Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Bioactivity in the Rice Lamina Inclination Assay of Brassinosteroids Analogs.
2.3. Molecular Docking
3. Materials and Methods
3.1. Biological Activity
3.1.1. Rice Lamina Inclination Test
3.1.2. Root Elongation of A. Thaliana
3.2. Molecular Docking
3.2.1. Ligand/Molecular Target Selection and Preparation
3.2.2. Docking Procedure and Analysis of Protein–Ligand Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BL | Brassinolide |
| BRs | Brassinosteroids |
| CS | Castasterone |
| RLIT | Rice leaf inclination test |
| BSIB | Bean second node bioassay |
| SAR | Structure-activity Relationships |
| BRI1LRR | BL receptor with extracellular leucine-rich domain |
| BAK1 | BRI1-associated kinase 1 |
References
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins—A New Family of Plant Hormones from Rape Pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Oklestkova, J.; Rarova, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072. [Google Scholar] [CrossRef]
- Clouse, S.D. A History of Brassinosteroid Research from 1970 through 2005: Thirty-Five Years of Phytochemistry, Physiology, Genes, and Mutants. J. Plant Growth Regul. 2015, 34, 828–844. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Sun, X.; Ding, T.; Lei, B.; Zhang, C. Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo MA, T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the Sixth Class of Phytohormones: A Molecular View from the Discovery to Hormonal Interactions in Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Zullo, M.A.T.; Adam, G. Brassinosteroid phytohormones: Structure, bioactivity and applications. Braz. J. Plant Physiol. 2002, 14, 143–181. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroids—Occurence and chemical structures in plants. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Fujioka, S. Natural Occurrence of Brassinosteroids in the Plant Kingdom. In Brassinosteroids: Steroidal Plant Hormones; Sakurai, A., Yokota, T., Clouse, S.D., Eds.; Springer: Tokyo, Japan, 1999; pp. 21–45. [Google Scholar]
- Thompson, M.J.; Meudt, W.J.; Mandava, N.B.; Dutky, S.R.; Lusby, W.R.; Spaulding, D.W. Synthesis of Brassinosteroids and Relationship of Structure to Plant Growth-Promoting Effects. Steroids 1982, 39, 89–105. [Google Scholar] [CrossRef]
- Takatsuto, S.; Yazawa, N.; Ikekawa, N.; Morishita, T.; Abe, H. Synthesis of (24R)-28-homobrassinolide analogues and structure-activity relationships of brassinosteroids in the rice-lamina inclination test. Phytochemistry 1983, 22, 1393–1397. [Google Scholar] [CrossRef]
- Takatsuto, S.; Ikekawa, N.; Morishita, T.; Abe, H. Structure Activity Relationship of Brassinosteroids with Respect to the A/B-Ring Functional-Groups. Chem. Pharm. Bull. 1987, 35, 211–216. [Google Scholar] [CrossRef]
- Yokota, T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997, 2, 137–143. [Google Scholar] [CrossRef]
- Kishi, T.; Wada, K.; Marumo, S.; Mori, K. Synthesis of Brassinolide Analogs with a Modified Ring B and Their Plant Growth-promoting Activity. Agric. Biol. Chem. 1986, 50, 1821–1830. [Google Scholar] [CrossRef]
- Mandava, N.B. Plant Growth-Promoting Brassinosteroids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 23–52. [Google Scholar] [CrossRef]
- Seto, H.; Fujioka, S.; Koshino, H.; Suenaga, T.; Yoshida, S.; Watanabe, T.; Takatsuto, S. Epimerization at C-5 of brassinolide with sodium methoxide and the biological activity of 5-epi-brassinolide in the rice lamina inclination test. J. Chem. Soc. Perkin Trans. 1 1998, 3355–3358. [Google Scholar] [CrossRef]
- Takatsuto, S.; Yazawa, N.; Ikekawa, N.; Takematsu, T.; Takeuchi, Y.; Koguchi, M. Structure Activity Relationship of Brassinosteroids. Phytochemistry 1983, 22, 2437–2441. [Google Scholar] [CrossRef]
- Černý, V.; Strnad, M.; Kamínek, M. Preparation of 2α,3α-dihydroxy-7-oxa-6-oxo-23,24-dinor-B-homo-5α-cholanic acid, its esters and amides as brassinolide analogues. Collect. Czechoslov. Chem. Commun. 1986, 51, 687–697. [Google Scholar] [CrossRef]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rarova, L.; Berka, K.; Strnad, M. Biological activities of new monohydroxylated brassinosteroid analogues with a carboxylic group in the side chain. Steroids 2014, 85, 58–64. [Google Scholar] [CrossRef]
- Uesusuki, S.; Watanabe, B.; Yamamoto, S.; Otsuki, J.; Nakagawa, Y.; Miyagawa, H. Synthesis of Brassinosteroids of Varying Acyl Side Chains and Evaluation of Their Brassinolide-like Activity. Biosci. Biotechnol. Biochem. 2004, 68, 1097–1105. [Google Scholar] [CrossRef]
- Šíša, M.; Hniličková, J.; Swaczynová, J.; Kohout, L. Syntheses of new androstane brassinosteroids with 17β-ester groups—Butyrates, heptafluorobutyrates, and laurates. Steroids 2005, 70, 755–762. [Google Scholar] [CrossRef]
- Gil, R.P.; Iglesias-Arteaga, M.A.; Martinez, C.P.; Manchado, F.C.; Garcia, D.C.; Rosado, A. Synthesis of analogues of brassinosteroids from chenodeoxycholic acid. Eur. J. Org. Chem. 1998, 11, 2405–2407. [Google Scholar]
- Iglesias-Arteaga, M.; Gil, R.P.; Leliebre-Lara, V.; Martinez, C.S.P.; Manchado, F. Synthesis and biological activity of (22R,25R)-5 alpha-furostan-2 alpha,3 alpha,26-triol. J. Chem. Res. 1996, 28, 504–505. [Google Scholar]
- Iglesias-Arteaga, M.; Gil, R.; Leliebre-Lara, V.; Manchado, F.; Pérez, C.S.; Rosado, A. Synthesis of (25R)-5α-Spirostan-2α,3α,6β-triol Triacetate. Synth. Commun. 1998, 28, 75–81. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.; Gil, R.P.; Leliebre-Lara, V.; Martinez, C.S.P.; Manchado, F.; Perez, A.R.; Rios, L.P. Synthesis of (22R,25R)-3 beta,26-dihydroxy-5 alpha-furostan-6-one. Synth. Commun. 1998, 28, 1381–1386. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.; Gil, R.P.; Leliebre-Lara, V.; Martinez, C.S.P.; Manchado, F. Synthesis of (22R,25R)-2 alpha,3 alpha,26-trihydroxy-5 alpha-furostanaone-6-one. Synth. Commun. 1998, 28, 1779–1784. [Google Scholar] [CrossRef]
- IglesiasArteaga, M.A.; PérezGil, R.; LeliebreLara, V.; CollManchado, F.; PérezMartínez, C.S. Synthesis of (25R)-2α,3α-Epoxy-5α-Spirostan-6,23-Dione. Synth. Commun. 1998, 28, 4387–4392. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.A.; Martinez, C.S.P.; Manchado, F.C. Synthesis and characterization of (25R)-2 alpha,3 alpha-epoxy-5 alpha-spirostan-12,23-dione. Synth. Commun. 1999, 29, 1811–1818. [Google Scholar] [CrossRef]
- Iglesias Arteaga, M.A.; Gil, R.P.; Pérez Martínez, C.S.; Manchado, F.C. Synthetic Steroidal Sapogenins. Part III1 23-Ketohecogenin and 23-Ketoisochiapagenin. Synth. Commun. 2000, 30, 163–170. [Google Scholar] [CrossRef]
- Huang, L.F.; Zhou, W.S. Studies on Steroidal Plant-Growth Regulators. Part 33. Novel Method for Construction of the Side-Chain of 23-Arylbrassinosteroids Via Heck Arylation and Asymmetric Dihydroxylation As Key Steps. J. Chem. Soc. Perkin Trans. 1 1994, 3579–3585. [Google Scholar] [CrossRef]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rárová, L.; Korinkova, P.; Mikulík, J.; Budesinsky, M.; Béres, T.; Berka, K.; Lu, Q.; et al. Design, synthesis and biological activities of new brassinosteroid analogues with a phenyl group in the side chain. Org. Biomol. Chem. 2016, 14, 8691–8701. [Google Scholar] [CrossRef]
- Zhou, W.S.; Tian, W.S. The Synthesis of Steroids Containing Structural Unit of A, B Ring of Brassinolide and Ecdysone from Hyodesoxycholic Acid. Acta Chim. Sin. 1984, 42, 1173–1177. [Google Scholar]
- Back, T.; Pharis, R. Structure-Activity Studies of Brassinosteroids and the Search for Novel Analogues and Mimetics with Improved Bioactivity. J. Plant Growth Regul. 2004, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Kovganko, N.V.; Ananich, S.K. Advances in the chemical synthesis of brassinosteroids. Chem. Nat. Compd. 1997, 33, 389–416. [Google Scholar] [CrossRef]
- Korinkova, P.; Bazgier, V.; Oklestkova, J.; Rarova, L.; Strnad, M.; Kvasnica, M. Synthesis of novel aryl brassinosteroids through alkene cross-metathesis and preliminary biological study. Steroids 2017, 127, 46–55. [Google Scholar] [CrossRef]
- Duran, M.I.; Gonzalez, C.; Acosta, A.; Olea, A.F.; Diaz, K.; Espinoza, L. Synthesis of Five Known Brassinosteroid Analogs from Hyodeoxycholic Acid and Their Activities as Plant-Growth Regulators. Int. J. Mol. Sci. 2017, 18, 516. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, B.; Shen, J. Synthesis of cholesteric lactones and analogs as plant growth regulators. Patent CN 1184113, 1998. [Google Scholar]
- Tian, W.S.; Zhou, W.S. Studies on steroidal plant growth hormone VI. Conversion of methyl 3,6-diketoallocholanate into methyl Δ^2-6-ketoallocholenate. Acta Chim. Sin. 1988, 46, 824–826. [Google Scholar]
- Brosa, C.; Capdevila, J.M.; Zamora, I. Brassinosteroids: A new way to define the structural requirements. Tetrahedron 1996, 52, 2435–2448. [Google Scholar] [CrossRef]
- Brosa, C.; Soca, L.; Terricabras, E.; Ferrer, J.C.; Alsina, A. New synthetic brassinosteroids: A 5 alpha-hydroxy-6-ketone analog with strong plant growth promoting activity. Tetrahedron 1998, 54, 12337–12348. [Google Scholar] [CrossRef]
- Ferrer-Pertuz, K.; Espinoza, L.; Mella, J. Insights into the Structural Requirements of Potent Brassinosteroids as Vegetable Growth Promoters Using Second-Internode Elongation as Biological Activity: CoMFA and CoMSIA Studies. Int. J. Mol. Sci. 2017, 18, 2734. [Google Scholar] [CrossRef]
- Carvajal, R.; Gonzalez, C.; Olea, A.F.; Fuentealba, M.; Espinoza, L. Synthesis of 2-Deoxybrassinosteroids Analogs with 24-nor, 22(S)-23-Dihydroxy-Type Side Chains from Hyodeoxycholic Acid. Molecules 2018, 23, 1306. [Google Scholar] [CrossRef]
- Oyarce, J.; Aitken, V.; Gonzalez, C.; Ferrer, K.; Olea, A.F.; Parella, T.; Espinoza, L. Synthesis and structural determination of new brassinosteroid 24-nor-5α-cholane type analogs. Molecules 2019, 24, 4612. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, H.; Jang, S. Rice Lamina Joint Inclination Assay. Bio-protocol 2017, 7, e2409. [Google Scholar] [CrossRef]
- Jang, S.; An, G.; Li, H.-Y. Rice Leaf Angle and Grain Size Are Affected by the OsBUL1 Transcriptional Activator Complex. Plant Physiol. 2017, 173, 688. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146. [Google Scholar] [CrossRef]
- Müssig, C.; Shin, G.-H.; Altmann, T. Brassinosteroids Promote Root Growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef]
- Martins, S.; Montiel Jorda, A.; Cayrel, A.; Huguet, S.; Roux, C.; Ljung, K.; Vert, G. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- González-García, M.-P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid perception in the epidermis controls root meristem size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef]
- Chaiwanon, J.; Wang, Z.-Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr. Biol. 2015, 25, 1031–1042. [Google Scholar] [CrossRef]
- Bao, F.; Shen, J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol. 2004, 134, 1624–1631. [Google Scholar] [CrossRef]
- Šíša, M.; Vilaplana-Polo, M.; Ballesteros, C.B.; Kohout, L. Brassinolide activities of 2α,3α-diols versus 3α,4α-diols in the bean second internode bioassay: Explanation by molecular modeling methods. Steroids 2007, 72, 740–750. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- She, J.; Han, Z.; Zhou, B.; Chai, J. Structural basis for differential recognition of brassinolide by its receptors. Protein Cell 2013, 4, 475–482. [Google Scholar] [CrossRef]
- Tang, J.; Han, Z.; Chai, J. Q&A: What are brassinosteroids and how do they act in plants? BMC Biol. 2016, 14, 113. [Google Scholar] [CrossRef]
- Hothorn, M.; Belkhadir, Y.; Dreux, M.; Dabi, T.; Noel, J.P.; Wilson, I.A.; Chory, J. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 2011, 474, 467–471. [Google Scholar] [CrossRef]
- Santiago, J.; Henzler, C.; Hothorn, M. Molecular Mechanism for Plant Steroid Receptor Activation by Somatic Embryogenesis Co-Receptor Kinases. Science 2013, 341, 889–892. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castillo, E.; Ramírez-Echemendía, D.P.; Hernández-Campoalegre, G.; Mesa-Tejeda, D.; Coll-Manchado, F.; Coll-García, Y. In silico identification of new potentially active brassinosteroid analogues. Steroids 2018, 138, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Liu, J.; Yao, X. Unveiling the molecular mechanism of brassinosteroids: Insights from structure-based molecular modeling studies. Steroids 2015, 104, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Marumo, S. Synthesis and Plant Growth-Promoting Activity of Brassinolide Analogs. Agric. Biol. Chem. 1981, 45, 2579–2585. [Google Scholar]
- Han, K.S.; Ko, K.W.; Nam, S.J.; Park, S.H.; Kim, S.K. Optimization of a rice lamina inclination assay for detection of brassinosteroids: I. effect of phytohormones on the inclination activity. J. Plant Biol. 1997, 40, 240–244. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Søndergaard, C.R.; Olsson, M.H.M.; Rostkowski, M.; Jensen, J.H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef]


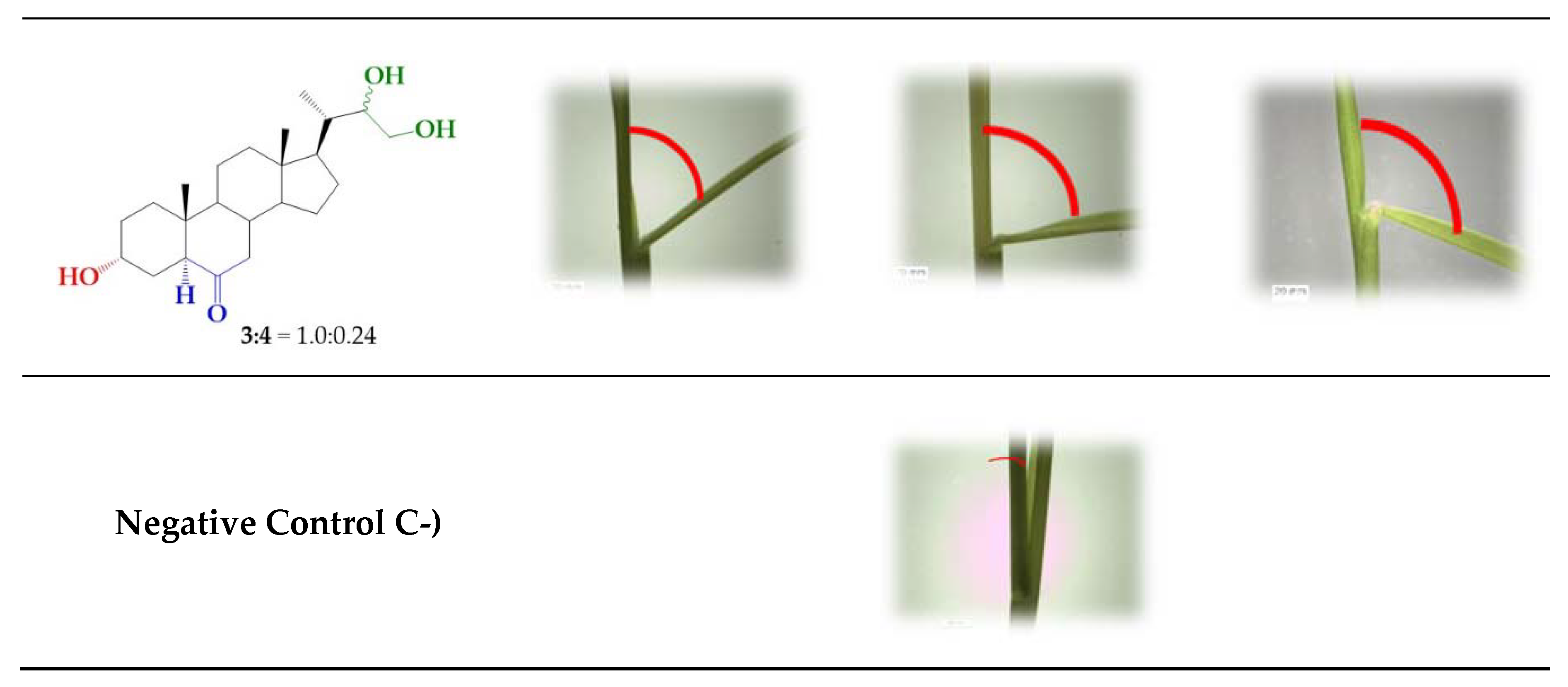


| Bending Angle Between Laminae and Sheaths (Degrees ± Standard Error) | |||
|---|---|---|---|
| Compounds | 1 × 10−8 M | 1 × 10−7 M | 1 × 10−6 M |
| BL (C+) | 31 ± 11 | 41 ± 4.5 | 70 ± 7.6 |
| 3 | 35 ± 3 | 60 ± 3 * | 62 ± 12 |
| 4 | 45 ± 9.5 * | 31 ± 5 | 24 ± 5.8 * |
| 5 | 5 ± 2.7 * | 13 ± 1.7 * | 28 ± 2.5 * |
| 6 | 49 ± 5 * | 60 ± 5 * | 78 ± 15 * |
| 7 | 28 ± 3 | 64 ± 3 * | 70 ± 10 |
| 8 | 24 ± 2.5 | 34 ± 2.5 | 35 ± 2.9 * |
| 9 | 34 ± 2.2 | 45 ± 2.7 | 53 ± 6.3 * |
| 3/4 (1.0:0.24) | 26 ± 2.5 | 63 ± 2.9 * | 66 ± 6.3 |
| 3/4 (1.0:0.9) | 35 ± 3.8 | 43 ± 5.0 | 38 ± 5 |
| Control (C-) | 7 ± 5 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, K.; Espinoza, L.; Carvajal, R.; Conde-González, M.; Niebla, V.; Olea, A.F.; Coll, Y. Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs. Int. J. Mol. Sci. 2020, 21, 1832. https://doi.org/10.3390/ijms21051832
Díaz K, Espinoza L, Carvajal R, Conde-González M, Niebla V, Olea AF, Coll Y. Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs. International Journal of Molecular Sciences. 2020; 21(5):1832. https://doi.org/10.3390/ijms21051832
Chicago/Turabian StyleDíaz, Katy, Luis Espinoza, Rodrigo Carvajal, Marcos Conde-González, Vladimir Niebla, Andrés F. Olea, and Yamilet Coll. 2020. "Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs" International Journal of Molecular Sciences 21, no. 5: 1832. https://doi.org/10.3390/ijms21051832
APA StyleDíaz, K., Espinoza, L., Carvajal, R., Conde-González, M., Niebla, V., Olea, A. F., & Coll, Y. (2020). Biological Activities and Molecular Docking of Brassinosteroids 24-Norcholane Type Analogs. International Journal of Molecular Sciences, 21(5), 1832. https://doi.org/10.3390/ijms21051832





