Reperfusion Arrhythmias Increase after Superior Cervical Ganglionectomy Due to Conduction Disorders and Changes in Repolarization
Abstract
1. Introduction
2. Results
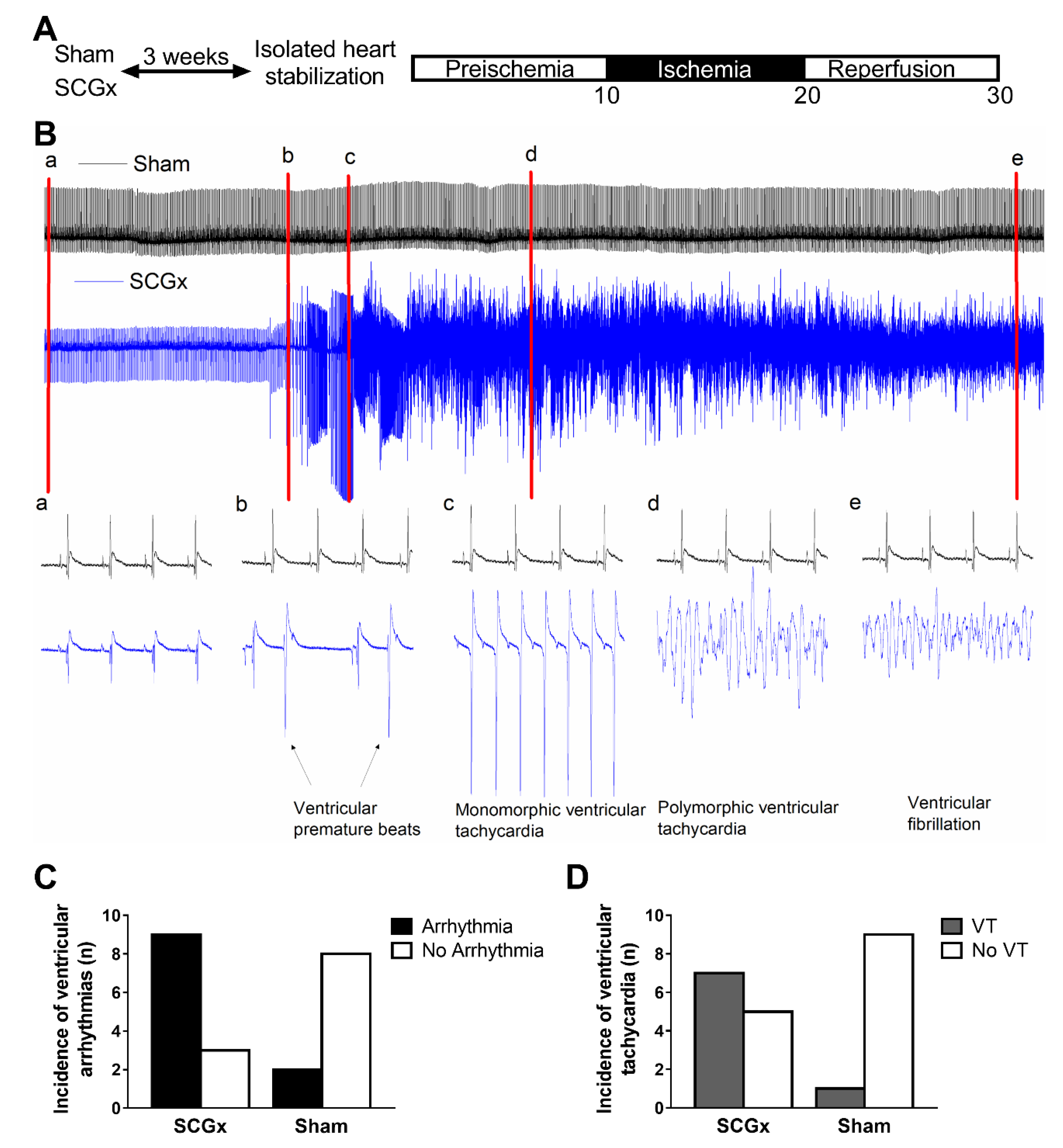
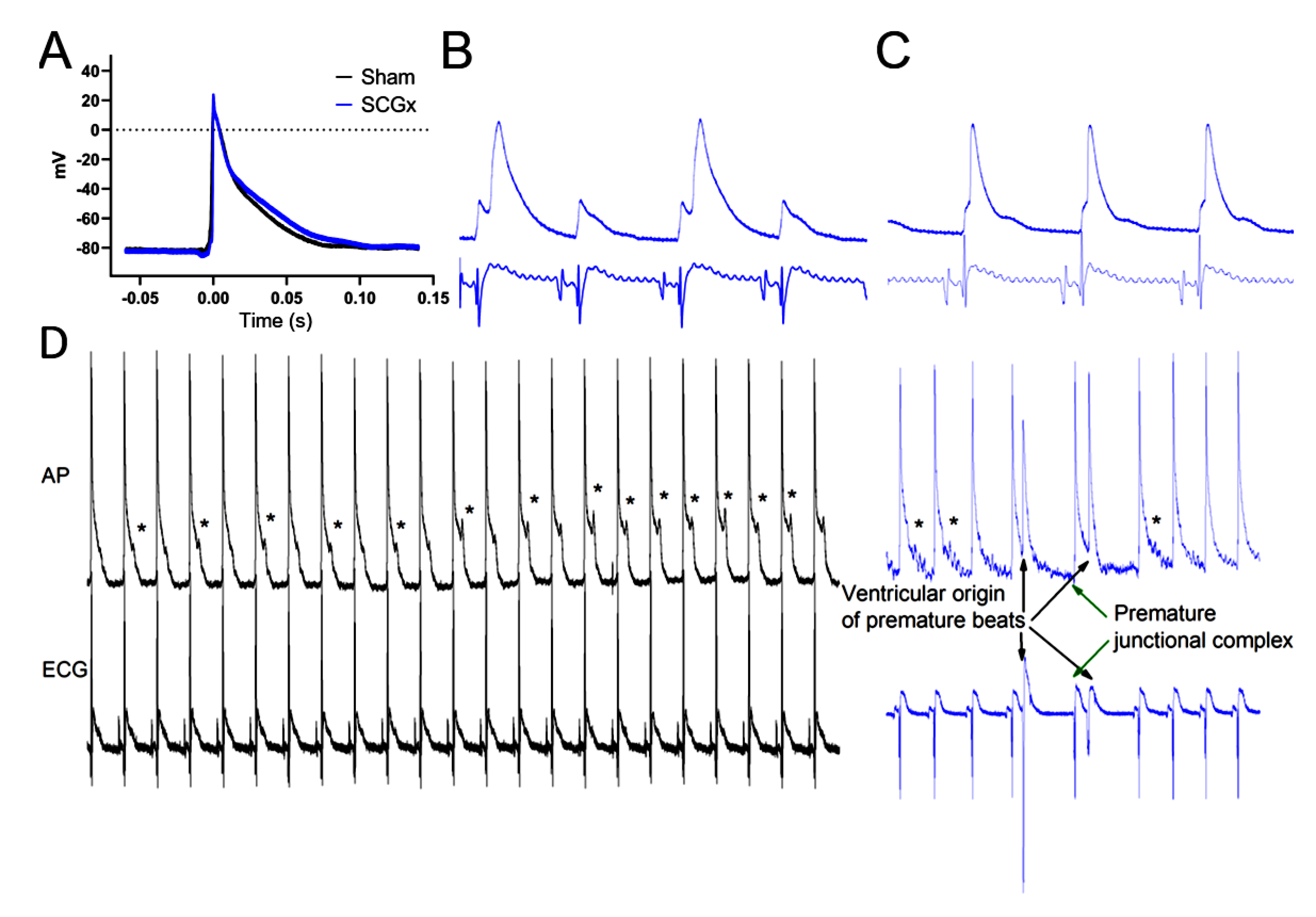
2.1. Electrophysiological Effects of SCGx in Isolated Rat Hearts Submitted to Regional Ischemia/Reperfusion
2.2. SCGx Reduced the Expression of Myocardial Melatonin Receptors and SERCA2A
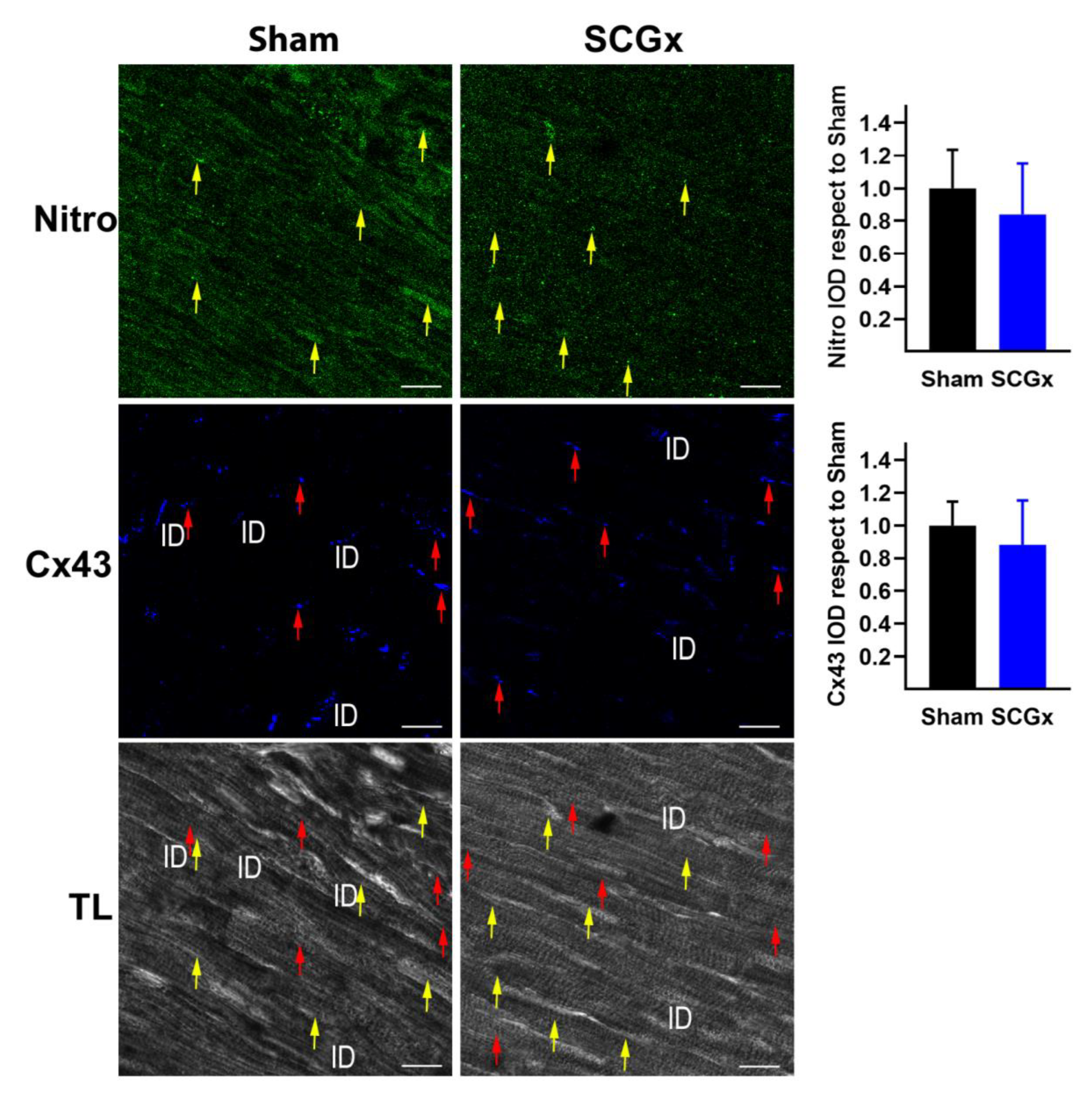
2.3. SCGx Increased KATP Channels and Connexin 43 Lateralization, Without Changing TNFα or Nitrotyrosine
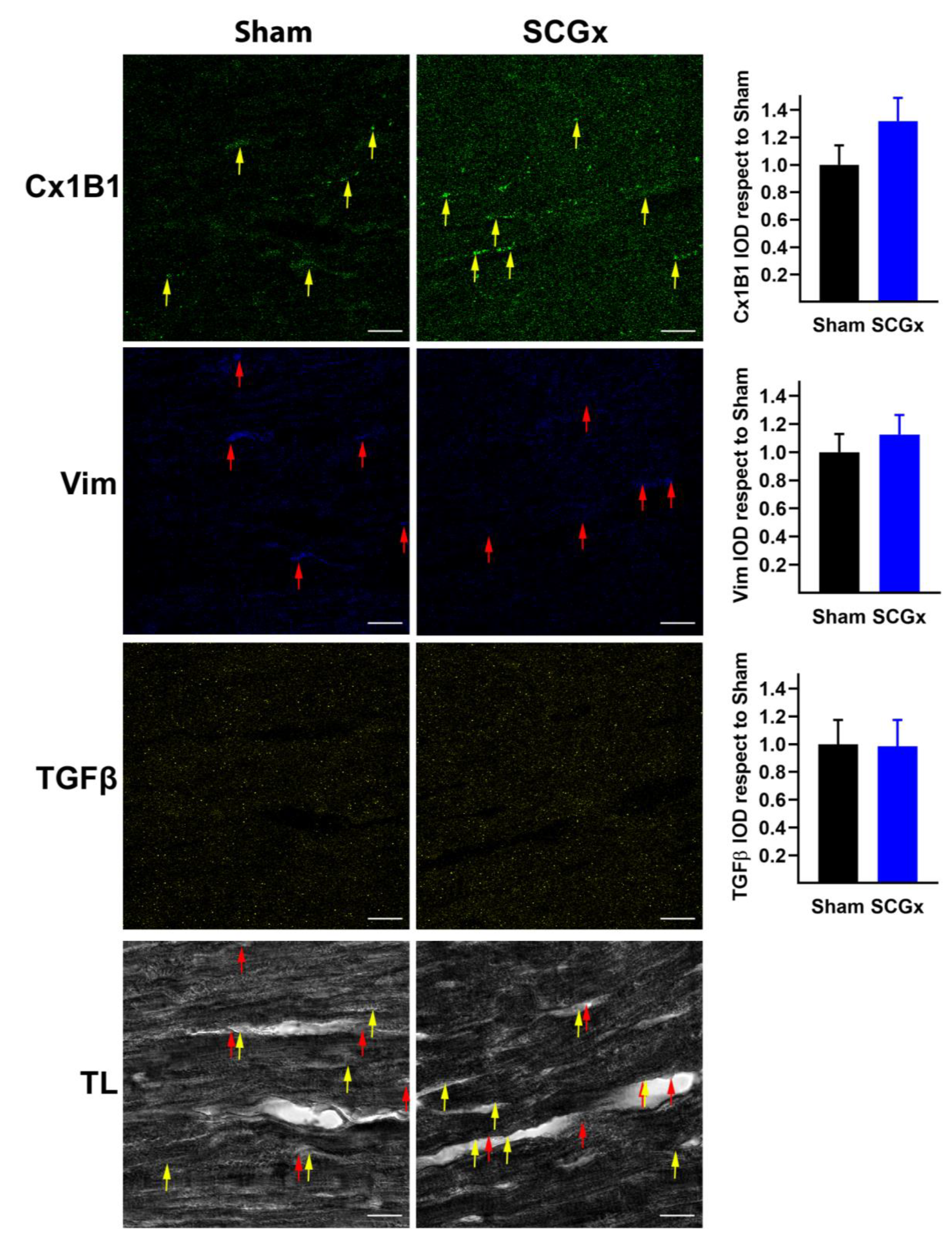
2.4. SCGx Did Not Increase Markers of Fibrosis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.3. Electrophysiological Studies
4.3.1. Arrhythmias
4.3.2. Electrocardiograms and Action Potentials
4.4. Confocal Immunofluorescence Microscopy
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | action potential duration |
| Cx43 | connexin 43 |
| IOD | integrated optical density |
| MT1 | melatonin type 1 receptor |
| MT2 | melatonin type 2 receptor |
| Nitro | nitrotyrosine |
| SCGx | superior cervical ganglionectomy |
| SERCA2A | sarco/endoplasmic reticulum Ca2+-ATPase, paralog 2A |
| TGFβ | transforming growing factor β |
| TL | transmitted light |
| TNFα | tumor necrosis factor α |
References
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart. Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R. Melatonin: Biological Basis of its Function in Health and Disease; CRC Press: London, UK, 2005. [Google Scholar]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Avanzas, P. The role of melatonin in acute myocardial infarction. Front. Biosci. 2012, 17, 2433. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Reiter, R.J. Circadian variation in acute myocardial infarction size: Likely involvement of the melatonin and suprachiasmatic nuclei. Int. J. Cardiol. 2017, 235, 191. [Google Scholar] [CrossRef] [PubMed]
- Sahna, E.; Olmez, E.; Acet, A. Effects of physiological and pharmacological concentrations of melatonin on ischemia-reperfusion arrhythmias in rats: Can the incidence of sudden cardiac death be reduced? J. Pineal Res. 2002, 32, 194–198. [Google Scholar] [CrossRef]
- Singhanat, K.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Roles of melatonin and its receptors in cardiac ischemia–reperfusion injury. Cell. Mol. Life Sci. 2018, 75, 4125–4149. [Google Scholar] [CrossRef]
- Black, N.; D’Souza, A.; Wang, Y.; Piggins, H.; Dobrzynski, H.; Morris, G.; Boyett, M.R. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Hear. Rhythm 2019, 16, 298–307. [Google Scholar] [CrossRef]
- Du Pré, B.C.; Dierickx, P.; Crnko, S.; Doevendans, P.A.; Vos, M.A.; Geijsen, N.; Neutel, D.; van Veen, T.A.B.; van Laake, L.W. Neonatal rat cardiomyocytes as an in vitro model for circadian rhythms in the heart. J. Mol. Cell. Cardiol. 2017, 112, 58–63. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Pereira, P.J.S.; Pugsley, M.K.; Troncy, E.; Tan, W.; Pouliot, M.; Harper, C.; Prefontaine, A.; Easter, A.; Wallis, R.; Miraucourt, L.; et al. Incidence of spontaneous arrhythmias in freely moving healthy untreated Sprague-Dawley rats. J. Pharmacol. Toxicol. Methods 2019, 99, 106589. [Google Scholar] [CrossRef]
- Svorc, P.; Bacova, I.; Gresova, S.; Svorc, P. Chronobiological perspectives on myocardial electrophysiological parameters under three types of general anesthesia in a rat model. Biol. Rhythm Res. 2017, 48, 343–351. [Google Scholar] [CrossRef]
- Gul-Kahraman, K.; Yilmaz-Bozoglan, M.; Sahna, E. Physiological and pharmacological effects of melatonin on remote ischemic perconditioning after myocardial ischemia-reperfusion injury in rats: Role of Cybb, Fas, NfκB, Irisin signaling pathway. J. Pineal Res. 2019, 67, e12589. [Google Scholar] [CrossRef] [PubMed]
- Sahna, E.; Acet, A.; Kaya Ozer, M.; Olmez, E. Myocardial ischemia-reperfusion in rats: Reduction of infarct size by either supplemental physiological or pharmacological doses of melatonin. J. Pineal Res. 2002, 33, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Diez, E.R.; Renna, N.F.; Prado, N.J.; Lembo, C.; Ponce Zumino, A.Z.; Vazquez-Prieto, M.; Miatello, R.M. Melatonin, given at the time of reperfusion, prevents ventricular arrhythmias in isolated hearts from fructose-fed rats and spontaneously hypertensive rats. J. Pineal Res. 2013, 55, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.J.; Ferder, L.; Manucha, W.; Diez, E.R. Anti-Inflammatory Effects of Melatonin in Obesity and Hypertension. Curr. Hypertens. Rep. 2018, 20, 45. [Google Scholar] [CrossRef]
- Mul Fedele, M.L.; Galiana, M.D.; Golombek, D.A.; Muñoz, E.M.; Plano, S.A. Alterations in Metabolism and Diurnal Rhythms following Bilateral Surgical Removal of the Superior Cervical Ganglia in Rats. Front. Endocrinol. 2018, 8, 370. [Google Scholar] [CrossRef]
- De Farias, T.D.S.M.; De Oliveira, A.C.; Andreotti, S.; Do Amaral, F.G.; Chimin, P.; De Proença, A.R.A.; Torres Leal, F.L.; Sertié, R.A.L.; Campana, A.B.; Lopes, A.B.; et al. Pinealectomy interferes with the circadian clock genes expression in white adipose tissue. J. Pineal Res. 2015, 58, 251–261. [Google Scholar] [CrossRef]
- Savastano, L.E.; Castro, A.E.; Fitt, M.R.; Rath, M.F.; Romeo, H.E.; Muñoz, E.M. A standardized surgical technique for rat superior cervical ganglionectomy. J. Neurosci. Methods 2010, 192, 22–33. [Google Scholar] [CrossRef]
- Castro, A.E.; Benitez, S.G.; Farias Altamirano, L.E.; Savastano, L.E.; Patterson, S.I.; Muñoz, E.M. Expression and cellular localization of the transcription factor NeuroD1 in the developing and adult rat pineal gland. J. Pineal Res. 2015, 58, 439–451. [Google Scholar] [CrossRef]
- Genade, S.; Genis, A.; Ytrehus, K.; Huisamen, B.; Lochner, A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: Role of its anti-adrenergic actions. J. Pineal Res. 2008, 45, 449–458. [Google Scholar] [CrossRef]
- Stroethoff, M.; Behmenburg, F.; Spittler, K.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Mathes, A. Activation of melatonin receptors by ramelteon induces cardioprotection by postconditioning in the rat heart. Anesth. Analg. 2018, 126, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.J.; Egan Beňová, T.; Diez, E.R.; Knezl, V.; Lipták, B.; Ponce Zumino, A.Z.; Llamedo-Soria, M.; Szeiffová Bačová, B.; Miatello, R.M.; Tribulová, N. Melatonin receptor activation protects against low potassium-induced ventricular fibrillation by preserving action potentials and connexin-43 topology in isolated rat hearts. J. Pineal Res. 2019, 67, e12605. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, Y.; Chen, J.; Zhang, J.; Yu, P.; Zhang, R.; Li, S.; Tao, B.; Wang, Y.; Qiu, Y.; et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res. 2019, 67, e12571. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.M.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Guerrero Montávez, J.M.; Carrascosa-Salmoral, M.D.P.; Naranjo Gutierrez, M.D.C.; Lardone, P.J.; de la Lastra Romero, C.A. Decreased MT1 and MT2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging. J. Pineal Res. 2009, 46, 29–35. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Grossini, E.; Molinari, C.; Uberti, F.; Mary, D.A.S.G.; Vacca, G.; Caimmi, P.P. Intracoronary melatonin increases coronary blood flow and cardiac function through β-adrenoreceptors, MT1/MT2 receptors, and nitric oxide in anesthetized pigs. J. Pineal Res. 2011, 51, 246–257. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Y.; Cheng, L.; Jin, Z.; Yang, Y.; Zhai, M.; Pei, H.; Wang, X.; Zhang, H.; Meng, Q.; et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: Role of SIRT1. J. Pineal Res. 2014, 57, 228–238. [Google Scholar] [CrossRef]
- Lamont, K.; Nduhirabandi, F.; Adam, T.; Thomas, D.P.; Opie, L.H.; Lecour, S. Role of melatonin, melatonin receptors and STAT3 in the cardioprotective effect of chronic and moderate consumption of red wine. Biochem. Biophys. Res. Commun. 2015, 465, 719–724. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, P.; Zhou, H.; Zhang, Y.; Chen, Y. Melatonin-induced protective effects on cardiomyocytes against reperfusion injury partly through modulation of IP3R and SERCA2a via activation of ERK1. Arq. Bras. Cardiol. 2018, 110, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gupta, S.; Sharma, B. Melatonin receptor and KATP channel modulation in experimental vascular dementia. Physiol. Behav. 2015, 142, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Shakiba, S.; Mehrzadi, S.; Afshari, K.; Rahimnia, A.H.; Dehpour, A.R. Anticonvulsant effect of melatonin through ATP-sensitive channels in mice. Fundam. Clin. Pharmacol. 2019, 34, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, H. Role of ATP-Sensitive K+ Channels in Cardiac Arrhythmias. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef]
- Diez, E.R.; Altamirano, L.B.; García, I.M.; Mazzei, L.; Prado, N.J.; Fornes, M.W.; Carrión, F.D.C.; Zumino, A.Z.P.; Ferder, L.; Manucha, W. Heart Remodeling and Ischemia–Reperfusion Arrhythmias Linked to Myocardial Vitamin D Receptors Deficiency in Obstructive Nephropathy Are Reversed by Paricalcitol. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 211–220. [Google Scholar] [CrossRef]
- Prado, N.J.; Casarotto, M.; Calvo, J.P.; Mazzei, L.; Ponce Zumino, A.Z.; García, I.M.; Cuello-Carrión, F.D.; Fornés, M.W.; Ferder, L.; Diez, E.R.; et al. Antiarrhythmic effect linked to melatonin cardiorenal protection involves AT 1 reduction and Hsp70-VDR increase. J. Pineal Res. 2018, 65, e12513. [Google Scholar] [CrossRef]
- Lee, Y.M.; Chen, H.R.; Hsiao, G.; Sheu, J.R.; Wang, J.J.; Yen, M.H. Protective effects of melatonin on myocardial ischemia/reperfusion injury in vivo. J. Pineal Res. 2002, 33, 72–80. [Google Scholar] [CrossRef]
- Said, M.; Becerra, R.; Palomeque, J.; Rinaldi, G.; Kaetzel, M.A.; Diaz-Sylvester, P.L.; Copello, J.A.; Dedman, J.R.; Mundiña-Weilenmann, C.; Vittone, L.; et al. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am. J. Physiol. Circ. Physiol. 2008, 295, H1669–H1683. [Google Scholar] [CrossRef]
- Xie, Y.; Sato, D.; Garfinkel, A.; Qu, Z.; Weiss, J.N. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys. J. 2010, 99, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Diez, E.; Barancik, M.; Tribulova, N. Protection of cardiac cell-to-cell coupling attenuate myocardial remodeling and proarrhythmia induced by hypertension. Physiol. Res. 2016, 65 (Suppl. S1), S29–S42. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.M.; Hung, M.W.; Lau, C.F.; Fung, M.L. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 2015, 58, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, G.; Sommese, L.; Palomeque, J.; Felice, J.I.; Di Carlo, M.N.; Fainstein, D.; Gonzalez, P.; Contreras, P.; Skapura, D.; McCauley, M.D.; et al. Phospholamban ablation rescues the enhanced propensity to arrhythmias of mice with CaMKII-constitutive phosphorylation of RyR2 at site S2814. J. Physiol. 2016, 594, 3005–3030. [Google Scholar] [CrossRef] [PubMed]
- Valverde, C.A.; Mazzocchi, G.; Di Carlo, M.N.; Ciocci Pardo, A.; Salas, N.; Ragone, M.I.; Felice, J.I.; Cely-Ortiz, A.; Consolini, A.E.; Portiansky, E.; et al. Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors. Cardiovasc. Res. 2019, 115, 556–569. [Google Scholar] [CrossRef]
- Nelson, C.S.; Marino, J.L.; Allen, C.N. Melatonin receptors activate heteromeric G-protein coupled Kir3 channels. Neuroreport 1996, 7, 717–720. [Google Scholar] [CrossRef]
- Diez, E.R.; Prados, L.V.; Carrión, A.; Ponce, Z.A.Z.; Miatello, R.M. A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J. Pineal Res. 2009, 46, 155–160. [Google Scholar] [CrossRef]
- Sedova, K.A.; Bernikova, O.G.; Cuprova, J.I.; Ivanova, A.D.; Kutaeva, G.A.; Pliss, M.G.; Lopatina, E.V.; Vaykshnorayte, M.A.; Diez, E.R.; Azarov, J.E. Association Between Antiarrhythmic, Electrophysiological, and Antioxidative Effects of Melatonin in Ischemia/Reperfusion. Int. J. Mol. Sci. 2019, 20, 6331. [Google Scholar] [CrossRef]
- Salvarani, N.; Crasto, S.; Miragoli, M.; Bertero, A.; Paulis, M.; Kunderfranco, P.; Serio, S.; Forni, A.; Lucarelli, C.; Dal Ferro, M.; et al. The K219T-Lamin mutation induces conduction defects through epigenetic inhibition of SCN5A in human cardiac laminopathy. Nat. Commun. 2019, 10, 2267. [Google Scholar] [CrossRef]
- Heinrich, M.; Oberbach, A.; Schlichting, N.; Stolzenburg, J.-U.; Neuhaus, J. Cytokine effects on gap junction communication and connexin expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS ONE 2011, 6, e20792. [Google Scholar] [CrossRef]
- Rahim, I.; Djerdjouri, B.; Sayed, R.K.; Fernández-Ortiz, M.; Fernández-Gil, B.; Hidalgo-Gutiérrez, A.; López, L.C.; Escames, G.; Reiter, R.J.; Acuña-Castroviejo, D. Melatonin administration to wild-type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J. Pineal Res. 2017, 63, e12410. [Google Scholar] [CrossRef]
- Cruz, J.S.; Machado, F.S.; Ropert, C.; Roman-Campos, D. Molecular mechanisms of cardiac electromechanical remodeling during Chagas disease: Role of TNF and TGF-β. Trends Cardiovasc. Med. 2017, 27, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ibañez Rodriguez, M.P.; Galiana, M.D.; Rásmussen, J.A.; Freites, C.L.; Noctor, S.C.; Muñoz, E.M. Differential response of pineal microglia to surgical versus pharmacological stimuli. J. Comp. Neurol. 2018, 526, 2462–2481. [Google Scholar] [CrossRef]
- Sánchez, S.; Sánchez, C.L.; Paredes, S.D.; Rodriguez, A.B.; Barriga, C. The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland of rats. J. Appl. Biomed 2008, 6, 177–186. [Google Scholar] [CrossRef]
- Curtis, M.J.; Hancox, J.C.; Farkas, A.; Wainwright, C.L.; Stables, C.L.; Saint, D.A.; Clements-Jewery, H.; Lambiase, P.D.; Billman, G.E.; Janse, M.J.; et al. The Lambeth Conventions (II): Guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther. 2013, 139, 213–248. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado, N.J.; Muñoz, E.M.; Farias Altamirano, L.E.; Aguiar, F.; Ponce Zumino, A.Z.; Sánchez, F.J.; Miatello, R.M.; Pueyo, E.; Diez, E.R. Reperfusion Arrhythmias Increase after Superior Cervical Ganglionectomy Due to Conduction Disorders and Changes in Repolarization. Int. J. Mol. Sci. 2020, 21, 1804. https://doi.org/10.3390/ijms21051804
Prado NJ, Muñoz EM, Farias Altamirano LE, Aguiar F, Ponce Zumino AZ, Sánchez FJ, Miatello RM, Pueyo E, Diez ER. Reperfusion Arrhythmias Increase after Superior Cervical Ganglionectomy Due to Conduction Disorders and Changes in Repolarization. International Journal of Molecular Sciences. 2020; 21(5):1804. https://doi.org/10.3390/ijms21051804
Chicago/Turabian StylePrado, Natalia Jorgelina, Estela Maris Muñoz, Luz Estefanía Farias Altamirano, Francisco Aguiar, Amira Zulma Ponce Zumino, Francisco Javier Sánchez, Roberto Miguel Miatello, Esther Pueyo, and Emiliano Raúl Diez. 2020. "Reperfusion Arrhythmias Increase after Superior Cervical Ganglionectomy Due to Conduction Disorders and Changes in Repolarization" International Journal of Molecular Sciences 21, no. 5: 1804. https://doi.org/10.3390/ijms21051804
APA StylePrado, N. J., Muñoz, E. M., Farias Altamirano, L. E., Aguiar, F., Ponce Zumino, A. Z., Sánchez, F. J., Miatello, R. M., Pueyo, E., & Diez, E. R. (2020). Reperfusion Arrhythmias Increase after Superior Cervical Ganglionectomy Due to Conduction Disorders and Changes in Repolarization. International Journal of Molecular Sciences, 21(5), 1804. https://doi.org/10.3390/ijms21051804




