A Possible Link of Genetic Variations in ER/IGF1R Pathway and Risk of Melanoma
Abstract
:1. Introduction
2. Results
2.1. Study Participants
2.2. SNP Selection
2.3. Genotyping and SNP Associations in the GEM Cohort
2.4. An Attempt to Validate the Top 2 SNPs in the GENEVA Dataset
2.5. Gender Disparity of the Association
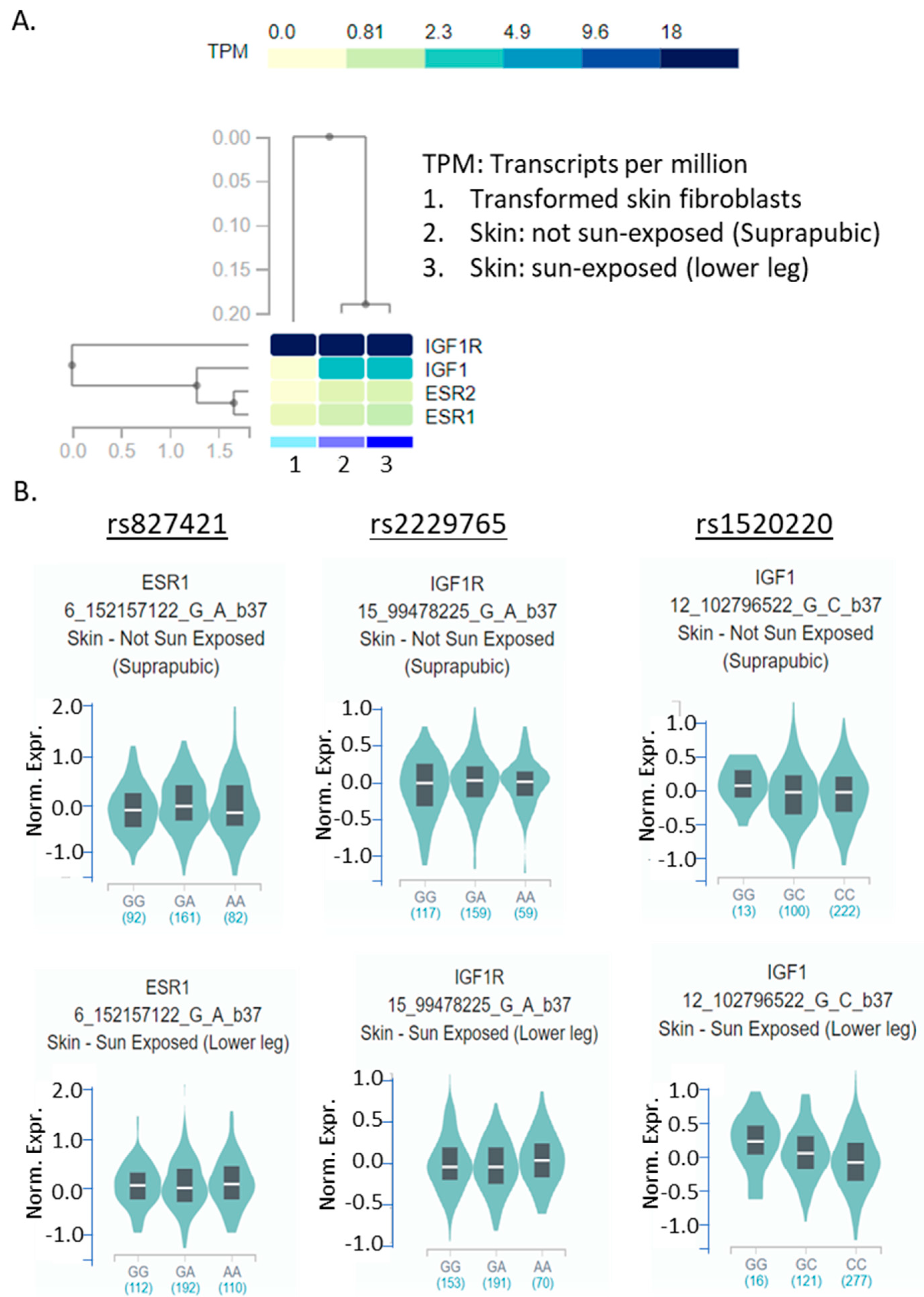
2.6. eQTL of the Three SNPs in Skin Tissue
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Population
4.3. Genotyping and Quality Control
4.4. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CM | Cutaneous melanoma |
| SNP | Single nucleotide polymorphism |
| OR | Odds ratio |
| ER | Estrogen receptor |
| IGF1 | Insulin-like growth factor 1 |
| GENEVA | The Gene Environment Association Studies Initiative |
| GEM | The International Genes, Environment, and Melanoma Study |
References
- SEER. Annual Report to the Nation 2019: National Trends in Rates of New Cancer Cases. 2019. Available online: https://seer.cancer.gov/report_to_nation/infographics/incidence.html (accessed on 28 June 2019).
- Liu-Smith, F.; Farhat, A.M.; Arce, A.; Ziogas, A.; Taylor, T.; Wang, Z.; Yourk, V.; Liu, J.; Wu, J.; McEligot, A.J.; et al. Sex differences in the association of cutaneous melanoma incidence rates and geographic ultraviolet light exposure. J. Am. Acad. Dermatol. 2017, 76, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Liu-Smith, F.; Ziogas, A. An age-dependent interaction between sex and geographical UV index in melanoma risk. J. Am. Acad. Dermatol. 2018, in press. [Google Scholar]
- Yuan, T.A.; Lu, Y.; Edwards, K.; Jakowatz, J.; Meyskens, F.L.; Liu-Smith, F. Race-, Age-, and Anatomic Site-Specific Gender Differences in Cutaneous Melanoma Suggest Differential Mechanisms of Early-and Late-Onset Melanoma. Int. J. Environ. Res. Public Health 2019, 16, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrett, L.D.; Nguyen, H.L.; Armstrong, B.K. Trends in the incidence of cutaneous malignant melanoma in New South Wales, 1983–1996. Int. J. Cancer 2001, 92, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Vranova, J.; Arenbergerova, M.; Arenberger, P.; Vrana, A.; Zivcak, J.; Kolarova, H.; Rosina, J. Malignant melanoma in the Czech Republic: Incidence and mortality according to sex, age and disease stage. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2014, 158, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bessonova, L.; Taylor, T.H.; Ziogas, A.; Meyskens, F.L.; Anton-Culver, H. A unique gender difference in early onset melanoma implies that in addition to ultraviolet light exposure other causative factors are important. Pigm. Cell Melanoma R. 2013, 26, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Hein, R.; Maranian, M.; Hopper, J.L.; Kapuscinski, M.K.; Southey, M.C.; Park, D.J.; Schmidt, M.K.; Broeks, A.; Hogervorst, F.B.; Bueno-de-Mesquit, H.B.; et al. Comparison of 6q25 breast cancer hits from Asian and European Genome Wide Association Studies in the Breast Cancer Association Consortium (BCAC). PLoS ONE 2012, 7, e42380. [Google Scholar] [CrossRef]
- Swetter, S.M.; Johnson, T.M.; Miller, D.R.; Layton, C.J.; Brooks, K.R.; Geller, A.C. Melanoma in middle-aged and older men: A multi-institutional survey study of factors related to tumor thickness. Arch. Dermatol. 2009, 145, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.A.H.; Storer, B.E. Further-Studies on Skin Melanomas Apparently Dependent on Female Sex-Hormones. Int. J. Epidemiol. 1982, 11, 127–131. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Reintgen, D.S.; Vollmer, R.T.; Seigler, H.F. Malignant-Melanoma Arising during Pregnancy—A Study of 100 Patients. Ann. Surg. 1990, 211, 552–559. [Google Scholar] [CrossRef]
- Karagas, M.R.; Stukel, T.A.; Dykes, J.; Miglionico, J.; Greene, M.A.; Carey, M.; Armstrong, B.; Elwood, J.M.; Gallagher, R.P.; Green, A.; et al. A pooled analysis of 10 case-control studies of melanoma and oral contraceptive use. Br. J. Cancer 2002, 86, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, S.; Iodice, S.; Koomen, E.; Di Pietro, A.; Sera, F.; Caini, S. Hormonal and reproductive factors in relation to melanoma in women: Current review and meta-analysis. Eur. J. Cancer 2011, 47, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Mahamat-Saleh, Y.; Savoye, I.; Dartois, L.; Boutron-Ruault, M.C.; Fournier, A.; Kvaskoff, M. Oral contraceptive use and cutaneous melanoma risk: A French prospective cohort study. Int. J. Cancer 2018, 143, 2390–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natale, C.A.; Li, J.; Zhang, J.; Dahal, A.; Dentchev, T.; Stanger, B.Z.; Ridky, T.W. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. Elife 2018, 7, e31770. [Google Scholar] [CrossRef]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, O.; Johnson, N.; Orr, N.; Hosking, F.J.; Gibson, L.J.; Walker, K.; Zelenika, D.; Gut, I.; Heath, S.; Palles, C.; et al. Novel Breast Cancer Susceptibility Locus at 9q31.2: Results of a Genome-Wide Association Study. J. Natl. Cancer Inst. 2011, 103, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Sakoda, L.C.; Blackston, C.R.; Doherty, J.A.; Ray, R.M.; Lin, M.G.; Gao, D.L.; Stalsberg, H.; Feng, Z.; Thomas, D.B.; Chen, C. Selected estrogen receptor 1 and androgen receptor gene polymorphisms in relation to risk of breast cancer and fibrocystic breast conditions among Chinese women. Cancer Epidemiol. 2011, 35, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Stevens, K.N.; Vachon, C.M.; Lee, A.M.; Slager, S.; Lesnick, T.; Olswold, C.; Fasching, P.A.; Miron, P.; Eccles, D.; Carpenter, J.E.; et al. Common breast cancer susceptibility loci are associated with triple-negative breast cancer. Cancer Res. 2011, 71, 6240–6249. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.C.; Hsiung, C.N.; Hsu, H.M.; Bao, B.Y.; Chen, S.T.; Hsu, G.C.; Chou, W.C.; Hu, L.Y.; Ding, S.L.; Cheng, C.W.; et al. Genetic variation in the genome-wide predicted estrogen response element-related sequences is associated with breast cancer development. Breast Cancer Res. 2011, 13, R13. [Google Scholar] [CrossRef] [Green Version]
- Backes, F.J.; Walker, C.J.; Goodfellow, P.J.; Hade, E.M.; Agarwal, G.; Mutch, D.; Cohn, D.E.; Suarez, A.A. Estrogen receptor-alpha as a predictive biomarker in endometrioid endometrial cancer. Gynecol. Oncol. 2016, 141, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Nüssler, N.C.; Reinbacher, K.; Shanny, N.; Schirmeier, A.; Glanemann, M.; Neuhaus, P.; Nussler, A.K.; Kirschner, M. Sex-specific differences in the expression levels of estrogen receptor subtypes in colorectal cancer. Gend. Med. 2008, 5, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Martineti, V.; Picariello, L.; Tognarini, I.; Sala, S.C.; Gozzini, A.; Azzari, C.; Mavilia, C.; Tanini, A.; Falchetti, A.; Fiorelli, G.; et al. ER beta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr. Relat. Cancer 2005, 12, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, C.; Melachrinou, M.; Vasilakos, P.; Tzorakoleftherakis, E. Expression of estrogen receptors in melanoma and sentinel lymph nodes; A “female” clinical entity or a possible treatment modality? Eur. J. Gynaecol. Oncol. 2015, 36, 123–130. [Google Scholar] [PubMed]
- Thornton, M.J.; Taylor, A.H.; Mulligan, K.; Al-Azzawi, F.; Lyon, C.C.; O’Driscoll, J.; Messenger, A.G. Oestrogen receptor beta is the predominant oestroryan receptor in human scalp skin. Exp. Dermatol. 2003, 12, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.N.; Nanney, L.B.; Boyd, A.S.; King, L.E., Jr.; Ellis, D.L. Oestrogen receptor-beta expression in melanocytic lesions. Exp. Dermatol. 2006, 15, 971–980. [Google Scholar] [CrossRef]
- Pollard, K.J.; Daniel, J.M. Nuclear estrogen receptor activation by insulin-like growth factor-1 in Neuro-2A neuroblastoma cells requires endogenous estrogen synthesis and is mediated by mutually repressive MAPK and PI3K cascades. Mol. Cell. Endocrinol. 2019, 490, 68–79. [Google Scholar] [CrossRef]
- Lee, A.V.; Jackson, J.G.; Gooch, J.L.; Hilsenbeck, S.G.; Coronado-Heinsohn, E.; Osborne, C.K.; Yee, D. Enhancement of insulin-like growth factor signaling in human breast cancer: Estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol. Endocrinol. 1999, 13, 787–796. [Google Scholar] [CrossRef]
- Kahlert, S.; Nuedling, S.; van Eickels, M.; Vetter, H.; Meyer, R.; Grohe, C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 2000, 275, 18447–18453. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.H.; Gao, W.M.; Jiang, E.Z.; Lu, F.; Zhang, L..; Shi, Z.R.; Wang, X.; Chen, L.; Lv, T. Interaction between IGF-IR and ER Induced by E2 and IGF-I. PLoS ONE 2013, 8, e62642. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Hummer, A.J.; Mujumdar, U.; Armstrong, B.K.; Kricker, A.; Marrett, L.D.; Millikan, R.C.; Gruber, S.B.; Culver, H.A.; Zanetti, R.; et al. A design for cancer case-control studies using only incident cases: Experience with the GEM study of melanoma. Int. J. Epidemiol. 2006, 35, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Hummer, A.; Mujumdar, U.; Armstrong, B.K.; Kricker, A.; Marrett, L.D.; Millikan, R.C.; Gruber, S.B.; Anton-Culver, H.; Klotz, J.B.; et al. Familial aggregation of melanoma risks in a large population-based sample of melanoma cases. Cancer Causes Control 2004, 15, 957–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhao, H.; Hu, Z.; Liu, Z.; Wang, L.E.; Gershenwald, J.E.; Prieto, V.G.; Lee, J.E.; Duvic, M.; Grimm, E.A.; et al. Genetic variants and haplotypes of the caspase-8 and caspase-10 genes contribute to susceptibility to cutaneous melanoma. Hum. Mutat. 2008, 29, 1443–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacey, S.N.; Sulem, P.; Zanon, C.; Gudjonsson, S.A.; Thorleifsson, G.; Helgason, A.; Jonasdottir, A.; Besenbacher, S.; Kostic, J.P.; Fackenthal, J.D.; et al. Ancestry-shift refinement mapping of the C6orf97-ESR1 breast cancer susceptibility locus. PLoS Genet. 2010, 6, e1001029. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.N.; Vachon, C.M.; Couch, F.J. Genetic susceptibility to triple-negative breast cancer. Cancer Res. 2013, 73, 2025–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; He, N.; Feng, T.; Geng, T.T.; Jin, T.B.; Chen, C. Association of five single nucleotide polymorphisms at 6q25.1 with breast cancer risk in northwestern China. Am. J. Cancer Res. 2015, 5, 2467. [Google Scholar] [PubMed]
- Sun, Y.; Ye, C.; Guo, X.; Wen, W.; Long, J.; Gao, Y.T.; Shu, X.O.; Zheng, W.; Cai, Q. Evaluation of potential regulatory function of breast cancer risk locus at 6q251. Carcinogenesis 2016, 37, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingjord-Dale, M.; Grotmol, T.; Lee, E.; Van Den Berg, D.J.; Hofvind, S.; Couto, E.; Sovio, U.; dos-Santos-Silva, I.; Ursin, G. Breast Cancer Susceptibility Variants and Mammographic Density Phenotypes in Norwegian Postmenopausal Women. Cancer Epidem. Biomar. 2014, 23, 1752–1763. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.L.; Yu, J.C.; Chen, S.T.; Hsu, G.C.; Hsu, H.M.; Ho, J.Y.; Lin, Y.H.; Chang, C.C.; Fann, C.S.; Cheng, C.W.; et al. Diverse Associations between ESR1 Polymorphism and Breast Cancer Development and Progression. Clin. Cancer Res. 2010, 16, 3473–3484. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Siegfried, J.M.; Diergaarde, B.; Land, S.R.; Bowser, R.; Stabile, L.P.; Dacic, S.; Dhir, R.; Nukui, T.; Romkes, M.; et al. Genetic variation in ESR2 and estrogen receptor-beta expression in lung tumors. Cancer Epidemiol. 2013, 37, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Xu, L.; Chen, J.; Hu, J.; Yu, S.; Hu, G.; Huang, L.; Chen, X.; Yuan, X.; Li, G. Association of estrogen receptor beta variants and serum levels of estradiol with risk of colorectal cancer: A case control study. BMC Cancer 2012, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.H.; Yang, B.; Wang, X.H.; Xu, C.L.; Gao, X.F.; Gao, X.; Wang, L.H. Association between single-nucleotide polymorphisms in estrogen receptor beta gene and risk of prostate cancer. Zhonghua Wai Ke Za Zhi 2005, 43, 948–951. [Google Scholar] [PubMed]
- Muendlein, A.; Lang, A.H.; Geller-Rhomberg, S.; Winder, T.; Gasser, K.; Drexel, H.; Decker, T.; Mueller-Holzner, E.; Chamson, M.; Marth, C.; et al. Association of a common genetic variant of the IGF-1 gene with event-free survival in patients with HER2-positive breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, S.L.; Rensing, K.L.; Holly, J.M.; Chen, L.; Young, E.H.; Luben, R.; Ashford, S.; Song, K.; Yuan, X.; Dehghan, A.; et al. Prospective study of insulin-like growth factor-I, insulin-like growth factor-binding protein 3, genetic variants in the IGF1 and IGFBP3 genes and risk of coronary artery disease. Int. J. Mol. Epidemiol. Genet. 2011, 2, 261–285. [Google Scholar] [PubMed]
- Cho, S.H.; Kim, S.K.; Kwon, E.; Park, H.J.; Kwon, K.H.; Chung, J.H. Polymorphism of IGF1R Is Associated with Papillary Thyroid Carcinoma in a Korean Population. J. Interf. Cytok. Res. 2012, 32, 401–406. [Google Scholar] [CrossRef]
- Stanilov, N.S.; Karakolev, I.A.; Deliysky, T.S.; Jovchev, J.P.; Stanilova, S.A. Association of insulin-like growth factor-I receptor polymorphism with colorectal cancer development. Mol. Biol. Rep. 2014, 41, 8099–8106. [Google Scholar] [CrossRef]
- Reinmuth, N.; Kloos, S.; Warth, A.; Risch, A.; Muley, T.; Hoffmann, H.; Thomas, M.; Meister, M. Insulin-like growth factor 1 pathway mutations and protein expression in resected non-small cell lung cancer. Hum. Pathol. 2014, 45, 1162–1168. [Google Scholar] [CrossRef]
- Neuhausen, S.L.; Brummel, S.; Ding, Y.C.; Singer, C.F.; Pfeiler, G.; Lynch, H.T.; Nathanson, K.L.; Rebbeck, T.R.; Garber, J.E.; Couch, F.; et al. Genetic variation in insulin-like growth factor signaling genes and breast cancer risk among BRCA1 and BRCA2 carriers. Breast Cancer Res. 2009, 11, R76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, M.; Yuan, X.; Zhang, Z.; Zhang, P.; Chao, H.; Jiang, L.; Jiang, J. Association Between ESR1 PvuII, XbaI, and P325P Polymorphisms and Breast Cancer Susceptibility: A Meta-Analysis. Med Sci. Monit. 2015, 21, 2986–2996. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Yamano, Y.; Takahashi, H.; Koda, M.; Fujiwara, Y.; Hisada, A.; Miyazaki, W.; Katoh, T. Associations between estrogen receptor genetic polymorphisms, smoking status, and prostate cancer risk: A case-control study in Japanese men. Environ. Health Prev. Med. 2015, 20, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Gold, B.; Kalush, F.; Bergeron, J.; Scott, K.; Mitra, N.; Wilson, K.; Ellis, N.; Huang, H.; Chen, M.; Lippert, R.; et al. Estrogen receptor genotypes and haplotypes associated with breast cancer risk. Cancer Res. 2004, 64, 8891–8900. [Google Scholar] [CrossRef] [Green Version]
- Razavi, P.; Lee, E.; Bernstein, L.; Van Den Berg, D.; Horn-Ross, P.L.; Ursin, G. Variations in sex hormone metabolism genes, postmenopausal hormone therapy and risk of endometrial cancer. Int. J. Cancer 2012, 130, 1629–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, M.; Hamada, G.S.; Nishimoto, I.N.; Netto, M.M.; Motola Jr, J.; Laginha, F.M.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; et al. Isoflavone, polymorphisms in estrogen receptor genes and breast cancer risk in case-control studies in Japanese, Japanese Brazilians and non-Japanese Brazilians. Cancer Sci. 2009, 100, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Permuth-Wey, J.; Chen, Y.A.; Tsai, Y.Y.; Chen, Z.; Qu, X.; Lancaster, J.M.; Stockwell, H.; Dagne, G.; Iversen, E.; Risch, H.; et al. Inherited Variants in Mitochondrial Biogenesis Genes May Influence Epithelial Ovarian Cancer Risk. Cancer Epidem. Biomar. 2011, 20, 1131–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, E.M.; Tworoger, S.S.; Hankinson, S.E.; Baer, H.J. Genetic variability in IGF-1 and IGFBP-3 and body size in early life. BMC Public Health 2012, 12, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, M.; Hosono, S.; Ito, H.; Watanabe, M.; Mizuno, N.; Yatabe, Y.; Yamao, K.; Ueda, R.; Tajima, K.; Tanaka, H.; et al. Interaction between IGF-1 polymorphisms and overweight for the risk of pancreatic cancer in Japanese. Int. J. Mol. Epidemiol. Genet. 2011, 2, 354–366. [Google Scholar]
- Simons, C.C.; Schouten, L.J.; Godschalk, R.W.; Van Engeland, M.; Van Den Brandt, P.A.; Van Schooten, F.J.; Weijenberg, M.P. Genetic Variants in the Insulin-like Growth Factor Pathway and Colorectal Cancer Risk in the Netherlands Cohort Study. Sci. Rep. 2015, 5, 14126. [Google Scholar] [CrossRef] [Green Version]
- Berwick, M.; Reiner, A.S.; Paine, S.; Armstrong, B.K.; Kricker, A.; Goumas, C.; Cust, A.E.; Thomas, N.E.; Groben, P.A.; From, L.; et al. Sun exposure and melanoma survival: A GEM study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2145–2152. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.A.; Yourk, V.; Farhat, A.; Ziogas, A.; Meyskens, F.L.; Anton-Culver, H.; Liu-Smith, F. A Case-Control Study of the Genetic Variability in Reactive Oxygen Species-Metabolizing Enzymes in Melanoma Risk. Int. J. Mol. Sci. 2018, 19, 242. [Google Scholar] [CrossRef] [Green Version]
- Clarke, G.M.; Anderson, C.A.; Pettersson, F.H.; Cardon, L.R.; Morris, A.P.; Zondervan, K.T. Basic statistical analysis in genetic case-control studies. Nat. Protoc. 2011, 6, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Kido, T.; Sikora-Wohlfeld, W.; Kawashima, M.; Kikuchi, S.; Kamatani, N.; Patwardhan, A.; Chen, R.; Sirota, M.; Kodama, K.; Hadley, D.; et al. Are minor alleles more likely to be risk alleles? BMC Med. Genom. 2018, 11, 3. [Google Scholar] [CrossRef]
- Chen, S.Y.; Feng, Z.; Yi, X. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 2017, 9, 1725–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- LaMorte, W.W.; Sullivan, L. Confounding and Effect Measure Modification: Boston University School of Public Health. Available online: http://sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/BS704-EP713_Confounding-EM/BS704-EP713_Confounding-EM_print.html (accessed on 7 June 2019).
- Kucera, R.; Treskova, I.; Vrzalova, J.; Svobodova, S.; Topolcan, O.; Fuchsova, R.; Rousarova, M.; Treska, V.; Kydlicek, T. Evaluation of IGF1 serum levels in malignant melanoma and healthy subjects. Anticancer Res. 2014, 34, 5217–5220. [Google Scholar]
- Bradbury, K.E.; Appleby, P.N.; Tipper, S.J.; Travis, R.C.; Allen, N.E.; Kvaskoff, M.; Overvad, K.; Tjønneland, A.; Halkjær, J.; Cervenka, I.; et al. Circulating insulin-like growth factor I in relation to melanoma risk in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2019, 144, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Breathnach, A.S. The Epidermal Melanin Unit System. Derm. Wochenschr. 1963, 147, 481–489. [Google Scholar]
- Al-Zahrani, A.; Sandhu, M.S.; Luben, R.N.; Thompson, D.; Baynes, C.; Pooley, K.A.; Luccarini, C.; Munday, H.; Perkins, B.; Smith, P.; et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Hum. Mol. Genet. 2006, 15, 1–10. [Google Scholar] [CrossRef]
- Cheng, I.; Stram, D.O.; Penney, K.L.; Pike, M.; Le Marchand, L.; Kolonel, L.N.; Hirschhorn, J.; Altshuler, D.; Henderson, B.E.; Freedman, M.L. Common genetic variation in IGF1 and prostate cancer risk in the Multiethnic Cohort. J. Natl. Cancer Inst. 2006, 98, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; McKay, J.D.; Wiklund, F.; Rinaldi, S.; Verheus, M.; Van Gils, C.H.; Hallmans, G.; Bälter, K.; Adami, H.O.; Grönberg, H.; et al. Implications for prostate cancer of insulin-like growth factor-I (IGF-I) genetic variation and circulating IGF-I levels. J. Clin. Endocrinol. Metab. 2007, 92, 4820–4826. [Google Scholar] [CrossRef]
- Terry, K.L.; Tworoger, S.S.; Gates, M.A.; Cramer, D.W.; Hankinson, S.E. Common genetic variation in IGF1, IGFBP1 and IGFBP3 and ovarian cancer risk. Carcinogenesis 2009, 30, 2042–2046. [Google Scholar] [CrossRef]
- Ennishi, D.; Shitara, K.; Ito, H.; Hosono, S.; Watanabe, M.; Ito, S.; Sawaki, A.; Yatabe, Y.; Yamao, K.; Tajima, K.; et al. Between insulin-like growth factor-1 polymorphisms and stomach cancer risk in a Japanese population. Cancer Sci. 2011, 102, 2231–2235. [Google Scholar] [CrossRef]
- Kasprzak, A.; Kwasniewski, W.; Adamek, A.; Gozdzicka-Jozefiak, A. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat. Res. Rev. Mutat. Res. 2017, 772, 78–104. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Ahn, S.H.; Mishra, S.K.; Hong, K.M.; Lee, E.S.; Shin, K.H.; Ro, J.; Lee, K.S.; Kim, M.K. Association of polymorphisms and haplotypes in the insulin-like growth factor 1 receptor (IGF1R) gene with the risk of breast cancer in Korean women. PLoS ONE 2014, 9, e84532. [Google Scholar] [CrossRef] [PubMed]
- Gately, K.; Forde, L.; Gray, S.; Morris, D.; Corvin, A.; Tewari, P.; O’byrne, K. Mutational analysis of the insulin-like growth factor 1 receptor tyrosine kinase domain in non-small cell lung cancer patients. Mol. Clin. Oncol. 2015, 3, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deming, S.L.; Ren, Z.; Wen, W.; Shu, X.O.; Cai, Q.; Gao, Y.T.; Zheng, W. Genetic variation in IGF1, IGF-1R, IGFALS, and IGFBP3 in breast cancer survival among Chinese women: A report from the Shanghai breast cancer study. Breast Cancer Res. Treat. 2007, 104, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, S.C.; Armouti, M.; Ko, C.; Stocco, C. IGF1R Expression in Ovarian Granulosa Cells Is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology 2017, 158, 2309–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voudouri, K.; Nikitovic, D.; Berdiaki, A.; Kletsas, D.; Karamanos, N.K.; Tzanakakis, G.N. IGF-I/EGF and E2 signaling crosstalk through IGF-IR conduit point affects breast cancer cell adhesion. Matrix Biol. 2016, 56, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Zavodovskaya, M.; Youngren, J.F.; Campbell, M.; Diamond, M.; Jones, J.; Shiry, L.; Allan, G.; Maddux, B.A.; Goldfine, I.D. Inhibitory effects of nordihydroguaiaretic acid (NDGA) on the IGF-1 receptor and androgen dependent growth of LAPC-4 prostate cancer cells. Prostate 2008, 68, 1232–1240. [Google Scholar] [CrossRef]
- Albani, D.; Mazzuco, S.; Polito, L.; Batelli, S.; Biella, G.; Ongaro, F.; Gustafson, D.R.; Antuono, P.; Gajo, G.; Durante, E.; et al. Insulin-like growth factor 1 receptor polymorphism rs2229765 and circulating interleukin-6 level affect male longevity in a population-based prospective study (Treviso Longeva--TRELONG). Aging Male 2011, 14, 257–264. [Google Scholar] [CrossRef]
- Yuan, T.A.; Meyskens, F.; Liu-Smith, F. A cancer registry-based analysis on the non-white populations reveals a critical role of the female sex in early-onset melanoma. Cancer Cause Control 2018, 29, 405–415. [Google Scholar] [CrossRef]
- Castaldi, P. GWAS Exercise 6—Adjusting for Population Stratification. 2013. Available online: http://sites.tufts.edu/cbi/files/2013/02/GWAS_Exercise6_Stratification.pdf. (accessed on 7 June 2019).
- Li, C.; Liu, Z.; Wang, L.E.; Gershenwald, J.E.; Lee, J.E.; Prieto, V.G.; Duvic, M.; Grimm, E.A.; Wei, Q. Haplotype and genotypes of the VDR gene and cutaneous melanoma risk in non-Hispanic whites in Texas: A case-control study. Int. J. Cancer 2008, 122, 2077–2084. [Google Scholar] [CrossRef] [Green Version]
| Study Participants | GEM (N = 349) | GENEVA (N = 3114) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (N = 177) | Controls (N = 172) | Cases (N = 2054) | Controls (N = 1060) | |||||||||||||
| Men | Women | Men | Women | Men | Women | Men | Women | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Age (years) | ||||||||||||||||
| 0–29 | 0 | 0 | 2 | 1.1 | 0 | 0.0 | 0 | 0 | 55 | 2.7 | 100 | 4.9 | 16 | 1.5 | 46 | 4.3 |
| 30–49 | 13 | 13.8 | 28 | 15.8 | 20 | 11.6 | 37 | 21.5 | 373 | 18.2 | 352 | 17.1 | 198 | 18.7 | 184 | 17.4 |
| 50–69 | 43 | 24.3 | 37 | 20.9 | 59 | 34.3 | 29 | 16.9 | 606 | 29.5 | 336 | 16.4 | 366 | 34.5 | 186 | 17.5 |
| 70+ | 33 | 18.6 | 12 | 6.8 | 19 | 11.0 | 8 | 4.7 | 153 | 7.4 | 79 | 3.8 | 48 | 4.5 | 16 | 1.5 |
| Unknown | 5 | 2.8 | 4 | 2.3 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Family history of melanoma | ||||||||||||||||
| Yes | 10 | 5.6 | 18 | 10.2 | 1 | 0.6 | 3 | 1.7 | 388 | 18.9 | 280 | 13.6 | 399 | 37.6 | 273 | 25.8 |
| No | 78 | 44.1 | 60 | 33.9 | 97 | 56.4 | 71 | 41.3 | 231 | 11.2 | 157 | 7.6 | 227 | 21.4 | 159 | 15.0 |
| Unknown | 6 | 3.4 | 5 | 2.8 | 0 | 0.0 | 0 | 0.0 | 568 | 27.7 | 430 | 20.9 | 2 | 0.2 | 0 | 0.0 |
| Total | 94 | 53.1 | 83 | 46.9 | 98 | 57.0 | 74 | 43.0 | 1187 | 57.8 | 867 | 42.2 | 628 | 59.2 | 432 | 40.8 |
| Gene | SNP | Location | dbSNP ID | Minor Allele Disease Associations | References |
|---|---|---|---|---|---|
| ESR1 | T > G | Intron | rs12662670 | Common breast cancer locus | [19] |
| −397T > C | Promoter | rs2234693 | breast cancer susceptibility, prostate cancer risk | [49,50] | |
| G > A | Promoter | rs2046210 | Breast cancer risk | [36,37] | |
| A > C | Promoter | rs3734805 | Breast cancer risk | [36] | |
| A > G | Intron | rs827421 | Breast cancer risk | [51] | |
| ESR2 | C > G | 3’UTR | rs1255998 | Endometrial cancer | [52] |
| C > T | Exon | rs1256049 | Risks of breast cancer and colorectal cancer | [41,53] | |
| G > A | Intron | rs1256061 | Risks in lung tumors and ovarian cancer | [40,54] | |
| IGF1 | C > G | Intron | rs1520220 | Obesity, poor breast cancer survival, pancreatic cancer risk | [43,55,56] |
| A > G | Intron | rs2946834 | Poor outcome in patients with breast cancer | [43] | |
| C > A | Intron | rs5742694 | Colorectal cancer risk, poor breast cancer survival | [43,57] | |
| IGF1R | G > A | Exon | rs2229765 | Colorectal cancer risk, papillary thyroid carcinoma risk | [45,46] |
| T > C | Intron | rs8038415 | Risks in non-small cell lung cancer, breast cancer | [47,48] |
| SNP | Gene | Genotyping Rate a | Minor Allele Frequency (MAF) | Association (p-Value | Benjamini–Hochberg Critical Value) b | HWE c (p-Value) | dbSNP MAF d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 170) | Controls (n = 152) | Cases (n = 170) | Controls (n = 152) | Genotypic | Allelic | Recessive | Dominant | ||||
| rs12662670 | ESR1 | 97.1% | 82.2% | 6.7% | 9.6% | 0.083 | 0.077 | 0.255 | 0.115 | 0.034 | 0.077 | 0.638 | 0.173 | 0.014 | 10.7% |
| rs2046210 | ESR1 | 94.7% | 88.2% | 30.4% | 36.6% | 0.247 | 0.115 | 0.137 | 0.058 | 0.650 | 0.212 | 0.120 | 0.058 | 0.458 | 41.2% |
| rs2234693 | ESR1 | 98.2% | 96.1% | 38.3% | 36.0% | 0.254 | 0.135 | 0.598 | 0.212 | 0.588 | 0.173 | 0.238 | 0.077 | 0.007 | 44.6% |
| rs3734805 | ESR1 | 98.2% | 75.7% | 6.3% | 7.8% | 0.575 | 0.212 | 0.590 | 0.192 | N/A | 0.575 | 0.154 | 1.000 | 10.5% |
| rs827421 | ESR1 | 98.8% | 86.8% | 51.2% | 59.1% | 0.141 | 0.096 | 0.059| 0.038 | 0.214| 0.096 | 0.097 | 0.038 | 0.857 | 47.8% |
| rs1255998 | ESR2 | 98.8% | 96.1% | 8.6% | 11.3% | 0.349 | 0.154 | 0.325 | 0.135 | 0.465 | 0.154 | 0.370 | 0.115 | 0.696 | 36.9% |
| rs1256049 | ESR2 | 97.6% | 98.0% | 7.8% | 7.7% | 1.000 | 0.25 | 1.000 | 0.25 | N/A | 1.000 | 0.25 | 1.000 | 13.0% |
| rs1256061 | ESR2 | 98.2% | 88.8% | 49.1% | 47.4% | 0.384 | 0.173 | 0.740 | 0.231 | 0.335 | 0.115 | 0.786 | 0.212 | 0.731 | 40.0% |
| rs1520220 | IGF1 | 97.1% | 88.8% | 28.2% | 24.8% | 0.005 | 0.058 | 0.404 | 0.154 | 0.013 | 0.038 | 0.672 | 0.192 | 1.000 | 32.0% |
| rs2946834 | IGF1 | 97.6% | 74.3% | 35.2% | 40.7% | 0.445 | 0.192 | 0.222 | 0.077 | 0.370 | 0.135 | 0.362| 0.096 | 0.435 | 40.0% |
| rs5742694 | IGF1 | 98.8% | 93.4% | 50.6% | 41.2% | < 0.001 | 0.019 | 0.024| 0.019 | 0.025 | 0.058 | < 0.001 | 0.019 | < 0.001 | 21.6% |
| rs2229765 | IGF1R | 97.6% | 96.1% | 35.8% | 40.8% | 0.004 | 0.038 | 0.239 | 0.096 | 0.003 | 0.019 | 0.918 | 0.231 | 1.000 | 33.6% |
| rs8038415 | IGF1R | 97.1% | 98.1% | 51.5% | 54.3% | 0.654 | 0.231 | 0.411 | 0.173 | 0.648 | 0.192 | 0.458 | 0.135 | 1.000 | 42.5% |
| SNP a | Gene | Minor Allele Frequency (MAF) | Association (p-Value | Benjamini–Hochberg Critical Value) b | HWE c (p-Value) | dbSNP MAF d | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 1965) | Controls (n = 1038) | Genotypic | Allelic | Recessive | Dominant | ||||
| rs12662670 e | ESR1 | 14.1% | 14.3% | 0.995 | 0.231 | 0.446 | 0.212 | 0.961 | 0.231 | 0.069 | 0.077 | 0.997 | 10.7% |
| rs2046210 e | ESR1 | 54.2% | 51.1% | 0.640 | 0.135 | 0.248 | 0.135 | 0.936 | 0.212 | 0.155 | 0.154 | 0.984 | 41.2% |
| rs2234693 | ESR1 | 47.2% | 44.4% | 0.035 | 0.038 | 0.047 | 0.038 | 0.580 | 0.077 | 0.010 | 0.038 | 0.059 | 44.6% |
| rs3734805 | ESR1 | 8.0% | 6.9% | 0.181 | 0.096 | 0.129 | 0.115 | 0.660 | 0.115 | 0.090 | 0.115 | 0.624 | 10.5% |
| rs827421 | ESR1 | 50.7% | 47.6% | 0.018 | 0.019 | 0.027 | 0.019 | 0.440 | 0.058 | 0.005 | 0.019 | 0.192 | 47.8% |
| rs1255998 | ESR2 | 8.9% | 10.5% | 0.093 | 0.058 | 0.048 | 0.058 | 0.110 | 0.019 | 0.079 | 0.096 | 0.869 | 36.9% |
| rs1256049 | ESR2 | 2.8% | 3.5% | NA | 0.112 | 0.096 | NA | 0.104 | 0.135 | 0.634 | 13.0% |
| rs1256061 e | ESR2 | 60.7% | 62.6% | 0.971 | 0.212 | 0.404 | 0.192 | 0.678 | 0.154 | 0.581 | 0.231 | 0.979 | 40.0% |
| rs1520220 e | IGF1 | 63.7% | 62.6% | 0.752 | 0.173 | 0.281 | 0.154 | 0.642 | 0.096 | 0.257 | 0.173 | 0.985 | 32.0% |
| rs2946834 | IGF1 | 31.2% | 33.2% | 0.158 | 0.077 | 0.102 | 0.077 | 0.672 | 0.135 | 0.055 | 0.058 | 0.043 | 40.0% |
| rs5742694 e | IGF1 | 65.0% | 65.5% | 0.941 | 0.192 | 0.283 | 0.173 | 0.740 | 0.173 | 0.263 | 0.192 | 1.000 | 21.6% |
| rs2229765 e | IGF1R | 59.7% | 57.2% | 0.315 | 0.115 | 0.950 | 0.25 | 0.403 | 0.038 | 0.417 | 0.212 | 1.000 | 33.6% |
| rs8038415 e | IGF1R | 61.3% | 60.0% | 0.646 | 0.154 | 0.815 | 0.231 | 0.806 | 0.192 | 0.609 | 0.25 | 1.000 | 42.5% |
| Logistic Regression Models | Crude (Model A) | Model B b | Model C c | Model D d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs/Genetic Models | Genotypes | Cases n (%) | Controls n (%) | OR (95% CI) | p-Value a | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| rs1520220/IGF1 Recessive | CC + CG | 139 (81.8%) | 127 (83.6%) | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| GG | 26 (15.3%) | 8 (5.3%) | 2.97 (1.35, 7.23) | 0.010 | 2.86 (1.29, 6.98) | 0.014 | 2.79 (1.24, 6.92) | 0.018 | 2.80 (1.24, 6.93) | 0.018 | |
| Sex | -- | -- | -- | -- | 1.08 (0.68, 1.72) | 0.743 | -- | -- | 0.95 (0.58, 1.53) | 0.819 | |
| Family history | -- | -- | -- | -- | -- | -- | 6.60 (2.48, 22.86) | 0.0006 | 6.68 (2.50, 23.26) | 0.006 | |
| Model | -- | -- | -- | 0.006 | -- | 0.009 | -- | 0.0117 | -- | 0.0117 | |
| rs2229765/IGF1R Recessive | GG + GA | 157 (92.4%) | 122 (80.3%) | Reference | -- | Reference | -- | Reference | -- | Reference | -- |
| AA | 9 (5.3%) | 24 (15.8%) | 0.29 (0.12, 0.62) | 0.003 | 0.29 (0.12, 0.63) | 0.003 | 0.25 (0.10, 0.57) | 0.002 | 0.25 (0.10, 0.56) | 0.002 | |
| Sex | -- | -- | -- | -- | 0.98 (0.62, 1.56) | 0.948 | -- | -- | 0.84 (0.52, 1.36) | 0.484 | |
| Family history | -- | -- | -- | -- | -- | -- | 8.38 (3.08, 29.79) | 0.00017 | 8.77 (3.19, 31.44) | 0.00014 | |
| Model | -- | -- | -- | 0.001 | -- | 0.001 | -- | 0.002 | -- | 0.002 | |
| Model | Male OR (95% CI) | p-Value a | Female OR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| rs1520220/IGF1 Recessive | CC + CG | Reference | -- | Reference | -- |
| GG | 8.11 (2.20, 52.5) | 0.006 | 0.15 (0.018, 0.86) | 0.045 | |
| rs2229765/IGF1R Recessive | GG + GA | Reference | -- | Reference | -- |
| AA | 0.24 (0.07, 0.64) | 0.008 | 1.70 (0.32, 8.86) | 0.526 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.-A.; Yourk, V.; Farhat, A.; Guo, K.L.; Garcia, A.; Meyskens, F.L.; Liu-Smith, F. A Possible Link of Genetic Variations in ER/IGF1R Pathway and Risk of Melanoma. Int. J. Mol. Sci. 2020, 21, 1776. https://doi.org/10.3390/ijms21051776
Yuan T-A, Yourk V, Farhat A, Guo KL, Garcia A, Meyskens FL, Liu-Smith F. A Possible Link of Genetic Variations in ER/IGF1R Pathway and Risk of Melanoma. International Journal of Molecular Sciences. 2020; 21(5):1776. https://doi.org/10.3390/ijms21051776
Chicago/Turabian StyleYuan, Tze-An, Vandy Yourk, Ali Farhat, Katherine L. Guo, Angela Garcia, Frank L. Meyskens, and Feng Liu-Smith. 2020. "A Possible Link of Genetic Variations in ER/IGF1R Pathway and Risk of Melanoma" International Journal of Molecular Sciences 21, no. 5: 1776. https://doi.org/10.3390/ijms21051776
APA StyleYuan, T. -A., Yourk, V., Farhat, A., Guo, K. L., Garcia, A., Meyskens, F. L., & Liu-Smith, F. (2020). A Possible Link of Genetic Variations in ER/IGF1R Pathway and Risk of Melanoma. International Journal of Molecular Sciences, 21(5), 1776. https://doi.org/10.3390/ijms21051776





