Does the Pre-Ovulatory Pig Oviduct Rule Sperm Capacitation In Vivo Mediating Transcriptomics of Catsper Channels?
Abstract
1. Introduction
2. Results
2.1. Gene Expression is Altered in the Pre-Ovulatory Oviduct after Semen or Sperm-Free SP Exposure
2.2. Commonly Altered Genes between Pre-Ovulatory Oviduct Segments after Statistical Restrictive Analysis within Each Experimental Group
2.3. Analysis of Functional Categories: Enriched Tubal Genes During Pre-Ovulation are Differentially Associated with Sperm Motility, Acrosome Reaction, Single Fertilization, and Regulation of Signal Transduction
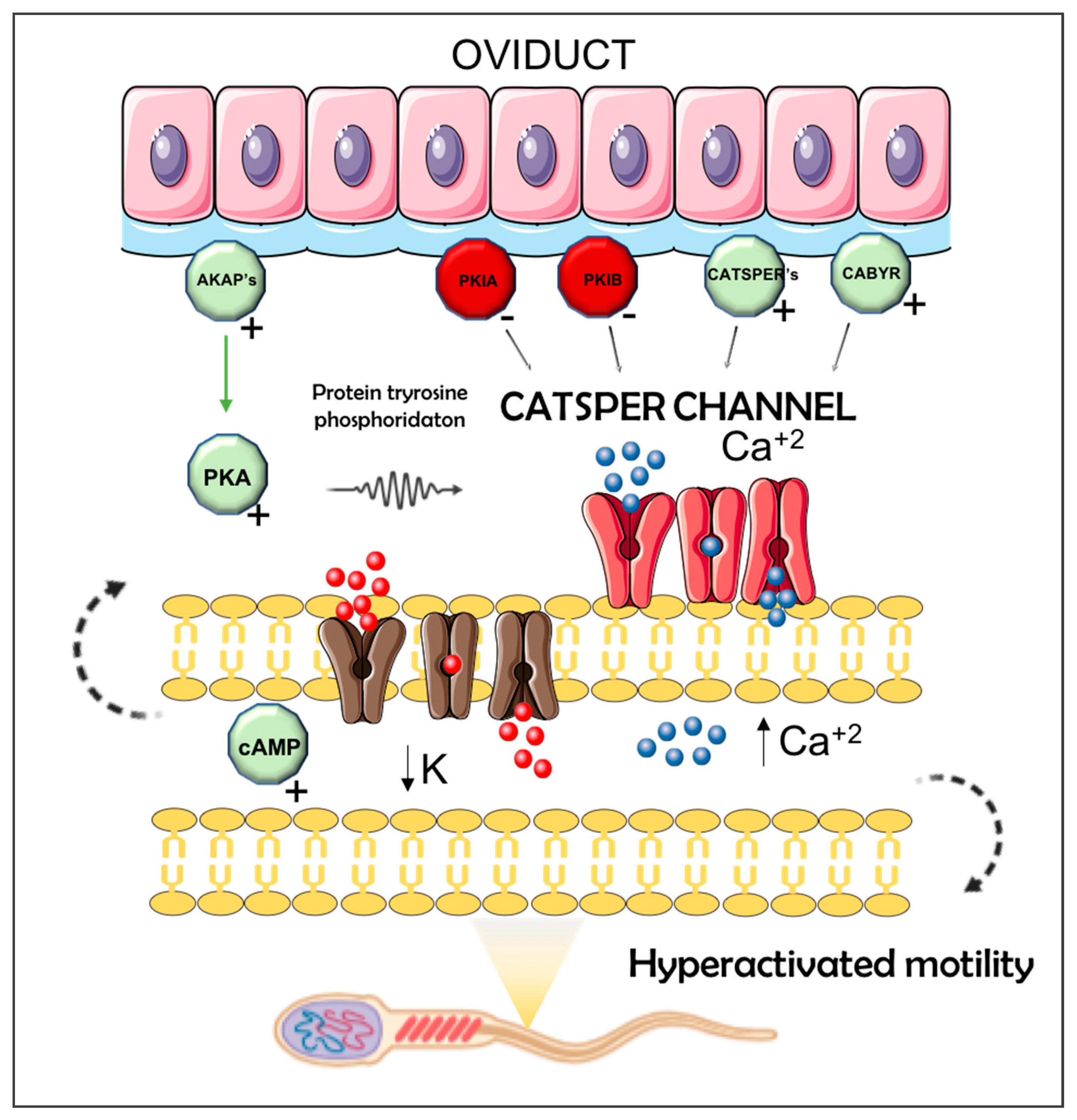
2.4. Antagonistic Influences of Sperm- or Seminal Plasma on Pre-Ovulatory Oviductal Gene Expression for Sperm Capacitation Genes
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Animal Management Including Ethics Statement
4.3. Semen Collection and SP Harvesting
4.4. Handling of Sows
4.5. Collection of Oviductal Samples
4.6. Transcriptome Analysis
4.7. Analysis of Microarray Data and Bioinformatics
4.8. Enrichment Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Biswas, D.; Yoon, J.D.; Jeon, Y.; Hyun, S.H. Effect of porcine uterus as ex vivo model of fertilizing ability and gene expression pattern on blastocysts. Theriogenology 2019, 129, 146–153. [Google Scholar] [CrossRef]
- Georgiou, A.S.; Snijders, A.P.L.; Sostaric, E.; Aflatoonian, R.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Martinez, E.A.; Wright, P.C.; Fazeli, A. Modulation of the oviductal environment by gametes. J. Proteome Res. 2007, 6, 4656–4666. [Google Scholar] [CrossRef]
- Rizos, D.; Maillo, V.; Sanchez-Calabuig, M.-J.; Lonergan, P. The Consequences of Maternal-Embryonic Cross Talk During the Periconception Period on Subsequent Embryonic Development. Adv. Exp. Med. Biol. 2017, 1014, 69–86. [Google Scholar]
- Hawk, H.W. Sperm survival and transport in the female reproductive tract. J. Dairy Sci. 1983, 66, 2645–2660. [Google Scholar] [CrossRef]
- Alminana, C.; Caballero, I.; Heath, P.R.; Maleki-Dizaji, S.; Parrilla, I.; Cuello, C.; Gil, M.A.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; et al. The battle of the sexes starts in the oviduct: Modulation of oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genom. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Schafer, J.; Bolling, A.; Scheld, M.; Henning, H.; Hambruch, N.; Schuberth, H.-J.; Pfarrer, C.; Wrenzycki, C.; Hunter, R.H.F. Seminal plasma modulates the immune-cytokine network in the porcine uterine tissue and pre-ovulatory follicles. PLoS ONE 2018, 13, e0202654. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A.; Affara, N.A.; Hubank, M.; Holt, W.V. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol. Reprod. 2004, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Pena, F.J.; Martinez, E.A.; Roca, J.; Vazquez, J.M.; et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009, 66, 1–21. [Google Scholar]
- Robertson, S.A.; Sharkey, D.J. The role of semen in induction of maternal immune tolerance to pregnancy. Semin. Immunol. 2001, 13, 243–254. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, M.; Atikuzzaman, M.; Venhoranta, H.; Wright, D.; Rodriguez-Martinez, H. Expression of Immune Regulatory Genes in the Porcine Internal Genital Tract Is Differentially Triggered by Spermatozoa and Seminal Plasma. Int. J. Mol. Sci. 2019, 20, 513. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef] [PubMed]
- Edgell, T.A.; Evans, J.; Lazzaro, L.; Boyes, K.; Sridhar, M.; Catt, S.; Rombauts, L.J.F.; Vollenhoven, B.J.; Salamonsen, L.A. Assessment of potential biomarkers of pre-receptive and receptive endometrium in uterine fluid and a functional evaluation of the potential role of CSF3 in fertility. Cytokine 2018, 111, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F.; Rodriguez-Martinez, H. Capacitation of mammalian spermatozoa in vivo, with a specific focus on events in the Fallopian tubes. Mol. Reprod. Dev. 2004, 67, 243–250. [Google Scholar] [CrossRef] [PubMed]
- AUSTIN, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Suarez, S.S. Hyperactivation of mammalian spermatozoa: Function and regulation. Reproduction 2001, 122, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Westbrook, V.A.; Chertihin, O.; Demarco, I.; Sleight, S.; Diekman, A.B. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002, 53, 133–150. [Google Scholar] [CrossRef]
- Jin, S.-K.; Yang, W.-X. Factors and pathways involved in capacitation: How are they regulated? Oncotarget 2017, 8, 3600–3627. [Google Scholar] [CrossRef]
- Niemann, H.; Rath, D. Progress in reproductive biotechnology in swine. Theriogenology 2001, 56, 1291–1304. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; Rodriguez-Martinez, H. Analysing mammalian fertilisation: Reservations and potential pitfalls with an in vitro approach. Zygote 2002, 10, 11–15. [Google Scholar] [CrossRef]
- Bergqvist, A.-S.; Ballester, J.; Johannisson, A.; Hernandez, M.; Lundeheim, N.; Rodriguez-Martinez, H. In vitro capacitation of bull spermatozoa by oviductal fluid and its components. Zygote 2006, 14, 259–273. [Google Scholar] [CrossRef]
- Morrell, J.M.; Rodriguez-Martinez, H. Practical applications of sperm selection techniques as a tool for improving reproductive efficiency. Vet. Med. Int. 2010, 2011. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Úbeda, C.; Romero-Aguirregomezcorta, J.; Matás, C.; Visconti, P.E.; García-Vázquez, F.A. Manipulation of bicarbonate concentration in sperm capacitation media improvesin vitro fertilisation output in porcine species. J. Anim. Sci. Biotechnol. 2019, 11, 19. [Google Scholar]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Trevino, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L. Factors regulating sperm capacitation. Syst. Biol. Reprod. Med. 2010, 56, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Asano, A.; Eriksson, B.; Niwa, K.; Nagai, T.; Rodriguez-Martinez, H. Capacitation status and in vitro fertility of boar spermatozoa: Effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int. J. Androl. 2002, 25, 84–93. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Tienthai, P.; Atikuzzaman, M.; Vicente-Carrillo, A.; Ruber, M.; Alvarez-Rodriguez, M. The ubiquitous hyaluronan: Functionally implicated in the oviduct? Theriogenology 2016, 86, 182–186. [Google Scholar] [CrossRef]
- Boilard, M.; Bailey, J.; Collin, S.; Dufour, M.; Sirard, M.-A. Effect of bovine oviduct epithelial cell apical plasma membranes on sperm function assessed by a novel flow cytometric approach. Biol. Reprod. 2002, 67, 1125–1132. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Role of the oviduct in sperm capacitation. Theriogenology 2007, 68 (Suppl. 1), S138–S146. [Google Scholar] [CrossRef]
- Tienthai, P.; Johannisson, A.; Rodriguez-Martinez, H. Sperm capacitation in the porcine oviduct. Anim. Reprod. Sci. 2004, 80, 131–146. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Tienthai, P.; Suzuki, K.; Funahashi, H.; Ekwall, H.; Johannisson, A. Involvement of oviduct in sperm capacitation and oocyte development in pigs. Reprod. Suppl. 2001, 58, 129–145. [Google Scholar] [PubMed]
- Carlson, A.E.; Westenbroek, R.E.; Quill, T.; Ren, D.; Clapham, D.E.; Hille, B.; Garbers, D.L.; Babcock, D.F. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA 2003, 100, 14864–14868. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-H.; Zhu, Y.-Y.; Wang, L.; Liu, H.-L.; Ling, Y.; Li, Z.-L.; Sun, L.-B. The Catsper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Kirichok, Y.; Chung, J.-J.; Clapham, D.E. Ion channels that control fertility in mammalian spermatozoa. Int. J. Dev. Biol. 2008, 52, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Alvarez-Rodriguez, M.; Rodriguez-Martinez, H. The CatSper channel modulates boar sperm motility during capacitation. Reprod. Biol. 2017, 17, 69–78. [Google Scholar] [CrossRef]
- Avidan, N.; Tamary, H.; Dgany, O.; Cattan, D.; Pariente, A.; Thulliez, M.; Borot, N.; Moati, L.; Barthelme, A.; Shalmon, L.; et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet. 2003, 11, 497–502. [Google Scholar] [CrossRef]
- Lobley, A.; Pierron, V.; Reynolds, L.; Allen, L.; Michalovich, D. Identification of human and mouse CatSper3 and CatSper4 genes: Characterisation of a common interaction domain and evidence for expression in testis. Reprod. Biol. Endocrinol. 2003, 1, 53. [Google Scholar] [CrossRef]
- Liu, J.; Xia, J.; Cho, K.-H.; Clapham, D.E.; Ren, D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J. Biol. Chem. 2007, 282, 18945–18952. [Google Scholar] [CrossRef]
- Marquez, B.; Ignotz, G.; Suarez, S.S. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 2007, 303, 214–221. [Google Scholar] [CrossRef]
- Alminana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [PubMed]
- Matamoros-Volante, A.; Moreno-Irusta, A.; Torres-Rodriguez, P.; Giojalas, L.; Gervasi, M.G.; Visconti, P.E.; Trevino, C.L. Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: The case of capacitation-induced tyrosine phosphorylation. Mol. Hum. Reprod. 2018, 24, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Orta, G.; de la Vega-Beltran, J.L.; Martin-Hidalgo, D.; Santi, C.M.; Visconti, P.E.; Darszon, A. CatSper channels are regulated by protein kinase A. J. Biol. Chem. 2018, 293, 16830–16841. [Google Scholar] [CrossRef]
- O’Brien, E.D.; Krapf, D.; Cabada, M.O.; Visconti, P.E.; Arranz, S.E. Transmembrane adenylyl cyclase regulates amphibian sperm motility through protein kinase A activation. Dev. Biol. 2011, 350, 80–88. [Google Scholar] [CrossRef]
- Reinton, N.; Collas, P.; Haugen, T.B.; Skalhegg, B.S.; Hansson, V.; Jahnsen, T.; Tasken, K. Localization of a novel human A-kinase-anchoring protein, hAKAP220, during spermatogenesis. Dev. Biol. 2000, 223, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Ficarro, S.; Chertihin, O.; Westbrook, V.A.; White, F.; Jayes, F.; Kalab, P.; Marto, J.A.; Shabanowitz, J.; Herr, J.C.; Hunt, D.F.; et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 2003, 278, 11579–11589. [Google Scholar] [CrossRef] [PubMed]
- Stival, C.; Ritagliati, C.; Xu, X.; Gervasi, M.G.; Luque, G.M.; Baro Graf, C.; De la Vega-Beltran, J.L.; Torres, N.; Darszon, A.; Krapf, D.; et al. Disruption of protein kinase A localization induces acrosomal exocytosis in capacitated mouse sperm. J. Biol. Chem. 2018, 293, 9435–9447. [Google Scholar] [CrossRef]
- Mburu, J.N.; Einarsson, S.; Lundeheim, N.; Rodriguez-Martinez, H. Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim. Reprod. Sci. 1996, 45, 109–121. [Google Scholar] [CrossRef]
- Colledge, M.; Scott, J.D. AKAPs: From structure to function. Trends Cell Biol. 1999, 9, 216–221. [Google Scholar] [CrossRef]
- Feliciello, A.; Rubin, C.S.; Avvedimento, E.V.; Gottesman, M.E. Expression of a kinase anchor protein 121 is regulated by hormones in thyroid and testicular germ cells. J. Biol. Chem. 1998, 273, 23361–23366. [Google Scholar] [CrossRef]
- Liu, S.-L.; Ni, B.; Wang, X.-W.; Huo, W.-Q.; Zhang, J.; Tian, Z.-Q.; Huang, Z.-M.; Tian, Y.; Tang, J.; Zheng, Y.-H.; et al. FSCB phosphorylation in mouse spermatozoa capacitation. BMB Rep. 2011, 44, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Naaby-Hansen, S. Functional and immunological analysis of the human sperm proteome. Dan. Med. J. 2012, 59, B4414. [Google Scholar] [PubMed]
- Li, Y.-F.; He, W.; Jha, K.N.; Klotz, K.; Kim, Y.-H.; Mandal, A.; Pulido, S.; Digilio, L.; Flickinger, C.J.; Herr, J.C. FSCB, a novel protein kinase A-phosphorylated calcium-binding protein, is a CABYR-binding partner involved in late steps of fibrous sheath biogenesis. J. Biol. Chem. 2007, 282, 34104–34119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, M.; Yu, R.; Liu, B.; Tian, Z.; Liu, S. FSCB phosphorylation regulates mouse spermatozoa capacitation through suppressing SUMOylation of ROPN1/ROPN1L. Am. J. Transl. Res. 2016, 8, 2776–2782. [Google Scholar]
- Newell, A.E.H.; Fiedler, S.E.; Ruan, J.M.; Pan, J.; Wang, P.J.; Deininger, J.; Corless, C.L.; Carr, D.W. Protein kinase A RII-like (R2D2) proteins exhibit differential localization and AKAP interaction. Cell Motil. Cytoskeleton 2008, 65, 539–552. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Blackmore, P.F.; Beebe, S.J.; Danforth, D.R.; Alexander, N. Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J. Biol. Chem. 1990, 265, 1376–1380. [Google Scholar]
- Park, K.-H.; Kim, B.-J.; Kang, J.; Nam, T.-S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.-W.; Kim, U.-H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, ra31. [Google Scholar] [CrossRef]
- Sagare-Patil, V.; Vernekar, M.; Galvankar, M.; Modi, D. Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol. Cell. Endocrinol. 2013, 374, 82–91. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Minetti, F.; Forti, G.; Muratori, M.; Baldi, E. The CatSper calcium channel in human sperm: Relation with motility and involvement in progesterone-induced acrosome reaction. Hum. Reprod. 2014, 29, 418–428. [Google Scholar] [CrossRef]
- Miller, M.R.; Mansell, S.A.; Meyers, S.A.; Lishko, P.V. Flagellar ion channels of sperm: Similarities and differences between species. Cell Calcium 2015, 58, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.-J. Dual Sensing of Physiologic pH and Calcium by EFCAB9 Regulates Sperm Motility. Cell 2019, 177, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.A.; Sharif, M.; Wang, H.; Bovin, N.; Miller, D.J. Release of Porcine Sperm from Oviduct Cells is Stimulated by Progesterone and Requires CatSper. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef]
- Martinez, E.A.; Vazquez, J.M.; Roca, J.; Lucas, X.; Gil, M.A.; Vazquez, J.L. Deep intrauterine insemination and embryo transfer in pigs. Reprod. Suppl. 2001, 58, 301–311. [Google Scholar]
- Kawano, N.; Yoshida, M. Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol. Reprod. 2007, 76, 353–361. [Google Scholar] [CrossRef]
- Kawano, N.; Yoshida, K.; Iwamoto, T.; Yoshida, M. Ganglioside GM1 mediates decapacitation effects of SVS2 on murine spermatozoa. Biol. Reprod. 2008, 79, 1153–1159. [Google Scholar] [CrossRef]
- Araki, N.; Kawano, N.; Kang, W.; Miyado, K.; Yoshida, K.; Yoshida, M. Seminal vesicle proteins SVS3 and SVS4 facilitate SVS2 effect on sperm capacitation. Reproduction 2016, 152, 313–321. [Google Scholar] [CrossRef]
- Wu, J.; Dong, X.; Liu, K.; Xia, Y.; Wang, X.; Shen, O.; Ding, X.; Zhang, J. Association of semenogelin (SEMG) gene variants in idiopathic male infertility in Chinese-Han population. J. Toxicol. Environ. Health. A 2019, 82, 928–934. [Google Scholar] [CrossRef]
- Yamasaki, K.; Yoshida, K.; Yoshiike, M.; Shimada, K.; Nishiyama, H.; Takamizawa, S.; Yanagida, K.; Iwamoto, T. Relationship between Semenogelins bound to human sperm and other semen parameters and pregnancy outcomes. Basic Clin. Androl. 2017, 27, 15. [Google Scholar] [CrossRef]
- Perez-Patino, C.; Parrilla, I.; Barranco, I.; Vergara-Barberan, M.; Simo-Alfonso, E.F.; Herrero-Martinez, J.M.; Rodriguez-Martinez, H.; Martinez, E.A.; Roca, J. New In-Depth Analytical Approach of the Porcine Seminal Plasma Proteome Reveals Potential Fertility Biomarkers. J. Proteome Res. 2018, 17, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66 (Suppl. 1), 11–22. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Weigel Munoz, M.; Battistone, M.A.; Carvajal, G.; Maldera, J.A.; Curci, L.; Torres, P.; Lombardo, D.; Pignataro, O.P.; Da Ros, V.G.; Cuasnicu, P.S. Influence of the genetic background on the reproductive phenotype of mice lacking Cysteine-Rich Secretory Protein 1 (CRISP1). Biol. Reprod. 2018, 99, 373–383. [Google Scholar] [CrossRef]
- Nixon, B.; MacIntyre, D.A.; Mitchell, L.A.; Gibbs, G.M.; O’Bryan, M.; Aitken, R.J. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol. Reprod. 2006, 74, 275–287. [Google Scholar] [CrossRef]
- Cohen, D.J.; Maldera, J.A.; Vasen, G.; Ernesto, J.I.; Munoz, M.W.; Battistone, M.A.; Cuasnicu, P.S. Epididymal protein CRISP1 plays different roles during the fertilization process. J. Androl. 2011, 32, 672–678. [Google Scholar] [CrossRef]
- Roberts, K.P.; Wamstad, J.A.; Ensrud, K.M.; Hamilton, D.W. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol. Reprod. 2003, 69, 572–581. [Google Scholar] [CrossRef]
- Roberts, K.P.; Ensrud, K.M.; Wooters, J.L.; Nolan, M.A.; Johnston, D.S.; Hamilton, D.W. Epididymal secreted protein Crisp-1 and sperm function. Mol. Cell. Endocrinol. 2006, 250, 122–127. [Google Scholar] [CrossRef]
- Carvajal, G.; Brukman, N.G.; Weigel Munoz, M.; Battistone, M.A.; Guazzone, V.A.; Ikawa, M.; Haruhiko, M.; Lustig, L.; Breton, S.; Cuasnicu, P.S. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci. Rep. 2018, 8, 17531. [Google Scholar] [CrossRef] [PubMed]
- Ernesto, J.I.; Weigel Munoz, M.; Battistone, M.A.; Vasen, G.; Martinez-Lopez, P.; Orta, G.; Figueiras-Fierro, D.; De la Vega-Beltran, J.L.; Moreno, I.A.; Guidobaldi, H.A.; et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell Biol. 2015, 210, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.A.; Washburn, C.A.; Sugiyama, H.; Xiang, X.; Olson, J.H.; Al-Anzi, B.; Bieber, A.L.; Chandler, D.E. Allurin, an amphibian sperm chemoattractant having implications for mammalian sperm physiology. Int. Rev. Cell Mol. Biol. 2012, 295, 1–61. [Google Scholar] [PubMed]
- Da Ros, V.G.; Maldera, J.A.; Willis, W.D.; Cohen, D.J.; Goulding, E.H.; Gelman, D.M.; Rubinstein, M.; Eddy, E.M.; Cuasnicu, P.S. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev. Biol. 2008, 320, 12–18. [Google Scholar] [CrossRef]
- Cohen, D.J.; Busso, D.; Da Ros, V.; Ellerman, D.A.; Maldera, J.A.; Goldweic, N.; Cuasnicu, P.S. Participation of cysteine-rich secretory proteins (CRISP) in mammalian sperm-egg interaction. Int. J. Dev. Biol. 2008, 52, 737–742. [Google Scholar] [CrossRef]
- Krutskikh, A.; Poliandri, A.; Cabrera-Sharp, V.; Dacheux, J.L.; Poutanen, M.; Huhtaniemi, I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 4198–4209. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Saravia, F.; Wallgren, M.; Tienthai, P.; Johannisson, A.; Vazquez, J.M.; Martinez, E.; Roca, J.; Sanz, L.; Calvete, J.J. Boar spermatozoa in the oviduct. Theriogenology 2005, 63, 514–535. [Google Scholar] [CrossRef]
- Lopez-Ubeda, R.; Garcia-Vazquez, F.A.; Romar, R.; Gadea, J.; Munoz, M.; Hunter, R.H.F.; Coy, P. Oviductal Transcriptome Is Modified after Insemination during Spontaneous Ovulation in the Sow. PLoS ONE 2015, 10, e0130128. [Google Scholar] [CrossRef]
- Terada, K.; Yomogida, K.; Imai, T.; Kiyonari, H.; Takeda, N.; Kadomatsu, T.; Yano, M.; Aizawa, S.; Mori, M. A type I DnaJ homolog, DjA1, regulates androgen receptor signaling and spermatogenesis. EMBO J. 2005, 24, 611–622. [Google Scholar] [CrossRef]
- Panneerdoss, S.; Siva, A.B.; Kameshwari, D.B.; Rangaraj, N.; Shivaji, S. Association of lactate, intracellular pH, and intracellular calcium during capacitation and acrosome reaction: Contribution of hamster sperm dihydrolipoamide dehydrogenase, the E3 subunit of pyruvate dehydrogenase complex. J. Androl. 2012, 33, 699–710. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, H.; Huang, B.; Liu, Q.; Wang, Y.; Shi, D.; Li, X. Expression pattern of prohibitin, capping actin protein of muscle Z-line beta subunit and tektin-2 gene in Murrah buffalo sperm and its relationship with sperm motility. Asian-Australas. J. Anim. Sci. 2018, 31, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Landers, E.A.; Burkin, H.R.; Bleck, G.T.; Howell-Skalla, L.; Miller, D.J. Porcine beta1,4-galactosyltransferase-I sequence and expression. Reprod. Domest. Anim. 2009, 44, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, K.A.; Anderson, A.L.; Dun, M.D.; McLaughlin, E.A.; O’Bryan, M.K.; Aitken, R.J.; Nixon, B. Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev. Biol. 2011, 356, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.A.; Baibakov, B.; Dean, J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J. Cell Biol. 2014, 205, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Inoue, N.; Shimizu, T. Structure of IZUMO1-JUNO reveals sperm-oocyte recognition during mammalian fertilization. Nature 2016, 534, 566–569. [Google Scholar] [CrossRef]
- Caballero, I.; Vazquez, J.M.; Gil, M.A.; Calvete, J.J.; Roca, J.; Sanz, L.; Parrilla, I.; Garcia, E.M.; Rodriguez-Martinez, H.; Martinez, E.A. Does seminal plasma PSP-I/PSP-II spermadhesin modulate the ability of boar spermatozoa to penetrate homologous oocytes in vitro? J. Androl. 2004, 25, 1004–1012. [Google Scholar] [CrossRef]
- Pursel, V.G.; Johnson, L.A. Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
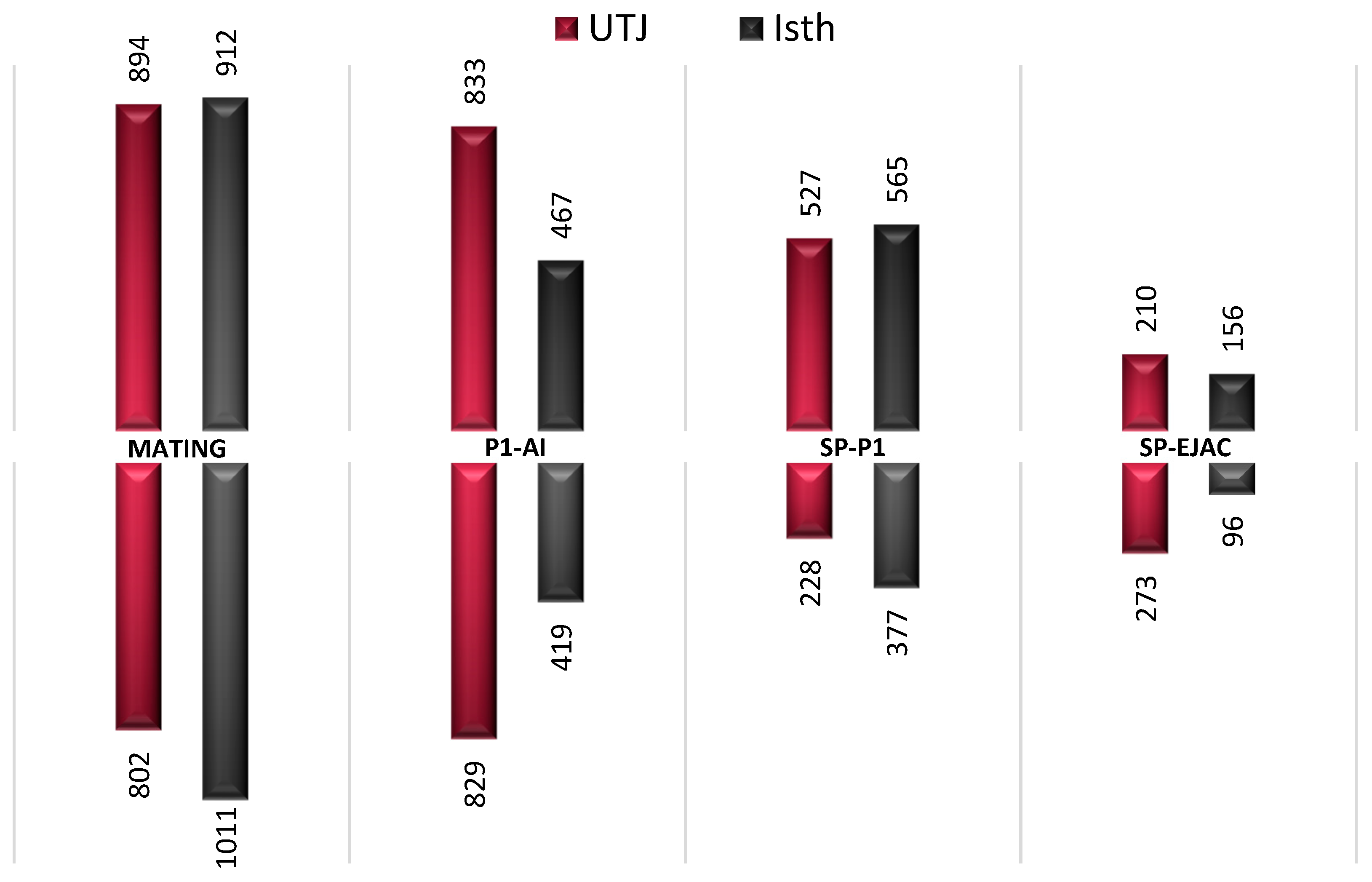


| MATING | |||||||
| UTJ | ISTH | ||||||
| Gene ID | Fold Change | p-value | Description | Gene ID | Fold Change | p-value | Description |
| ABHD2 | −1.66 | 0.004 | Monoacylglycerol lipase protease | ABHD2 | −2.6 | 0.0003 | Monoacylglycerol lipase protease |
| CATSPER2 | 1.53 | 0.01 | Cation channelsperm-associated protein 2 | CATSPERγ | 1.3 | 0.03 | Cation channelsperm-associated subunit gamma |
| CATSPERγ | 1.56 | 0.006 | Cation channelsperm-associated subunit gamma | ING2 | 1.25 | 0.003 | Inhibitor of growth protein 2 |
| GAS8 | 1.37 | 0.002 | Dynein regulatory complex subunit 8 | AAAS | −1.38 | 0.03 | Aladin WD repeat nucleoporin |
| RLN2 | −1.16 | 0.02 | Prorelaxin precursor | B4GALT1 | −1.89 | 0.0005 | Beta-1,4-galactosyltransferase |
| TEKT2 | 1.62 | 0.03 | Tektin-2 non-motor microtubule binding protein | BCL2L1 | 1.28 | 0.009 | Anti-apoptotic signaling molecule |
| TEKT3 | 1.45 | 0.04 | Tektin-3 non-motor microtubule binding protein | CCT3 | −1.19 | 0.04 | T-complex protein 1 subunit gamma |
| B4GALT1 | −1.69 | 0.0009 | Beta-1,4-galactosyltransferase | EHMT2 | 1.18 | 0.02 | Histone-Lysine N-methyltransferase |
| BCL2L1 | 1.37 | 0.007 | Anti-apoptotic signaling molecule | MFGE8 | −4.37 | <0.0001 | Lactadherin membrane-bound signaling molecule |
| MFGE8 | −2.23 | 0.004 | Lactadherin membrane-bound signaling molecule | NR2F2 | 1.73 | 0.001 | C4 Zinc finger nuclear binding receptor |
| RNASE10 | −1.15 | 0.03 | Inactive ribonuclease-like protein 10 | SPINK2 | 1.33 | 0.03 | Serine protease inhibitor Kazal type 2 |
| SPAM1 | −1.21 | 0.04 | Sperm adhesión member | TARBP2 | −1.26 | 0.004 | RISC-loading complex subunit 2 |
| SPEF2 | 1.61 | 0.03 | Sperm flagelar protein 2 | ZP4 | −4.09 | <0.0001 | Zona pellucida sperm-binding protein 4 |
| TARBP2 | −1.47 | 0.001 | RISC-loading complex subunit 2 | AKAP11 | 1.18 | 0.04 | A-Kinase Anchor protein 11 |
| TRPC3 | 1.71 | 0.003 | Short transitient receptor potential channel 3 | AKAP12 | 1.55 | 0.02 | A-Kinase Anchor protein 12 |
| ZP4 | −1.76 | 0.03 | Zona pellucida sperm-binding protein 4 | PKIA | −3.4 | 0.0002 | cAMP-dependent kinase inhibitor alpha |
| AKAP11 | 1.43 | 0.007 | A-Kinase Anchor protein 11 | CABYR | 1.22 | 0.01 | Calcium-binding tyrosine phosphorylation-regulated protein |
| AKAP13 | 1.37 | 0.007 | A-Kinase Anchor protein 13 | ||||
| PKIA | −2.33 | 0.02 | cAMP-dependent kinase inhibitor alpha | ||||
| CABYR | 1.33 | 0.007 | Calcium-binding tyrosine phosphorylation-regulated protein | ||||
| P1-AI | |||||||
| UTJ | ISTH | ||||||
| CACNA1I | 1.56 | 0.006 | Voltage-dependent L-type calcium channel | ACRBP | 1.38 | 0.04 | Acrosin-binding protein |
| CFTR | −2.38 | 0.009 | ATP-binding cassette transporter | CATSPERγ | 1.42 | 0.008 | Cation channelsperm-associated subunit gamma |
| DPCD | −1.49 | 0.04 | Deleted in primary ciliary dyskinesia | ING2 | 1.17 | 0.03 | Inhibitor of growth protein 2 |
| SPAG6 | −2.58 | 0.03 | Sperm-associated antigen 6 | AQN-1 | −1.336 | 0.04 | Carbohydrate-binding protein |
| HOXD9 | 1.52 | 0.02 | Homeobox protein 9 | PRDM14 | 1.19 | 0.04 | PR domain Zinc finger protein 14 |
| HOXD10 | 2.42 | 0.04 | Homeobox protein 10 | TARBP2 | −1.18 | 0.01 | RISC-loading complex subunit 2 |
| SPA17 | −1.7 | 0.03 | Sperm Surface protein | ZP4 | −3.01 | 0.0007 | Zona pellucida sperm-binding protein |
| TARBP2 | −1.25 | 0.02 | RISC-loading complex subunit 2 | ||||
| TRPC3 | 1.65 | 0.01 | Short transient receptor potential cannel 3 | ||||
| AKAP11 | 1.57 | 0.009 | A-Kinase Anchor protein 11 | ||||
| AKAP13 | 1.4 | 0.04 | A-Kinase Anchor protein 13 | ||||
| PKIB | −1.81 | 0.01 | cAMP-dependent kinase inhibitor beta | ||||
| SP-P1 | |||||||
| UTJ | ISTH | ||||||
| Gene ID | Fold Change | p-value | Description | Gene ID | Fold Change | p-value | Description |
| INSL6 | 1.39 | 0.0007 | Insulin-like 6 peptide | CACNA1D | 1.65 | 0.0008 | Voltage-dependent L-type calcium channel |
| RNASE10 | −1.21 | 0.02 | Inactive ribonuclease-like protein 10 | TEKT3 | −1.14 | 0.04 | Tektin-3 non-motor microtubule binding protein |
| SLC26A8 | 1.28 | 0.02 | Anion transporter 1 | EHHMT2 | 1.16 | 0.04 | Histone-Lysine N-methyltransferase |
| CRISP1 | 1.3 | 0.04 | Custein-rich secretory protein 1 | HOXA11 | −1.31 | 0.02 | Homeobox protein A-11 |
| PLCZ1 | 1.17 | 0.02 | Calcium-binding protein phospholipase signaling molecule | SYCP2 | 1.92 | 0.01 | Synaptonemal complexprotein 2 |
| TRPC3 | 1.45 | 0.04 | Short transient receptor potential cannel 3 | TARBP2 | −1.33 | 0.0007 | RISC-loading complex subunit 2 |
| SP-Ejac | |||||||
| UTJ | ISTH | ||||||
| CATSPER1 | −1.37 | 0.01 | Cation channelsperm-associated protein 1 | AAAS | 1.16 | 0.04 | Aladin WD repeat nucleoporin |
| RLN2 | −1.22 | 0.04 | Prorelaxin precursor | ||||
| RNASE10 | −1.16 | 0.03 | Inactive ribonuclease-like protein 10 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, C.A.; Alvarez-Rodriguez, M.; Wright, D.; Rodriguez-Martinez, H. Does the Pre-Ovulatory Pig Oviduct Rule Sperm Capacitation In Vivo Mediating Transcriptomics of Catsper Channels? Int. J. Mol. Sci. 2020, 21, 1840. https://doi.org/10.3390/ijms21051840
Martinez CA, Alvarez-Rodriguez M, Wright D, Rodriguez-Martinez H. Does the Pre-Ovulatory Pig Oviduct Rule Sperm Capacitation In Vivo Mediating Transcriptomics of Catsper Channels? International Journal of Molecular Sciences. 2020; 21(5):1840. https://doi.org/10.3390/ijms21051840
Chicago/Turabian StyleMartinez, Cristina A., Manuel Alvarez-Rodriguez, Dominic Wright, and Heriberto Rodriguez-Martinez. 2020. "Does the Pre-Ovulatory Pig Oviduct Rule Sperm Capacitation In Vivo Mediating Transcriptomics of Catsper Channels?" International Journal of Molecular Sciences 21, no. 5: 1840. https://doi.org/10.3390/ijms21051840
APA StyleMartinez, C. A., Alvarez-Rodriguez, M., Wright, D., & Rodriguez-Martinez, H. (2020). Does the Pre-Ovulatory Pig Oviduct Rule Sperm Capacitation In Vivo Mediating Transcriptomics of Catsper Channels? International Journal of Molecular Sciences, 21(5), 1840. https://doi.org/10.3390/ijms21051840








