The Role of Gibberellins in Regulation of Nitrogen Uptake and Physiological Traits in Maize Responding to Nitrogen Availability
Abstract
:1. Introduction
2. Results
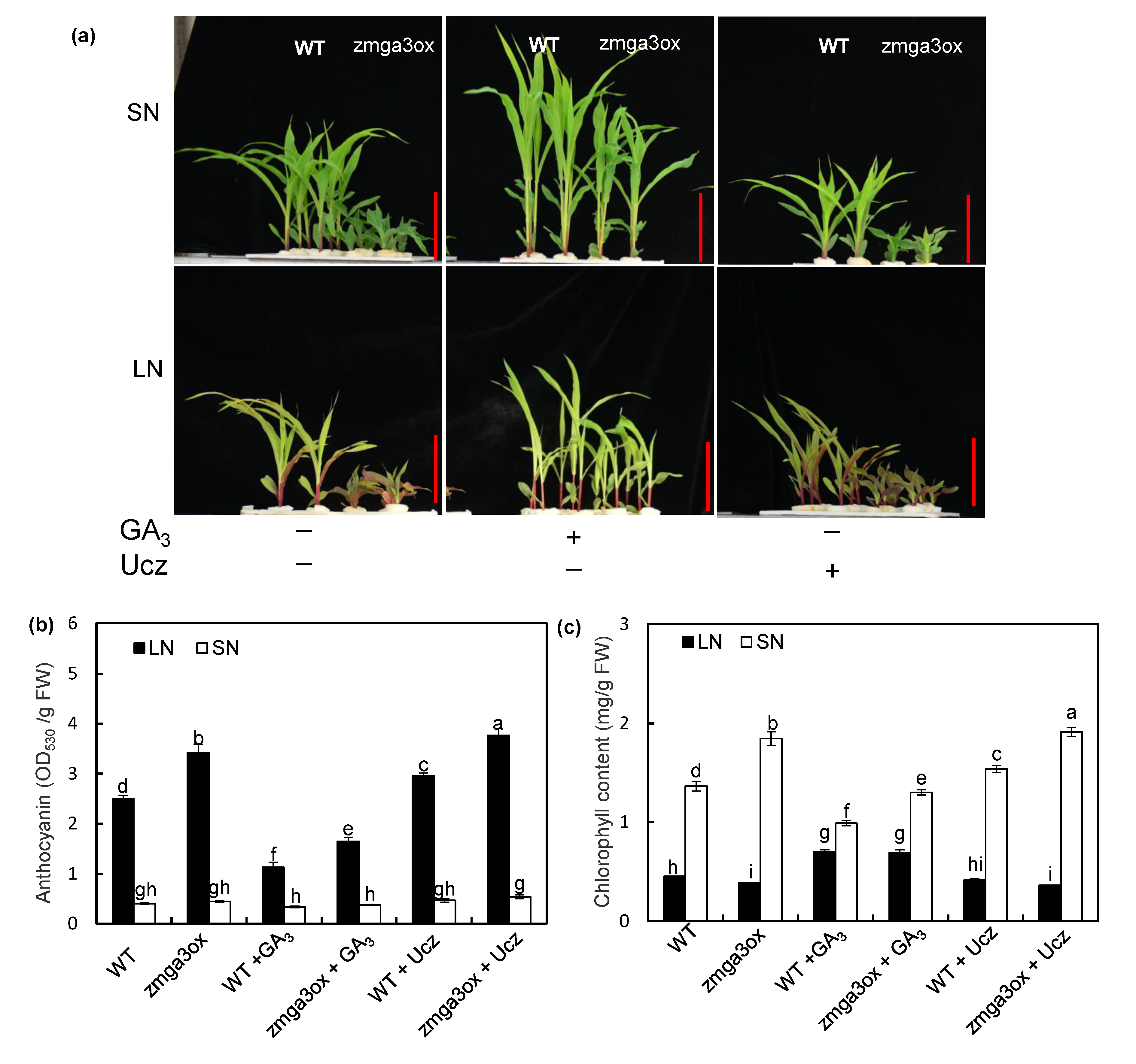
2.1. Characterization of zmga3ox Mutant in Maize
2.2. GAs Altered the Anthocyanin Accumulation and Chlorophyll Content in Leaves in Response to NO3− Supply
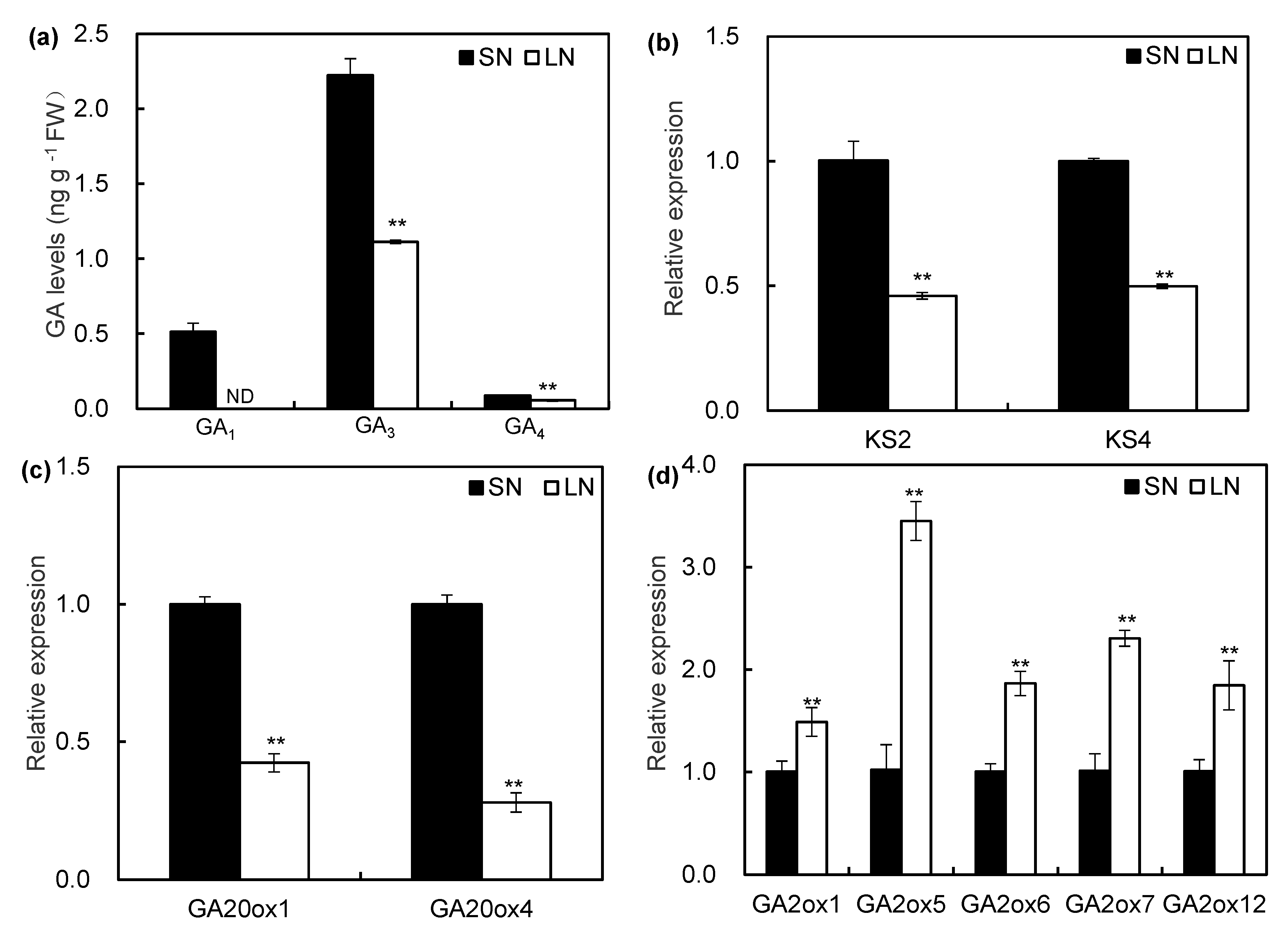
2.3. Supply of NO3− Modulated the Transcript Expression of GA Biosynthesis- and Metabolism-Related Genes
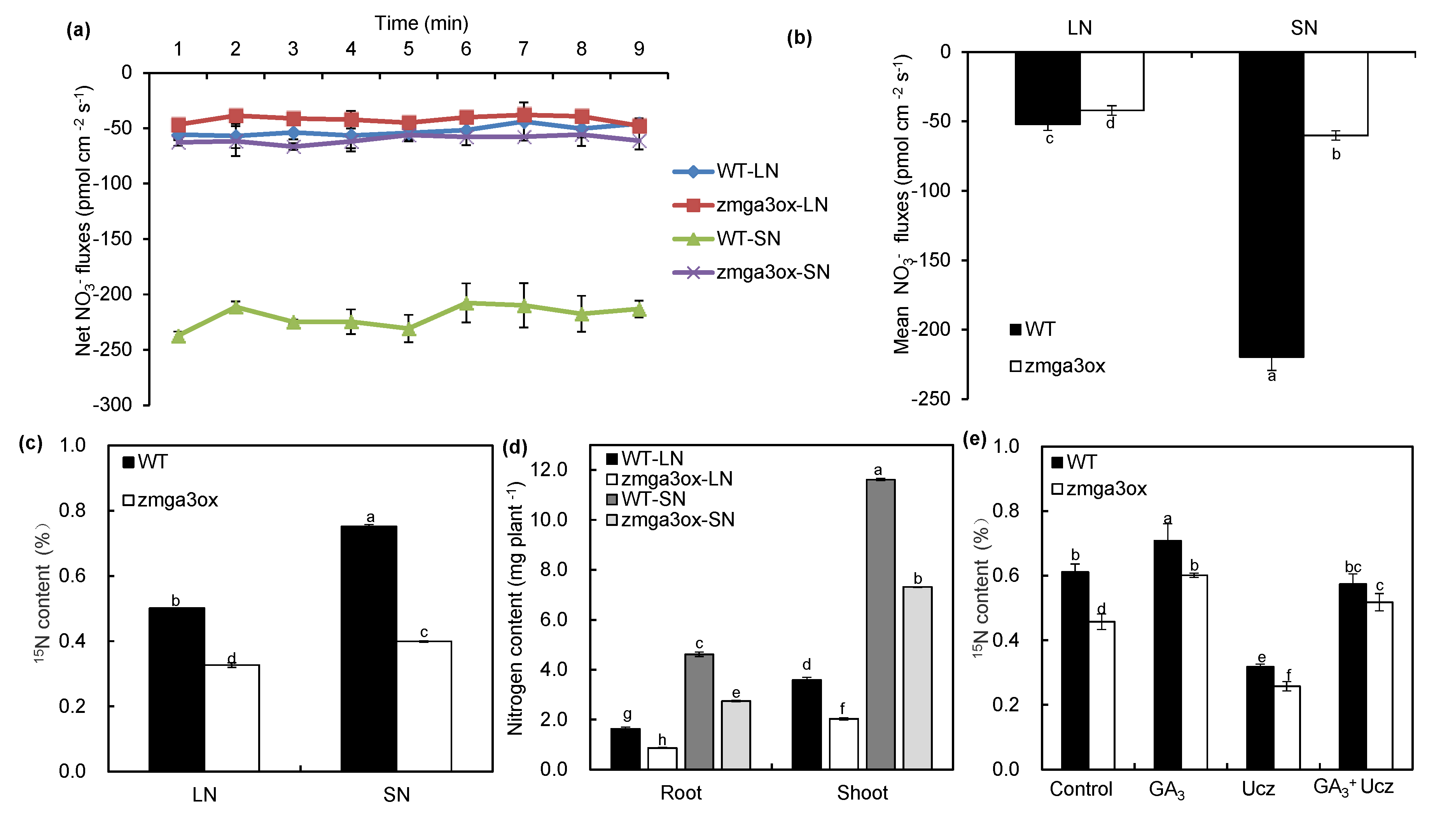
2.4. GAs Involved in Manipulating the NO3− Uptake
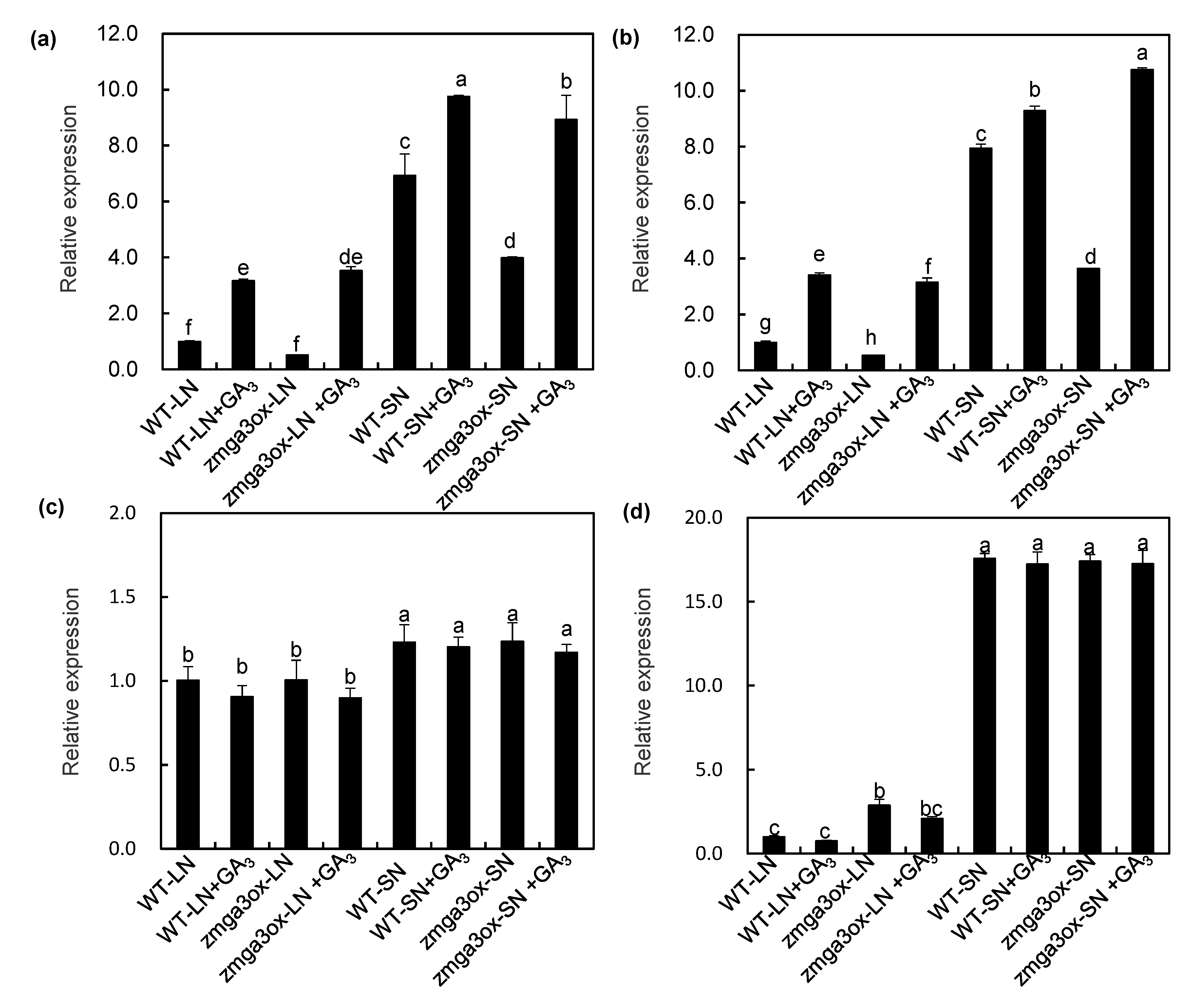
2.5. GAs Modulated the Transcript Expression of NO3− Uptake-Related Genes
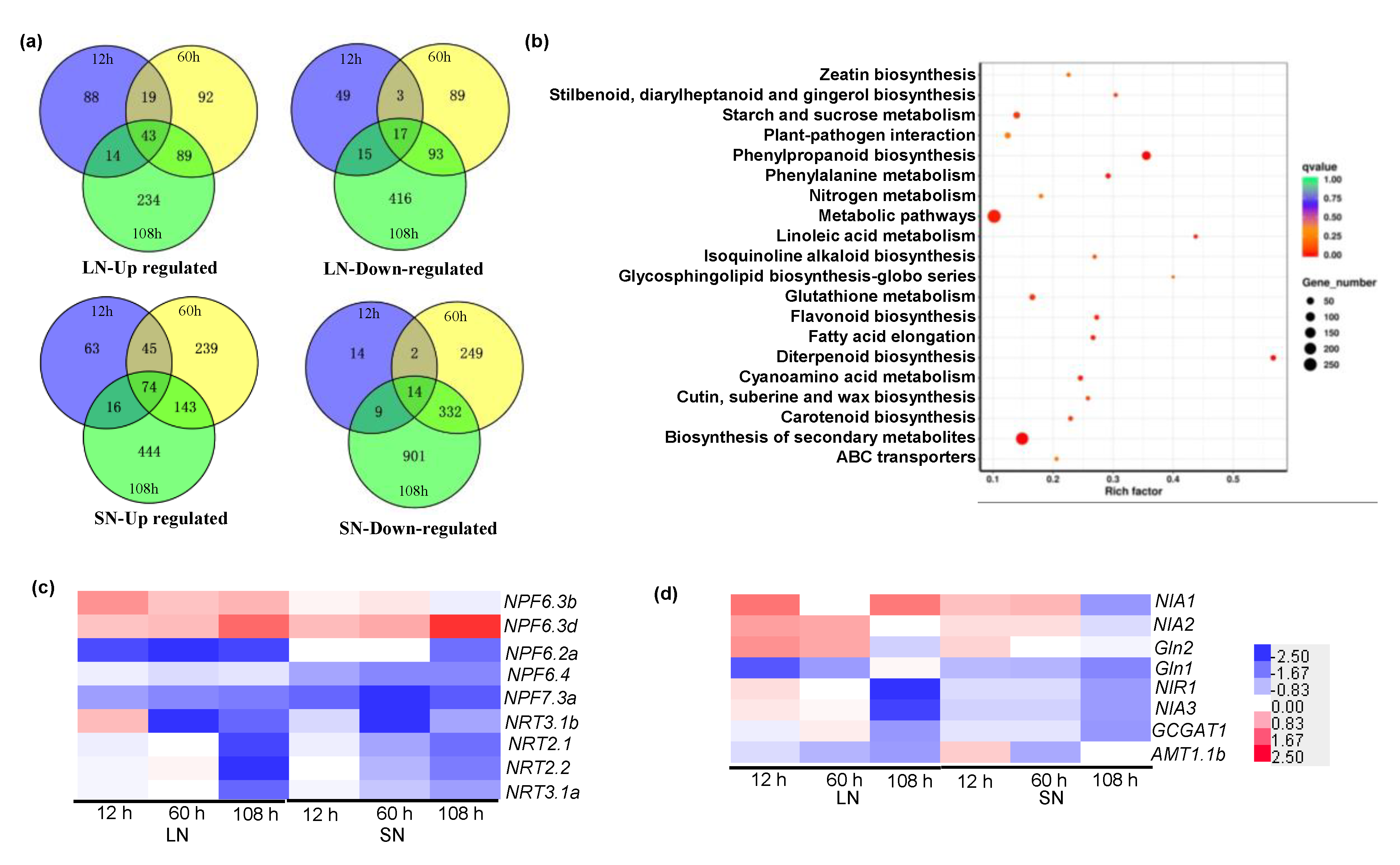
2.6. RNA-Sequencing Revealed Differentially Expressed Genes (DEGs) in the Wild-Type and zmga3ox Plants in Response to NO3− Supply
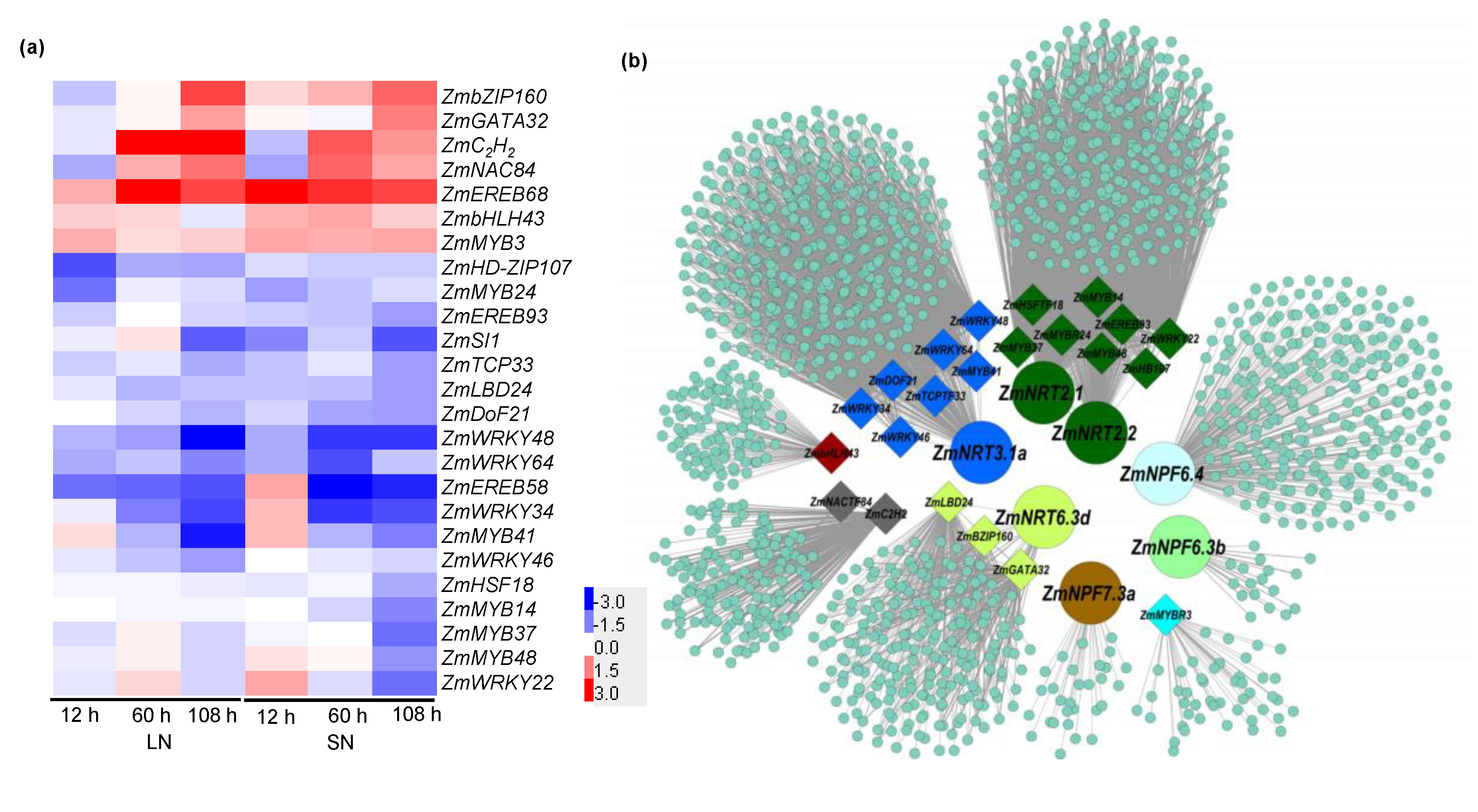
2.7. Transcription Factors Involved in GA-Mediated NO3− Uptake
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatment
4.2. The Measurement of Net NO3− Flux, 15NO3− Uptake and Total N Content
4.3. RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
4.4. RNA-Seq Analysis
4.5. Weighted Gene co-Expression Network Analysis (WGCNA)
4.6. Transcription Factor-binding Site Prediction
4.7. GAs Concentration Analysis
4.8. Assay of Physiological and Biochemical Properties
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GA | Gibberellin |
| GS | Glutamine Synthase |
| NR | Nitrate Reductase |
| LN | Low Nitrate |
| NUE | Nitrogen Use Efficiency |
| NMT | Non-Invasive Micro-Test System |
| NRT | Nitrate Transport |
| PR | Primary Root |
| SN | Sufficient Nitrate |
| Ucz | Uniconazole |
| FPKM | Fragments per Kilobase of Transcript per Million Reads |
| FDR | False Discovery Rate |
| DEGs | Differentially Expressed Genes |
| NRT | Nitrate Transporter |
| GRF4 | Growth-Regulattion Factor 4 |
| HAK5 | High-Affinity Potassium Transporter 5; |
| SLR1 | Slender Rice1 |
| SlPT2 | Tomato Phosphate Transporter |
| IRT1 | Iron-Regulated Transporter 1 |
| FRO2 | Iron Regulated Ferric Chelate Reductase |
| WGCNA | Weighted Gene co-Expression Network Analysis |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NPF | Nitrate Transporter1/Peptide Transporter Family |
References
- Yamaguchi, S. Gibberellin metabolism and its regulation. J. Plant Growth Regul. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Hou, M.M.; Liu, L.J.; Wu, S.; Shen, Y.; Ishiyama, K.; Kobayashi, M.; McCarty, D.R.; Tan, B.-C. The maize DWARF1 encodes a Gibberellin 3-Oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 2014, 166, 2028–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plackett, A.R.; Powers, S.J.; Fernandez-Garcia, N.; Urbanova, T.; Takebayashi, Y.; Seo, M.; Jikumaru, Y.; Benlloch, R.; Nilsson, O.; Ruiz-Rivero, O.; et al. Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and-3 are the dominant paralogs. Plant Cell 2012, 24, 941–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harberd, N.P.; Belfield, E.; Yasumura, Y.K. The angiosperm Gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 2009, 21, 1328–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedden, P. Constructing dwarf rice. Nat. Biotechnol. 2003, 21, 873–874. [Google Scholar] [CrossRef]
- Peng, J.R.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Harberd, N.P. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Itoh, H.; Ueguchi-tanaka, M.; Sato, Y.; Ashikari, M.; Matsuoka, M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 2002, 14, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Asano, K.; Yamasaki, M.; Takuno, S.; Miura, K.; Katagiri, S.; Tomoko, I.; Doi, K.; Wu, J.; Ebana, K.; Matsumoto, T.; et al. Artificial selection for a green revolution gene during japonica rice domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11034–11039. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tian, Y.H.; Wu, K.; Ye, Y.F.; Yu, J.P.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth-metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef]
- Jiang, C.F.; Gao, X.H.; Liao, L.L.; Harberd, N.P.; Fu, X.D. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the Gibberellin-DELLA signaling pathway in Arabidopsis. Plant Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Zhou, Y.W.; Chen, S.Y.; Liu, J.L.; Fan, K.; Li, Z.W. Gibberellins play dual roles in response to phosphate starvation of tomato seedlings, negatively in shoots but positively in roots. J. Plant Physiol. 2019, 234, 145–153. [Google Scholar] [CrossRef]
- Oliferuk, S.; Pérez, A.; Martinez, V.; Ródenas, R.; Ribio, F.; María, G.E.S. How DELLAs contribute to control potassium uptake under conditions of potassium scarcity? hypotheses and uncertainties. Plant Signal. Behav. 2017, 12, 1559–2324. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Liu, Z.J.; Liu, J.P.; Liu, S.; Wang, J.F.; Liu, W.X.; Xu, W.F. GA-DELLA pathway is involved in regulation of nitrogen deficiency-induced anthocyanin accumulation. Plant Cell Rep. 2017, 36, 557–569. [Google Scholar] [CrossRef]
- Khan, N.A. Effect of gibberellic acid on carbonic anhydrase, photosynthesis, growth and yield of mustard. Biol. Plant. 1996, 38, 145–147. [Google Scholar] [CrossRef]
- Nagel, O.W.; Lambers, H. Changes in the acquisition and partitioning of carbon and nitrogen in the gibberellin-deficient mutants A70 and W335 of tomato (Solanum lycopersicum L.). Plant Cell Environ. 2002, 25, 883–891. [Google Scholar] [CrossRef]
- Bai, L.Q.; Deng, H.Q.; Zhang, X.C.; Yu, X.C.; Li, Y.S. Gibberellin is involved in inhibition of cucumber growth and nitrogen uptake at suboptimal root-zone temperatures. PLoS ONE 2016, 11, e0156188. [Google Scholar] [CrossRef]
- Gooding, M.J.; Addisu, M.; Uppal, R.K.; Snape, J.W.; Jones, H.E. Effect of wheat dwarfing genes on nitrogen-use efficiency. J. Agric. Sci. 2012, 150, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.H.; Fan, X.R.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [Green Version]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [Green Version]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: Implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Hirel, B.; Gouis, J.L.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Valadier, M.H.; Migge, A.; Becker, T.W. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol. 1998, 1, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsay, Y.F.; Chiu, C.C.; Tsai, C.B.; Ho, C.H.; Hsu, P.K. Nitrate transporters and peptide transporters. FEBS Lett. 2007, 581, 2290–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 19, 1360–1385. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Mu, X.H.; Chen, Q.W.; Wu, X.Y.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Gibberellins synthesis is involved in the reduction of cell flux and elemental growth rate in maize leaf under low nitrogen supply. Environ. Exp. Bot. 2018, 150, 198–208. [Google Scholar] [CrossRef]
- Xing, H.L.; Dong, L.; Wang, Z.P.; Zhang, H.Y.; Han, C.Y.; Liu, B.; Wang, X.C.; Chen, Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.F.; Liu, F.; Crawford, N.M.; Yong, W. Molecular regulation of nitrate response in plants. Int. J. Mol. Sci. 2018, 19, 2039. [Google Scholar] [CrossRef] [Green Version]
- Naing, A.H.; Park, K.I.; Ai, T.N.; Chung, M.Y.; Han, J.S.; Kang, Y.W.; Lim, K.B.; Kim, C.K. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.J.; Kim, M.D.; Deng, X.P.; Kwak, S.S.; Chen, W. Enhanced salt stress tolerance in transgenic potato plants expressing IbMYB1, a sweet potato transcription factor. J. Microbiol. Biotechnol. 2013, 23, 1737–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, W.; Beeckman, T.; Xu, G.H. Plant nitrogen nutrition: Sensing and signaling. Curr. Biol. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Tyerman, S.D.; Dechorgnat, J.; Ovchinnikova, E.; Dhugga, K.S.; Kaiser, B.N. Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 2017, 29, 2581–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hu, D.; Yuan, D.Y.; Liu, Y.Q.; Che, R.H.; Hu, Y.Q.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Sun, Q.; Wang, K.; Du, Q.; Li, W.X. Nitrogen Limitation Adaptation (NLA) is involved in source-to-sink remobilization of nitrate by mediating the degradation of NRT1.7 in Arabidopsis. New Physiol. 2017, 214, 734–744. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, A.D. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007, 143, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.Q.; Yan, Y.; Liu, W.Q.; Zhang, W.N.; Guo, L.H.; Tian, Y.Q. Knock-Down of CsNRT2.1, a Cucumber Nitrate Transporter, Reduces Nitrate Uptake, Root length, and Lateral Root Number at Low External Nitrate Concentration. Front. Plant Sci. 2018, 9, 722. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Fan, X.; Feng, H.M.; Miller, A.J.; Shen, Q.R.; Xu, G.H. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 34, 1360–1372. [Google Scholar] [CrossRef]
- Garnett, T.; Conn, V.; Plett, D.; Conn, S.; Zanghellini, J.; Mackenzie, N.; Enju, A.; Francis, K.; Holtham, L.; Roessner, U.; et al. The response of the maize nitrate transport system to nitrogen demand and supply across the lifecycle. New Phytol. 2013, 198, 82–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarasinghe, B.H.R.; de Bruxelles, G.L.; Braddon, M.; Onyeocha, I.; Forde, B.G.; Udvardi, M.K. Regulation of GmNRT2 expression and nitrate transport activity in roots of soybean (Glycine max). Planta 1998, 206, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, J.J.; Zhuo, D.; Siddiqi, M.Y.; Schjoerring, J.K.; Touraine, B.; Glass, A.D. Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen pools in roots of barley. Plant Physiol. 2000, 123, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsel, M.; Filleur, S.; Fraisier, V.; Daniel-Vedele, F.D. Nitrate transport in plants: Which gene and which control? J. Exp. Bot. 2002, 53, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, M.Y.; Wu, W.H.; Wang, Y. Phosphorylation at Ser28 stabilizes the Arabidopsis nitrate transporter NRT2.1 in response to nitrate limitation. J. Integr. Plant Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, D.G.; Okamoto, M.; Vidmar, J.J.; Glass, A.D.M. Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J. 1999, 17, 563–568. [Google Scholar] [CrossRef]
- Lejay, L.; Gansel, X.; Cerezo, M.; Tillard, P.; Muller, C.; Krapp, A.; von Wiren, N.; Daniel-Vedele, F.; Gojon, A. Regulation of root ion transporters by photosynthesis: Functional importance and relation with hexokinase. Plant Cell 2003, 15, 2218–2232. [Google Scholar] [CrossRef] [Green Version]
- Wild, M.; Davie, J.M.; Regnault, T.; Sakvarelidze-Achard, L.; Carrera, E.; Diaz, I.L.; Cayrel, A.; Dubeaux, G.; Vert, G.; Vert, G.; et al. Tissue-specific regulation of gibberellin signaling article tissue-specific regulation of gibberellin signaling fine-tunes Arabidopsis iron-deficiency responses. Dev. Cell 2016, 37, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Daviere, J.M.; Wild, M.; Regnault, T.; Baumberger, N.; Eisler, H.; Genschik, P.; Achard, P. Report class I TCP-DELLA interactions in inflorescence shoot apex determine plant height. Curr. Biol. 2014, 24, 1923–1928. [Google Scholar] [CrossRef] [Green Version]
- Mengel, K.; Robin, P.; Salsac, L. Nitrate reductase activity in shoots and roots of maize seedlings as affected by the form of nitrogen nutrition and the pH of the nutrient solution. Plant Physiol. 1983, 71, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Qin, J.J.; He, F.F.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Net fluxes of ammonium and nitrate in association with H+ fluxes in fine roots of Populus popularis. Planta 2013, 237, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Xin, W.J.; Sun, S.B. Physiological and molecular responses of nitrogen-starved rice plants to re-supply of different nitrogen sources. Plant Soil 2006, 287, 145–159. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Wu, W.H.; Wang, Y. The K+ channel KZM2 is involved in stomatal movement by modulating inward K+ currents in maize guard cells. Plant J. 2017, 92, 662–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, C.; Trapnell, C. TopHat2 accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.H.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Mao, X.Z.; Cai, T.; Luo, J.C.; Wei, L.P. KOBAS server: A web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 2006, 34, W720–W724. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Genet. Mol. Biol. 2005, 4, 1544–6115. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Saito, R.; Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Lotia, S.; Pico, A.R.; Bader, G.D.; Ideker, T. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.P.; Tian, F.; Yang, Y.Q.; Kong, M.L.; Luo, J.C.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.D.; Ma, J.; Zhai, H.H.; Xin, P.Y.; Chu, J.F.; Qiao, Y.L.; Han, L.Z. CHR729 is a CHD3 protein that controls seedling development in rice. PLoS ONE 2015, 10, e0138934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.Y.; Ding, C.H.; Baerson, S.R.; Lian, F.H.; Lin, X.H.; Zhang, L.Q.; Wu, C.F.; Hwang, S.Y.; Zeng, R.S.; Song, Y.Y. The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ. 2018, 42, 659–672. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Habricot, Y.; Condiff, A.S.; Maldiney, R.; Straeten, V.D.; Ahmad, M. HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J. 2007, 49, 428–441. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Li, G.X.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol. Plant. 2016, 60, 139–147. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yao, Q.; Zhang, Y.; Zhang, Y.; Xing, J.; Yang, B.; Mi, G.; Li, Z.; Zhang, M. The Role of Gibberellins in Regulation of Nitrogen Uptake and Physiological Traits in Maize Responding to Nitrogen Availability. Int. J. Mol. Sci. 2020, 21, 1824. https://doi.org/10.3390/ijms21051824
Wang Y, Yao Q, Zhang Y, Zhang Y, Xing J, Yang B, Mi G, Li Z, Zhang M. The Role of Gibberellins in Regulation of Nitrogen Uptake and Physiological Traits in Maize Responding to Nitrogen Availability. International Journal of Molecular Sciences. 2020; 21(5):1824. https://doi.org/10.3390/ijms21051824
Chicago/Turabian StyleWang, Yubin, Qingqing Yao, Yushi Zhang, Yuexia Zhang, Jiapeng Xing, Benzhou Yang, Guohua Mi, Zhaohu Li, and Mingcai Zhang. 2020. "The Role of Gibberellins in Regulation of Nitrogen Uptake and Physiological Traits in Maize Responding to Nitrogen Availability" International Journal of Molecular Sciences 21, no. 5: 1824. https://doi.org/10.3390/ijms21051824




