homocysteine level and the risk of diabetic retinopathy: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Mohamed, R.; Elsherbiny, N.M.; DeAngelis, M.M.; Bartoli, M.; Al-Shabrawey, M. Homocysteine: A potential biomarker for diabetic retinopathy. J. Clin. Med. 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A.; Mohammad, G.; Sahajpal, N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, N.; Mishra, P.K.; Tyagi, S.C. Homocysteine, hydrogen sulfide (h2s) and nmda-receptor in heart failure. Ind. Biochem. Biophys. 2009, 46, 441–446. [Google Scholar]
- Tyagi, N.; Givvimani, S.; Qipshidze, N.; Kundu, S.; Kapoor, S.; Vacek, J.C.; Tyagi, S.C. Hydrogen sulfide mitigates matrix metalloproteinase-9 activity and neurovascular permeability in hyperhomocysteinemic mice. Neurochem. Int. 2010, 56, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.H.; Shih, T.S.; Huang, H.R.; Huang, S.C.; Lee, L.H.; Huang, Y.C. Plasma homocysteine is associated with increased oxidative stress and antioxidant enzyme activity in welders. Sci. World J. 2013, 2013, 370487. [Google Scholar] [CrossRef]
- Weber, G.J.; Pushpakumar, S.; Tyagi, S.C.; Sen, U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharm. Res. 2016, 113, 300–312. [Google Scholar] [CrossRef] [Green Version]
- Chiang, P.K.; Gordon, R.K.; Tal, J.; Zeng, G.C.; Doctor, B.P.; Pardhasaradhi, K.; McCann, P.P. S-adenosylmethionine and methylation. FASEB J. 1996, 10, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Shan, Y.; Mishra, M. Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab. Investig. 2016, 96, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Shan, Y. Role of oxidative stress in epigenetic modification of mmp-9 promoter in the development of diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Kowluru, R.A. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3832–3841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujumdar, V.S.; Aru, G.M.; Tyagi, S.C. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J. Cell. Biochem. 2001, 82, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Tyagi, S.C. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutr. J. 2004, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalayel, I.; Vallee, Y. Chemistry of homocysteine thiolactone in a prebiotic perspective. Life 2019, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial dysfunction: The link between homocysteine and hydrogen sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef]
- Kumar, M.; Sandhir, R. Hydrogen sulfide attenuates hyperhomocysteinemia-induced mitochondrial dysfunctions in brain. Mitochondrion 2020, 50, 158–169. [Google Scholar] [CrossRef]
- Okamoto, T.; Akuta, T.; Tamura, F.; van Der Vliet, A.; Akaike, T. Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol. Chem. 2004, 385, 997–1006. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Rodriguez, W.P.A.; Roberts, A.M.; Falcone, J.C.; Passmore, J.C.; Fleming, J.T.; Joshua, I.G. Hyperhomocysteinemic diabetic cardiomyopathy: Oxidative stress, remodeling, and endothelial-myocyte uncoupling. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 1–10. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Santos, J.M.; Zhong, Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5653–5660. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Solini, A.; Santini, E.; Nannipieri, M.; Ferrannini, E. High glucose and homocysteine synergistically affect the metalloproteinases-tissue inhibitors of metalloproteinases pattern, but not TGFB expression, in human fibroblasts. Diabetologia 2006, 49, 2499–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A.; Mishra, M. Regulation of matrix metalloproteinase in the pathogenesis of diabetic retinopathy. Prog. Mol. Biol. Transl. Sci. 2017, 148, 67–85. [Google Scholar] [PubMed]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Kundu, S.; Narayanan, N.; Sen, U. DNA hypermethylation in hyperhomocysteinemia contributes to abnormal extracellular matrix metabolism in the kidney. FASEB J. 2015, 29, 4713–4725. [Google Scholar] [CrossRef] [Green Version]
- Elmasry, K.; Mohamed, R.; Sharma, I.; Elsherbiny, N.M.; Liu, Y.; Al-Shabrawey, M.; Tawfik, A. Epigenetic modifications in hyperhomocysteinemia: Potential role in diabetic retinopathy and age-related macular degeneration. Oncotarget 2018, 9, 12562–12590. [Google Scholar] [CrossRef] [Green Version]
- Kamat, P.K.; Kalani, A.; Tyagi, S.C.; Tyagi, N. Hydrogen sulfide epigenetically attenuates homocysteine-induced mitochondrial toxicity mediated through nmda receptor in mouse brain endothelial (bend3) cells. J. Cell. Physiol. 2015, 230, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Santos, J.M.; Mishra, M. Epigenetic modifications and diabetic retinopathy. BioMed Res. Int. 2013, 2013, 635284. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Kowluru, R.A. Regulation of matrix metalloproteinase-9 by epigenetic modifications and the development of diabetic retinopathy. Diabetes 2013, 62, 2559–2568. [Google Scholar] [CrossRef] [Green Version]
- Duraisamy, A.J.; Mishra, M.; Kowluru, R.A. Crosstalk between histone and DNA methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6440–6448. [Google Scholar] [CrossRef] [Green Version]
- Sudduth, T.L.; Powell, D.K.; Smith, C.D.; Greenstein, A.; Wilcock, D.M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 708–715. [Google Scholar] [CrossRef] [Green Version]
- George, A.K.; Singh, M.; Pushpakumar, S.; Homme, R.P.; Hardin, S.J.; Tyagi, S.C. Dysbiotic 1-carbon metabolism in cardiac muscle remodeling. J. Cell. Physiol. 2020, 235, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab. Investig. 2010, 90, 1365–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, R.; Sharma, I.; Ibrahim, A.S.; Saleh, H.; Elsherbiny, N.M.; Fulzele, S.; Elmasry, K.; Smith, S.B.; Al-Shabrawey, M.; Tawfik, A.; et al. Hyperhomocysteinemia alters retinal endothelial cells barrier function and angiogenic potential via activation of oxidative stress. Sci. Rep. 2017, 7, 11952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A. Role of matrix metalloproteinase-9 in the development of diabetic retinopathy and its regulation by H-Ras. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4320–4326. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, G.; Radhakrishnan, R.; Kowluru, R.A. Epigenetic modifications compromise mitochondrial DNA quality control in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3943–3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraisamy, A.J.; Mohammad, G.; Kowluru, R.A. Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1617–1626. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Jirousek, M.R.; Stramm, L.; Farid, N.; Engerman, R.L.; Kern, T.S. Abnormalities of retinal metabolism in diabetes or experimental galactosemia: V. Relationship between protein kinase c and ATPases. Diabetes 1998, 47, 464–469. [Google Scholar] [CrossRef]
- Mohammad, G.; Kowluru, R.A. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J. Cell. Physiol. 2012, 227, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Kowluru, A.; Kowluru, R.A. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2985–2992. [Google Scholar] [CrossRef] [Green Version]
| Gene | Sequence |
|---|---|
| Human | |
| MMP-9 | Fwd- CACTGTCCACCCCTCAGAGC |
| Rev- GCCACTTGTCGGCGATAAGG | |
| Timp1 | Fwd- GAAGAGCCTGAACCACAGGT |
| Rev- CGGGGAGGAGATGTAGCAC | |
| MMP-9 promoter | Fwd- GAGTCAGCACTTGCCTGTCA |
| Rev- CTGCTGTTGTGGGGGCTTTA | |
| Timp1 promoter | Fwd- CTGATGGTGGGTGGATGAGTAATG |
| Rev- GCCTGAGCGCTAGAGGATAAATG | |
| β-Actin | Fwd- AGCCTCGCCTTTGCCGATCCG |
| Rev- TCTCTTGCTCTGGGCCTCGTCG | |
| Rat | |
| MMP-9 | Fwd- GAAGCAGCTGTCCCTGCCCC |
| Rev- GATGGTGCCACTTGAGGTCG | |
| Timp1 | Fwd- GTTTCCGGTTCGCCTACACC |
| Rev- GGGCTCAGATTATGCCAGGG | |
| MMP-9 promoter | Fwd- GACTGTGGGCAGGGCATAAA |
| Rev- GGGTGAAGCAGAATTTGCGG | |
| Timp1 promoter | Fwd- GCAGTGGGTGGATGAGTAAT |
| Rev- GACTGGCGGTGGAGAATA AA | |
| β-Actin | Fwd- TCTGTGTGGATTGGTGGCTCTA |
| Rev- AAC AGTCCGCCTAGAAGCATTTG | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, G.; Kowluru, R.A. Homocysteine Disrupts Balance between MMP-9 and Its Tissue Inhibitor in Diabetic Retinopathy: The Role of DNA Methylation. Int. J. Mol. Sci. 2020, 21, 1771. https://doi.org/10.3390/ijms21051771
Mohammad G, Kowluru RA. Homocysteine Disrupts Balance between MMP-9 and Its Tissue Inhibitor in Diabetic Retinopathy: The Role of DNA Methylation. International Journal of Molecular Sciences. 2020; 21(5):1771. https://doi.org/10.3390/ijms21051771
Chicago/Turabian StyleMohammad, Ghulam, and Renu A. Kowluru. 2020. "Homocysteine Disrupts Balance between MMP-9 and Its Tissue Inhibitor in Diabetic Retinopathy: The Role of DNA Methylation" International Journal of Molecular Sciences 21, no. 5: 1771. https://doi.org/10.3390/ijms21051771