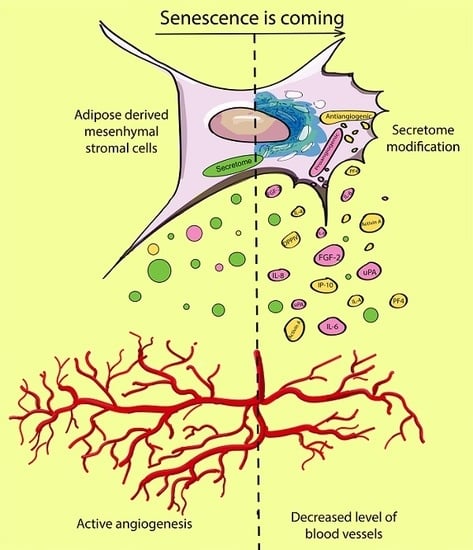
Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis
Abstract
1. Introduction
2. Results
2.1. ASC Identification
2.2. Cell Senescence Identification
2.3. Angiogenic Potential of Senescent ASCs
2.4. Characterization of Angiogenesis-Associated Secretome of Senescent ASCs
3. Discussion
4. Materials and Methods
4.1. ASCs Isolation and Expansion
4.2. Cell Senescence Identification
4.3. Isolation and Culture of ECs
4.4. Chorioallantoic Membrane Assay in Ovo
4.5. Capillary-Like Tube Formation
4.6. Nontargeted Cell Migration Assay (“Wound Healing”)
4.7. Analysis of Proteins Secreted by ASCs
4.8. Quantitative PCR Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| ASCs | Adipose-derived mesenchymal stem cells |
| IFATS | International Federation for Adipose Therapeutics and Science |
| ISCT | International Society for Cell and Gene Therapy |
| CD | Cluster of differentiation |
| PD | Population doubling |
| FSC | Forward scatter |
| SSC | Side scatter |
| SA-β-gal | Senescence associated-β-galactosidase |
| CM | Conditioned medium |
| CAM | Chorioallantoic membrane |
| HUVECs | Human umbilical vein endothelial cells |
| ECs | Endothelial cells |
| SASP | Senescence-associated secretory phenotype |
| FBS | Fetal bovine serum |
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, 54. [Google Scholar] [CrossRef]
- Lunyak, V.V.; Amaro-Ortiz, A.; Gaur, M. Mesenchymal stem cells secretory responses: Senescence messaging secretome and immunomodulation perspective. Front. Genet. 2017, 8, 220. [Google Scholar] [CrossRef]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.C.; Rogante, M.; Mitrečić, D.; Ferrari, R.; Zavan, B. Exosome in cardiovascular diseases: A complex world full of hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 1–3. [Google Scholar] [CrossRef]
- McLeod, C.M.; Mauck, R.L. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cell Mater. 2017, 34, 217–231. [Google Scholar] [CrossRef]
- Schäffler, A.; Büchler, C. Concise review: Adipose tissue-derived stromal cells—Basic and clinical implications for novel cell-based therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.K.; Cho, H.H.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Barba, M.; Cicione, C.; Bernardini, C.; Michetti, F.; Lattanzi, W. Adipose-derived mesenchymal cells for bone regereneration: State of the art. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Dufrane, D. Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant. 2017, 26, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.H.; Kim, S.Y.; Kim, Y.J.; Kim, S.J.; Lee, J.B.; Bae, Y.C.; Sung, S.M.; Jung, J.S. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol. Biochem. 2006, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Kondo, K.; Shintani, S.; Shibata, R.; Murakami, H.; Murakami, R.; Imaizumi, M.; Kitagawa, Y.; Murohara, T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 61–66. [Google Scholar] [CrossRef]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef]
- Madonna, R.; De Caterina, R. Adipose tissue: A new source for cardiovascular repair. J. Cardiovasc. Med. 2010, 11, 71–80. [Google Scholar] [CrossRef]
- Nahar, S.; Nakashima, Y.; Miyagi-Shiohira, C.; Kinjo, T.; Toyoda, Z.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, H.; Fujita, J. Cytokines in adipose-derived mesenchymal stem cells promote the healing of liver disease. World J. Stem Cells 2018, 10, 146. [Google Scholar] [CrossRef]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef]
- Gu, Y.; Li, T.; Ding, Y.; Sun, L.; Tu, T.; Zhu, W.; Hu, J.; Sun, X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 2016, 13, 5207–5215. [Google Scholar] [CrossRef] [PubMed]
- Legzdina, D.; Romanausk, A.; Nikulshin, S.; Kozlovska, T.; Berzins, U. Characterization of senescence of culture-expanded human adipose-derived mesenchymal stem cells. Int. J. Stem Cells 2016, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Ratushnyy, A.; Lobanova, M.; Buravkova, L.B. Expansion of adipose tissue-derived stromal cells at “physiologic” hypoxia attenuates replicative senescence. Cell Biochem. Funct. 2017, 35, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; Von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Cell senescence: Hypertrophic arrest beyond the restriction point. J. Cell Physiol. 2006, 209, 592–597. [Google Scholar] [CrossRef]
- Pili, R.; Guo, Y.; Chang, J.; Nakanishi, H.; Martin, G.; Passaniti, A. Altered angiogenesis underlying age-dependent changes in tumor growth. J. Natl. Cancer Inst. 1994, 86, 1303–1314. [Google Scholar] [CrossRef]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Efimenko, A.Y.; Starostina, E.E.; Kalinina, N.I.; Parfyenova, E.V. Age effects on angiogenic properties of adipose tissue mesenchymal stem cells. Cell. Transplant. Tissue Eng. 2011, 6, 48–57. [Google Scholar]
- De Barros, S.; Dehez, S.; Arnaud, E.; Barreau, C.; Cazavet, A.; Perez, G.; Planat-Bénard, V. Aging-related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol. Ther. 2013, 21, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kazama, T.; Nagaoka, Y.; Inamo, Y.; Mugishima, H.; Takahashi, S.; Matsumoto, T. Influence of donor age and passage number on angiogenic activity in human adipose-derived stem cell-conditioned media. J. Stem Cell Res. 2015, 5, 307. [Google Scholar] [CrossRef]
- Liekens, S.; De Clercq, E.; Neyts, J. Angiogenesis: Regulators and clinical applications. Biochem. Pharmacol. 2001, 61, 253–270. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Greisler, H.P. In vitro models of angiogenesis. World J. Surg. 2007, 31, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Schlieve, C.R.; Mojica, S.G.; Holoyda, K.A.; Hou, X.; Fowler, K.L.; Grikscheit, T.C. Vascular endothelial growth factor (VEGF) bioavailability regulates angiogenesis and intestinal stem and progenitor cell proliferation during postnatal small intestinal development. PLoS ONE 2016, 11, e0151396. [Google Scholar] [CrossRef]
- Sarkanen, J.R.; Mannerstrom, M.; Vuorenpaa, H.; Uotila, J.; Ylikomi, T.; Heinonen, T. Intra-laboratory pre-validation of a human cell based in vitro angiogenesis assay for testing angiogenesis modulators. Front. Pharmacol. 2011, 1, 147. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Rubina, K.A.; Tkachuk, V.A. Molecular and cellular mechanisms of angiogenesis in physiological and pathological conditions. Ross. Fiziol. Zhurnal Im. Sechenova 2017, 103, 121–137. [Google Scholar]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Cho, M.L.; Min, S.Y.; Shin, Y.J.; Yoo, S.A.; Choi, J.J.; Cho, C.S. Effect of interleukin-4 on vascular endothelial growth factor production in rheumatoid synovial fibroblasts. Clin. Exp. Immunol. 2007, 147, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef]
- Kaneda, H.; Arao, T.; Matsumoto, K.; De Velasco, M.A.; Tamura, D.; Aomatsu, K.; Tanaka, K. Activin A inhibits vascular endothelial cell growth and suppresses tumour angiogenesis in gastric cancer. Br. J. Cancer 2011, 105, 1210–1217. [Google Scholar] [CrossRef]
- Bikfalvi, A. Recent developments in the inhibition of angiogenesis: Examples from studies on platelet factor-4 and the VEGF/VEGFR system. Biochem. Pharmacol. 2004, 68, 1017–1021. [Google Scholar] [CrossRef]
- Albini, A.; Marchisone, C.; Del Grosso, F.; Benelli, R.; Masiello, L.; Tacchetti, C.; Belardelli, F. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: A gene therapy approach. Am. J. Pathol. 2000, 156, 1381–1393. [Google Scholar] [CrossRef]
- Maurer, A.M.; Zhou, B.; Han, Z.C. Roles of platelet factor 4 in hematopoiesis and angiogenesis. Growth Factors 2006, 24, 242–252. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, L.; Yang, G.; Piao, L.; Jin, M.; Cheng, X. Dipeptidyl Peptidase-IV Inhibition for the Treatment of Cardiovascular Disease―Recent Insights Focusing on Angiogenesis and Neovascularization. Circ. J. 2017, 81, 770–776. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M.R.; Bianchi, C.; Sellke, F.W. Hypoxia inducible factor-1α, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: A unifying hypothesis. Curr. Drug Targets 2008, 9, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.W.; Li, H.; Guedez, L.; Wingfield, P.T.; Diaz, T.; Salloum, R.; Stetler-Stevenson, W.G. TIMP-2 mediated inhibition of angiogenesis: An MMP-independent mechanism. Cell 2003, 114, 171–180. [Google Scholar] [CrossRef]
- Qi, J.H.; Anand-Apte, B. Tissue inhibitor of metalloproteinase-3 (TIMP3) promotes endothelial apoptosis via a caspase-independent mechanism. Apoptosis 2015, 20, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Carpizo, D.R.; Iruela-Arispe, M.L. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003, 278, 23656–23665. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Grinakovskaya, O.S.; Andreeva, E.R.; Zhambalova, A.P.; Kozionova, M.P. Characteristics of human lipoaspirate isolated mesenchymal stromal cells cultivated under lower oxygen tension. Tsitologiya 2009, 51, 4–10. [Google Scholar] [CrossRef]
- Greenwood, S.K.; Hill, R.B.; Sun, J.T.; Armstrong, M.J.; Johnson, T.E.; Gara, J.P.; Galloway, S.M. Population doubling: A simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduces irrelevant positive results. Environ. Mol. Mutagen. 2004, 43, 36–44. [Google Scholar] [CrossRef]
- Miyazawa, K.; Hondo, T.; Kanaya, T.; Tanaka, S.; Takakura, I.; Itani, W.; Aso, H. Characterization of newly established bovine intestinal epithelial cell line. Histochem. Cell Biol. 2010, 133, 125–134. [Google Scholar] [CrossRef]
- Gruber, H.E.; Somayaji, S.; Riley, F.; Hoelscher, G.L.; Norton, H.J.; Ingram, J.; Hanley, E.N., Jr. Human adipose-derived mesenchymal stem cells: Serial passaging, doubling time and cell senescence. Biotech. Histochem. 2012, 87, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Udartseva, O.O.; Lobanova, M.V.; Andreeva, E.R.; Buravkov, S.V.; Ogneva, I.V.; Buravkova, L.B. Acute hypoxic stress affects migration machinery of tissue O2-adapted adipose stromal cells. Stem Cells Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.R.; Udartseva, O.O.; Zhidkova, O.V.; Buravkov, S.V.; Ezdakova, M.I.; Buravkova, L.B. IFN-gamma priming of adipose-derived stromal cells at “physiological” hypoxia. J. Cell. Physiol. 2018, 233, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. https://doi.org/10.3390/ijms21051802
Ratushnyy A, Ezdakova M, Buravkova L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. International Journal of Molecular Sciences. 2020; 21(5):1802. https://doi.org/10.3390/ijms21051802
Chicago/Turabian StyleRatushnyy, Andrey, Mariia Ezdakova, and Ludmila Buravkova. 2020. "Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis" International Journal of Molecular Sciences 21, no. 5: 1802. https://doi.org/10.3390/ijms21051802
APA StyleRatushnyy, A., Ezdakova, M., & Buravkova, L. (2020). Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. International Journal of Molecular Sciences, 21(5), 1802. https://doi.org/10.3390/ijms21051802