Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression
Abstract
:1. Introduction
2. Results
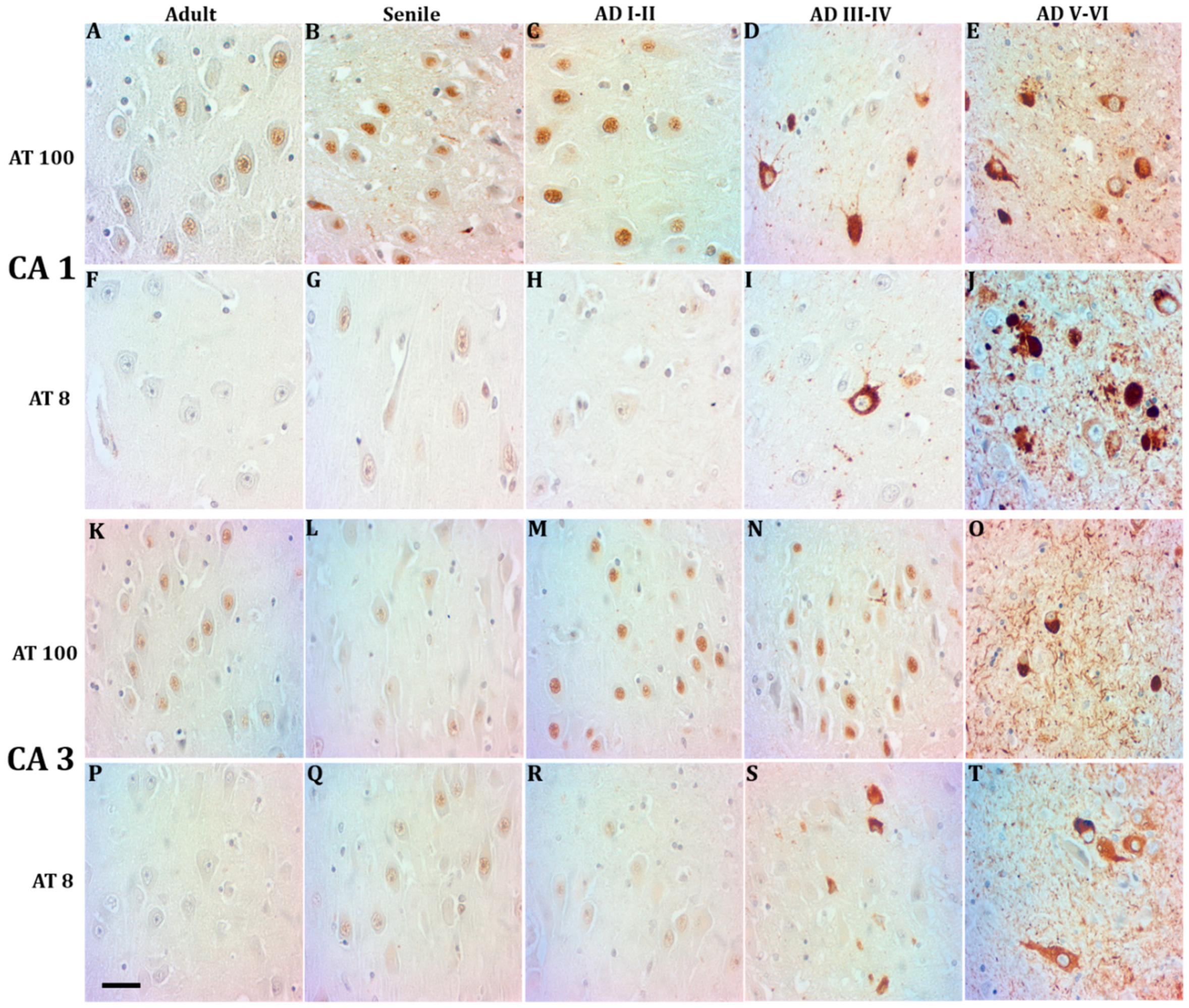
2.1. Phosphorylated Tau Shifts from Nucleus to Cytosol through AD Progression
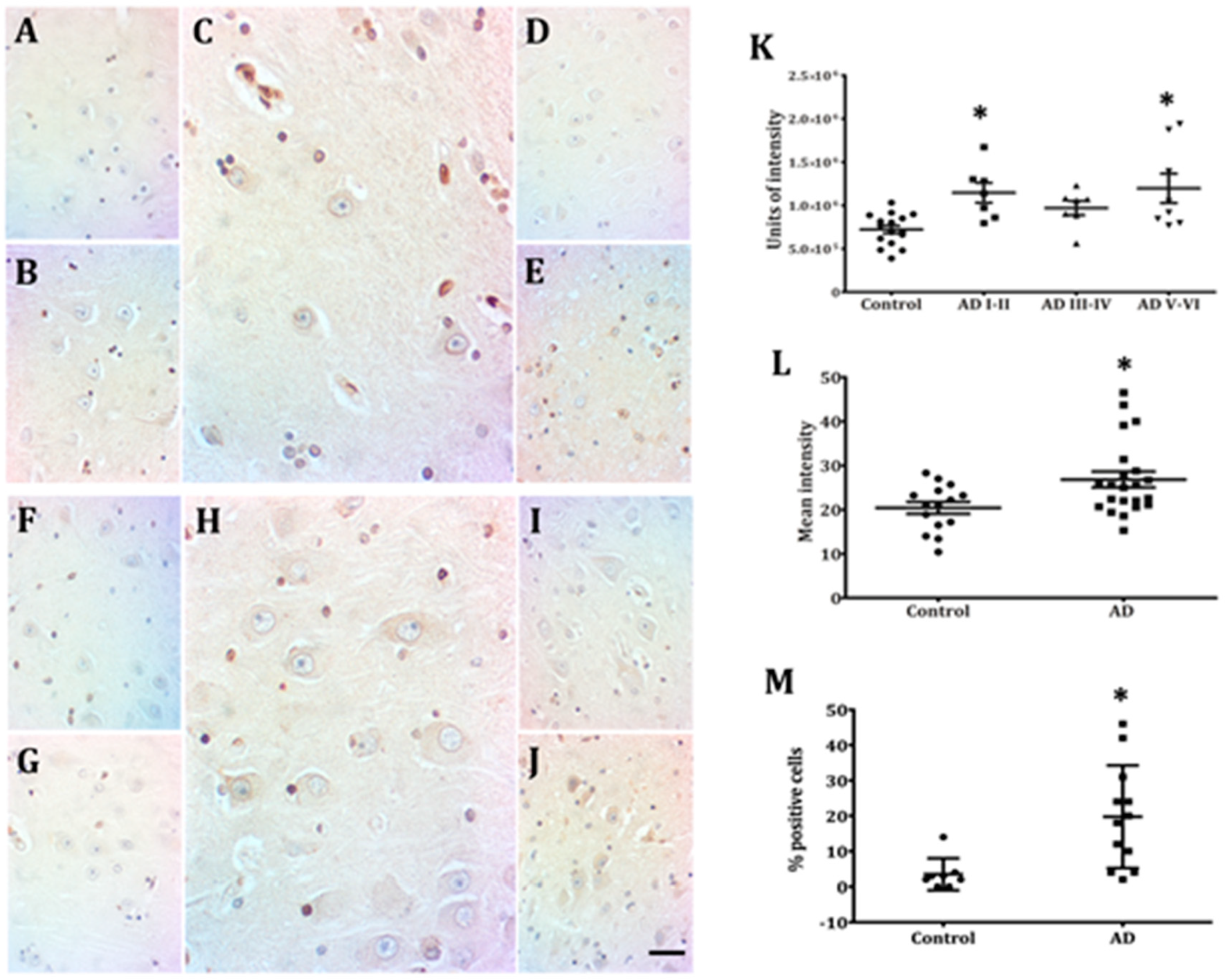
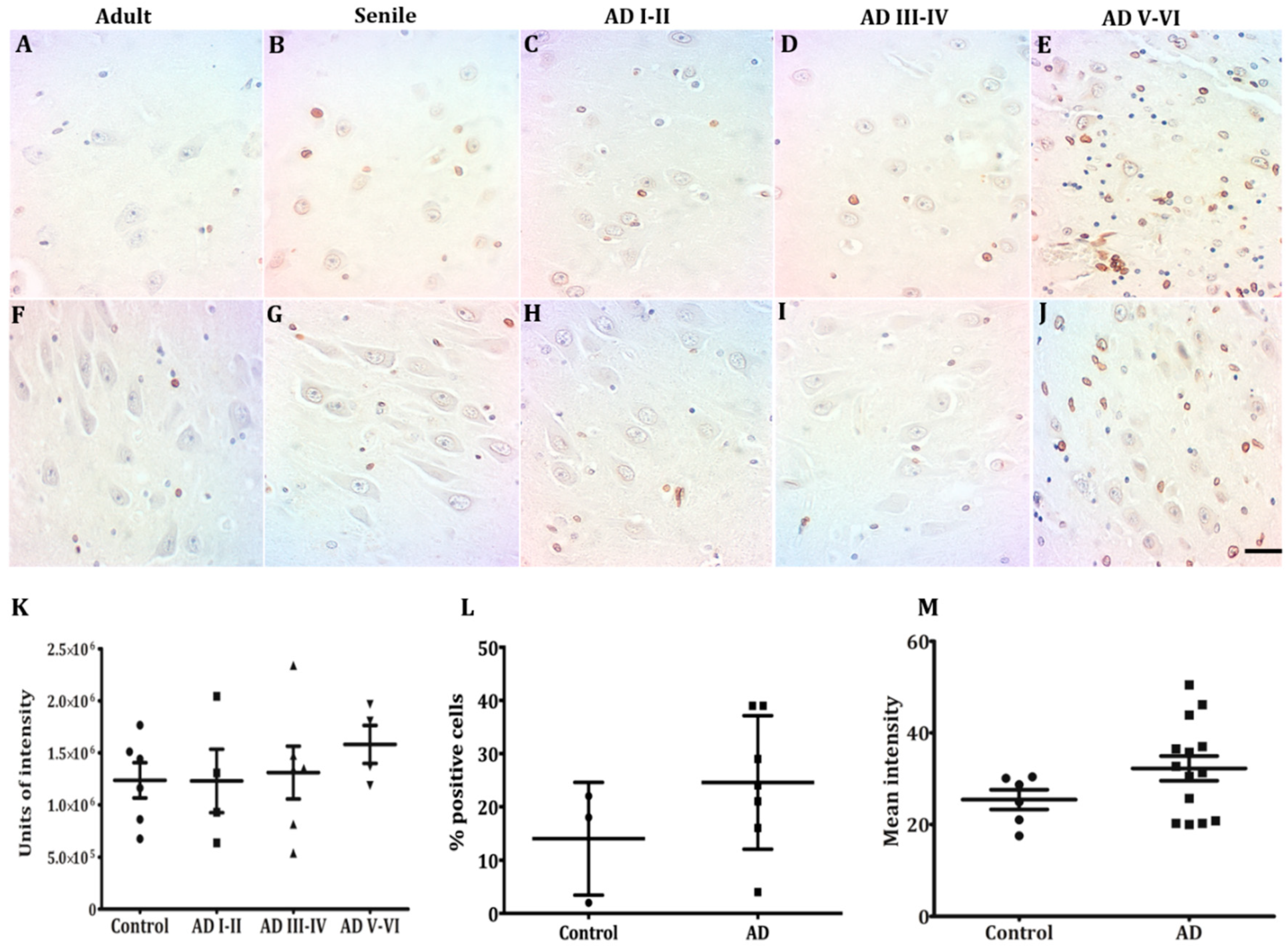
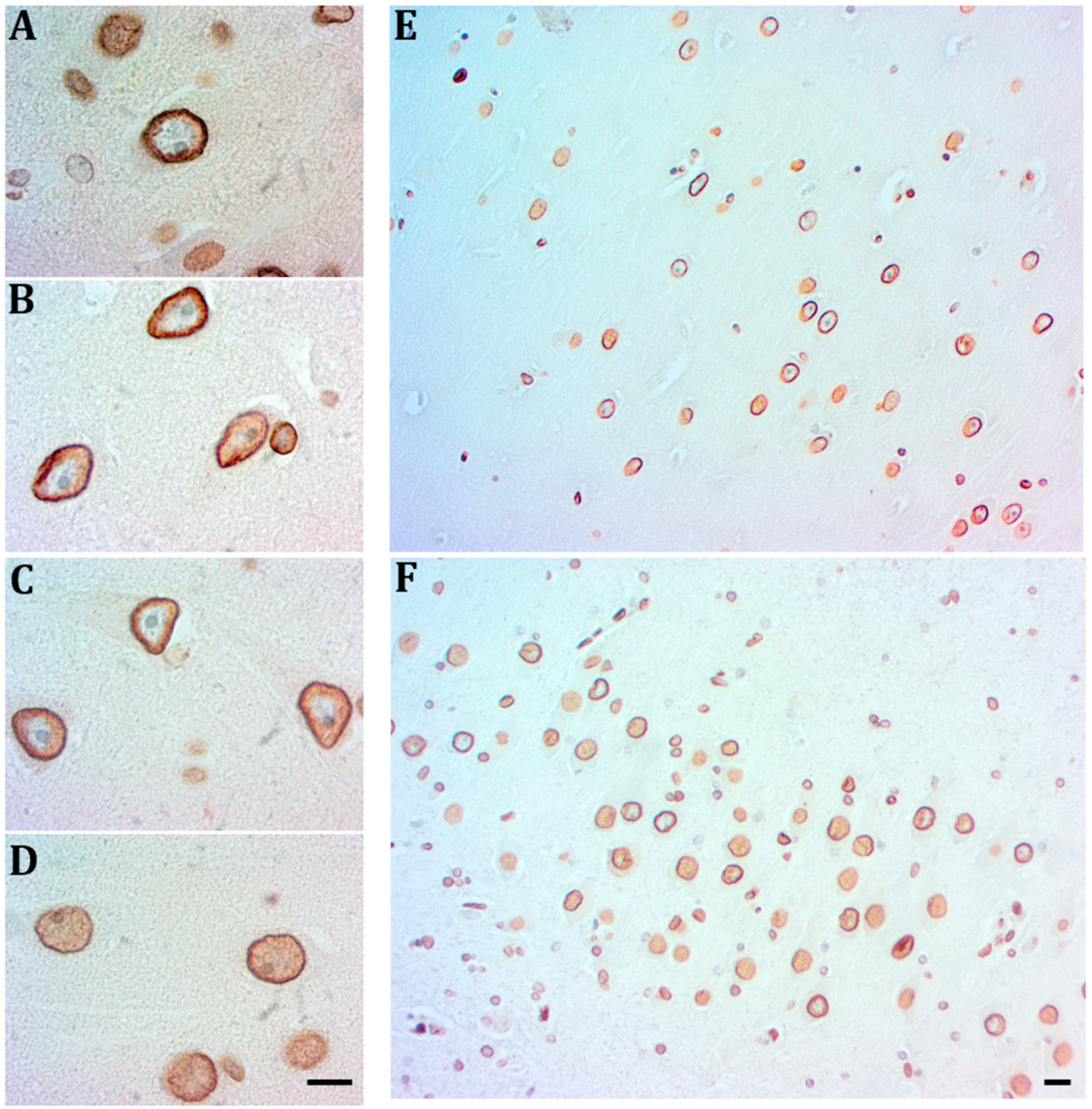
2.2. Hippocampal Neurons Express Lamin A from Early to Late AD Stages
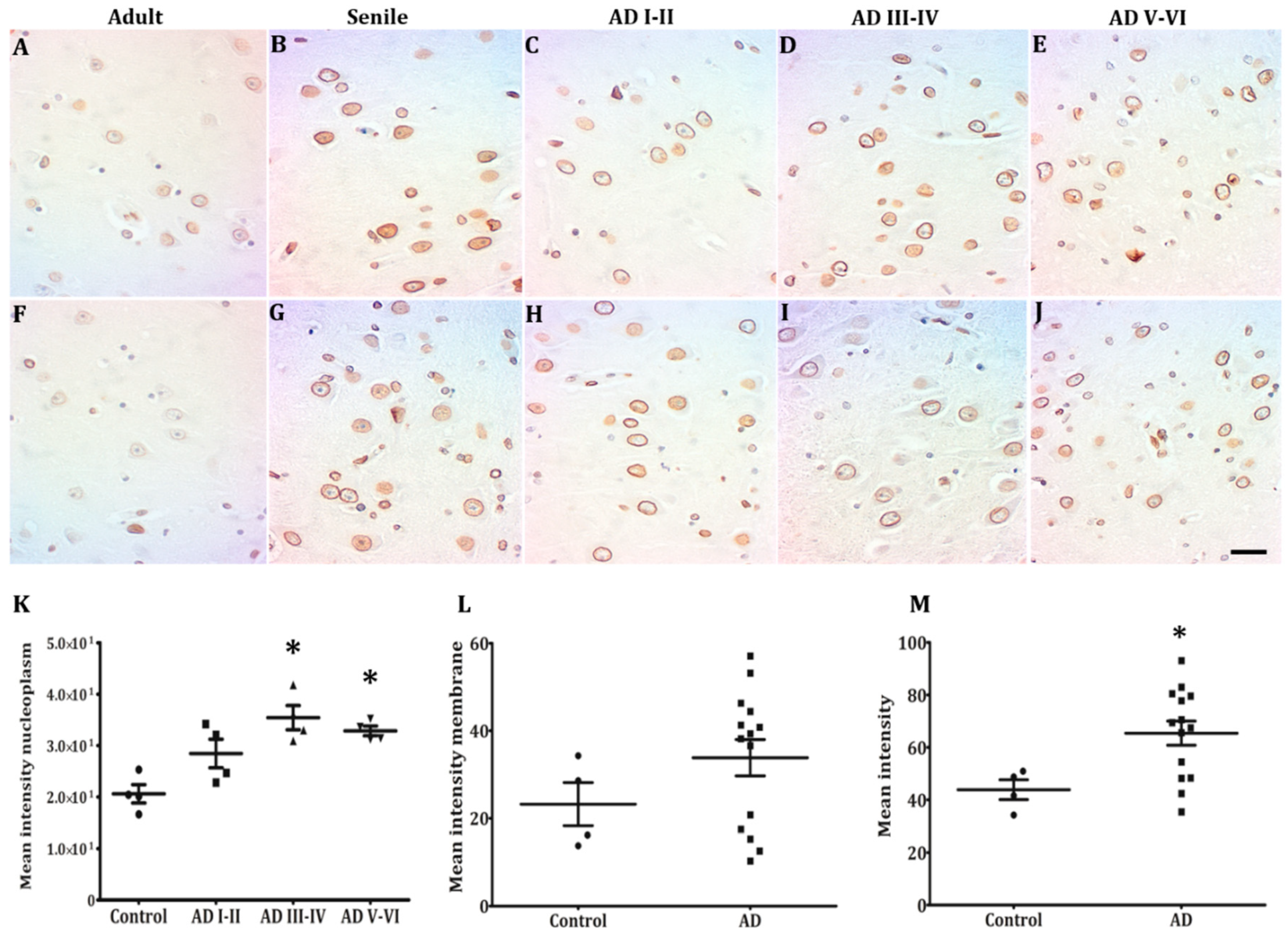
2.3. Lamin B2 Redistributes in the Nucleoplasm along AD Stages
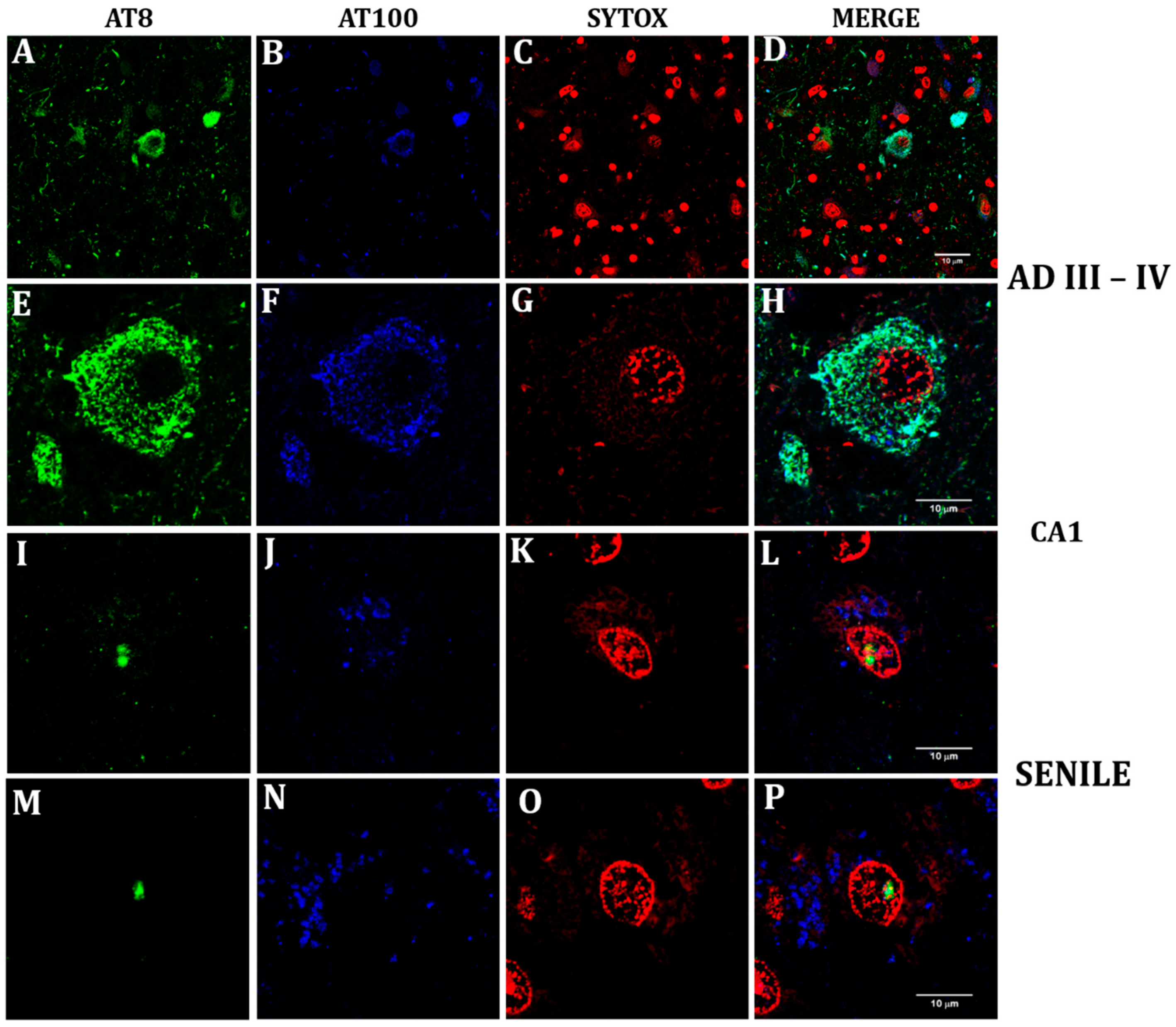
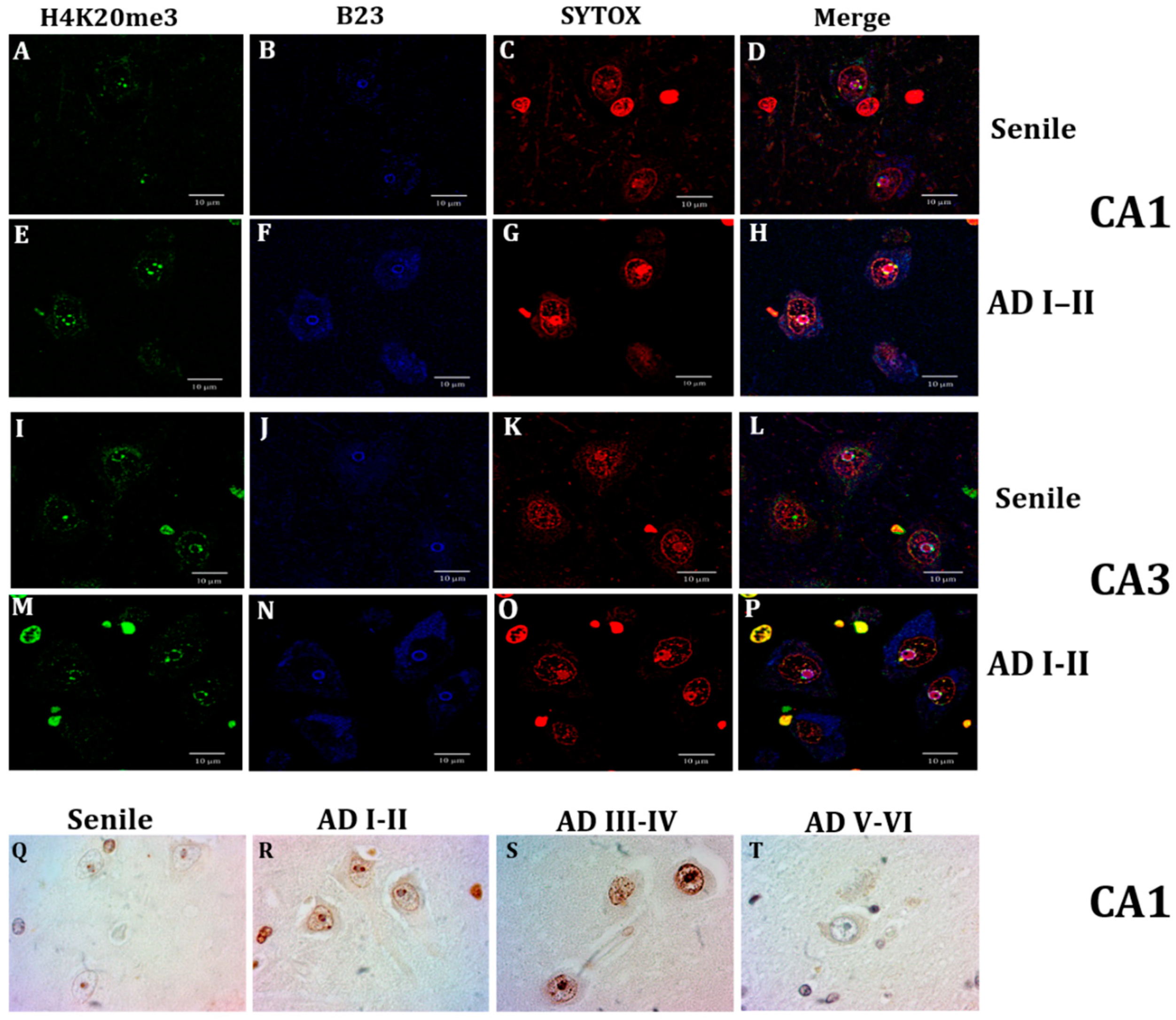
2.4. Nuclear Lamin and Nuclear Tau Changes are associated with Constitutive Heterochromatin Modifications
2.5. Lamin A Expression in Cells with Perinuclear Lamin B2
3. Discussion
3.1. Neurons Express Lamin A from Early AD Stages on
3.2. Lamin B2 in the Nucleoplasm of AD Neurons
3.3. Lamin A Associated Epigenetic Changes at Early AD Stages
3.4. Nuclear AT8 in Aging Cycling Neurons
3.5. Lamin A and Tau Oligomers in Neurons with NFTs
3.6. Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aebi, U.; Cohn, J.; Buhle, L.; Gerace, L. The nuclear lamina is a meshwork of intermediate-Type filaments. Nature 1986, 323, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, D.K.; Dechat, T.; Kohlmaier, A.; Adam, S.A.; Bozovsky, M.R.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Khuon, S.; Collins, F.S.; et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA 2006, 103, 8703–8708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Las Heras, J.I.; Meinke, P.; Batrakou, D.G.; Srsen, V.; Zuleger, N.; Kerr, A.R.; Schirmer, E.C. Tissue specificity in the nuclear envelope supports its functional complexity. Nucleus 2013, 4, 460–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittmer, T.A.; Misteli, T. The lamin protein family. Genome Biol. 2011, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozák, P.; Sasseville, A.M.; Raymond, Y.; Cook, P.R. Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. J. Cell Sci. 1995, 108 Pt 2, 635–644. [Google Scholar]
- Shimi, T.; Pfleghaar, K.; Kojima, S.; Pack, C.G.; Solovei, I.; Goldman, A.E.; Adam, S.A.; Shumaker, D.K.; Kinjo, M.; Cremer, T.; et al. The A- and B-Type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008, 223, 409–3421. [Google Scholar] [CrossRef] [Green Version]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo, S. DNA damage and lamins. Adv. Exp. Med. Biol. 2014, 773, 377–399. [Google Scholar]
- van Steensel, B.; Belmont, A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 2017, 169, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Zheng, X.; Zheng, Y. Role of lamins in 3D genome organization and global gene expression. Nucleus 2019, 10, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Németh, A.; Conesa, A.; Santoyo-Lopez, J.; Medina, I.; Montaner, D.; Péterfia, B.; Solovei, I.; Cremer, T.; Dopazo, J.; Langst, G. Initial genomics of the human nucleolus. PLoS Genet. 2010, 6, e1000889. [Google Scholar]
- van Koningsbruggen, S.; Gierlinski, M.; Schofield, P.; Martin, D.; Barton, G.J.; Ariyurek, Y.; den Dunnen, J.T.; Lamond, A.I. High-Resolution whole-Genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli. Mol. Biol. Cell 2010, 21, 3735–3748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotta, G.; Lachner, M.; Sarma, K.; Ebert, A.; Sengupta, R.; Reuter, G.; Reinberg, D.; Jenuwein, T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes. Dev. 2004, 18, 1251–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Yao, J. Identifying Novel Transcriptional and Epigenetic Features of Nuclear Lamina-Associated Genes. Sci. Rep. 2017, 7, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, J.; van Steensel, B. Stochastic genome-Nuclear lamina interactions: Modulating roles of Lamin A and BAF. Nucleus 2014, 5, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-Related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, K.; García-Esparcia, P.; Gil, L.; Lucas, J.J.; Ferrer, I. Altered Machinery of Protein Synthesis in Alzheimer’s: From the Nucleolus to the Ribosome. Brain Pathol. 2016, 26, 593–605. [Google Scholar] [CrossRef]
- Bukar Maina, M.; Al-Hilaly, Y.K.; Serpell, L.C. Nuclear Tau and Its Potential Role in Alzheimer’s Disease. Biomolecules 2016, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Frost, B. Alzheimer’s disease: An acquired neurodegenerative laminopathy. Nucleus 2016, 7, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Corces, V.G.; Salas, J.; Salas, M.L.; Avila, J. Binding of microtubule proteins to DNA: Specificity of the interaction. Eur. J. Biochem. 1978, 86, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; He, R.Q.; Haque, N.; Qu, M.H.; del Carmen Alonson, A.; Grundke-Iqbal, I.; Iqbal, K. Microtubule associated protein tau binds to double-Stranded but not single-Stranded DNA. Cell Mol. Life Sci. 2003, 60, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Cantrelle, F.X.; Benhelli-Mokrani, H.; Smet-Nocca, C.; Buée, L.; Lippens, G.; Bonnefoy, E.; Galas, M.-C.; Landrieu, I. Nuclear magnetic resonance spectroscopy characterization of interaction of Tau with DNA and its regulation by phosphorylation. Biochemistry 2015, 54, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, M.K.; Shestakova, E.; Mansuroglu, Z.; Maccioni, R.B.; Bonnefoy, E. Tau protein binds to pericentromeric DNA: A putative role for nuclear tau in nucleolar organization. J. Cell Sci. 2006, 119 Pt 10, 2025–2034. [Google Scholar] [CrossRef] [Green Version]
- Mansuroglu, Z.; Benhelli-Mokrani, H.; Marcato, V.; Sultan, A.; Violet, M.; Chauderlier, A.; Delattre, L.; Loyens, A.; Talahari, S.; Bégard, S.; et al. Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci. Rep. 2016, 6, 33047. [Google Scholar] [CrossRef]
- Hua, Q.; He, R.Q. Tau could protect DNA double helix structure. Biochim. Biophys. Acta 2003, 1645, 205–211. [Google Scholar] [CrossRef]
- Wei, Y.; Qu, M.H.; Wang, X.S.; Chen, L.; Wang, D.L.; Liu, Y.; He, R.Q. Binding to the minor groove of the double-Strand, tau protein prevents DNA from damage by peroxidation. PLoS ONE 2008, 3, e2600. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.; Nesslany, F.; Violet, M.; Bégard, S.; Loyens, A.; Talahari, S.; Mansuroglu, Z.; Marzin, D.; Sergeant, N.; Humez, S.; et al. Nuclear tau, a key player in neuronal DNA protection. J. Biol. Chem. 2011, 286, 4566–4575. [Google Scholar] [CrossRef] [Green Version]
- Violet, M.; Delattre, L.; Tardivel, M.; Sultan, A.; Chauderlier, A.; Caillierez, R.; Talahari, S.; Nesslany, F.; Lefebvre, B.; Bonnefoy, E.; et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Siano, G.; Varisco, M.; Caiazza, M.C.; Quercioli, V.; Mainardi, M.; Ippolito, C.; Cattaneo, A.; Di Primio, C. Tau Modulates VGluT1 Expression. J. Mol. Biol. 2019, 431, 873–884. [Google Scholar] [CrossRef]
- Benhelli-Mokrani, H.; Mansuroglu, Z.; Chauderlier, A.; Albaud, B.; Gentien, D.; Sommer, S.; Schirmer, C.; Laqueuvre, L.; Josse, T.; Buée, L.; et al. Genome-Wide identification of genic and intergenic neuronal DNA regions bound by Tau protein under physiological and stress conditions. Nucleic Acids. Res. 2018, 46, 11405–11422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, L.; Federico, C.; Pinedo, F.; Bruno, F.; Rebolledo, A.B.; Montoya, J.J.; Olazabal, I.M.; Ferrer, I.; Saccone, S. Aging dependent effect of nuclear tau. Brain Res. 2017, 1677, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, B.; Bardai, F.H.; Feany, M.B. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr. Biol. 2016, 26, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, H.U.; McCabe, C.; Gjoneska, E.; Sullivan, S.S.; Kaskow, B.J.; Tang, A.; Smith, R.V.; Xu, J.; Pfenning, A.R.; Bernstein, B.E. Epigenome-Wide study uncovers large-Scale changes in histone acetylation driven by tau pathology in aging and Alzheimer’s human brains. Nat. Neurosci. 2019, 22, 37–46. [Google Scholar] [CrossRef]
- Pietrzak, M.; Rempala, G.; Nelson, P.T.; Zheng, J.J.; Hetman, M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. PLoS ONE 2011, 6, e22585. [Google Scholar] [CrossRef]
- Yang, Y.; Geldmacher, D.S.; Herrup, K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J. Neurosci. 2001, 21, 2661–2668. [Google Scholar] [CrossRef]
- Colangelo, V.; Schurr, J.; Ball, M.J.; Pelaez, R.P.; Bazan, N.G.; Lukiw, W.J. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: Transcription and neurotrophic factor down-Regulation and up-Regulation of apoptotic and pro-Inflammatory signaling. J. Neurosci. Res. 2002, 70, 462–473. [Google Scholar] [CrossRef]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Ramsey, K.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C.; et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: A reference data set. Physiol. Genom. 2008, 33, 240–256. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Chen, S.; Zheng, C.; Wei, H.; Song, X. Meta-Analysis of Gene Expression and Identification of Biological Regulatory Mechanisms in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 633. [Google Scholar] [CrossRef] [Green Version]
- Takamori, Y.; Tamura, Y.; Kataoka, Y.; Cui, Y.; Seo, S.; Kanazawa, T.; Kurokawa, K.; Yamada, H. Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur. J. Neurosci. 2007, 25, 1653–1662. [Google Scholar] [CrossRef]
- Coffinier, C.; Chang, S.Y.; Nobumori, C.; Tu, Y.; Farber, E.A.; Toth, J.I.; Fong, L.G.; Young, S.G. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 5076–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffinier, C.; Jung, H.J.; Nobumori, C.; Chang, S.; Tu, Y.; Barnes, R.H., 2nd; Yoshinaga, Y.; de Jong, P.J.; Vergnes, L.; Reue, K.; et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol. Biol. Cell 2011, 22, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of A-Type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-López, I.; Blanco-Luquin, I.; Sánchez-Ruiz de Gordoa, J.; Urdánoz-Casado, A.; Roldán, M.; Acha, B.; Echavarri, C.; Zelaya, V.; Jericó, I.; Mendioroz, M. Hippocampal LMNA Gene Expression is Increased in Late-Stage Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 878. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.J.; Coffinier, C.; Choe, Y.; Beigneux, A.P.; Davies, B.S.; Yang, S.H.; Barnes, R.H., 2nd; Hong, J.; Sun, T.; Pleasure, S.J.; et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl. Acad. Sci. USA 2012, 109, E423–E431. [Google Scholar] [CrossRef] [Green Version]
- Takamori, Y.; Hirahara, Y.; Wakabayashi, T.; Mori, T.; Koike, T.; Kataoka, Y.; Tamuracd, Y.; Kurebayashiae, S.; Kurokawaaf, K.; Yamadaa, H. Differential expression of nuclear lamin subtypes in the neural cells of the adult rat cerebral cortex. IBRO Rep. 2018, 5, 99–109. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Mori, T.; Hirahara, Y.; Koike, T.; Kubota, Y.; Takamori, Y.; Yamada, H. Nuclear lamins are differentially expressed in retinal neurons of the adult rat retina. Histochem. Cell Biol. 2011, 136, 427–436. [Google Scholar] [CrossRef]
- Schirmer, E.C.; Gerace, L. The stability of the nuclear lamina polymer changes with the composition of lamin subtypes according to their individual binding strengths. J. Biol. Chem. 2004, 279, 42811–42817. [Google Scholar] [CrossRef] [Green Version]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; Tewari, M. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.N.; Wilson, K.L. The nucleoskeleton as a genome-Associated dynamic ‘network of networks’. Nat. Rev. Mol. Cell Biol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell 2019, 49, 920.e5–935.e5. [Google Scholar] [CrossRef] [PubMed]
- Pugh, G.E.; Coates, P.J.; Lane, E.B.; Raymond, Y.; Quinlan, R.A. Distinct nuclear assembly pathways for lamins A and C lead to their increase during quiescence in Swiss 3T3 cells. J. Cell Sci. 1997, 110 Pt 19, 2483–2493. [Google Scholar]
- Ghosh, S.; Liu, B.; Wang, Y.; Hao, Q.; Zhou, Z. Lamin A is an Endogenous SIRT6 Activator and Promotes SIRT6-Mediated DNA Repair. Cell Rep. 2015, 13, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruman, I.I.; Wersto, R.P.; Cardozo-Pelaez, F.; Smilenov, L.; Chan, S.L.; Emokpae, R., Jr.; Gorospe, M.; Mattson, M.P. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 2004, 41, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Herrup, K. Cell division in the CNS: Protective response or lethal event in post-Mitotic neurons? Biochim. Biophys. Acta 2007, 1772, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.J.; Lee, J.M.; Yang, S.H.; Young, S.G.; Fong, L.G. Nuclear lamins in the brain-New insights into function and regulation. Mol. Neurobiol. 2013, 47, 290–301. [Google Scholar] [CrossRef] [Green Version]
- De Sandre-Giovannoli, A.; Bernard, R.; Cau, P.; Navarro, C.; Amiel, J.; Boccaccio, I.; Lyonnet, S.; Stewart, C.L.; Munnich, A.; Le Merrer, M.; et al. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef]
- Martin, C.; Chen, S.; Maya-Mendoza, A.; Lovric, J.; Sims, P.F.; Jackson, D.A. Lamin B1 maintains the functional plasticity of nucleoli. J. Cell Sci. 2009, 122 Pt 10, 1551–1562. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, F.J.; Pombo, A.; Jackson, D.A.; Cook, P.R. Active RNA polymerases are localized within discrete transcription “factories’ inhuman nuclei. J. Cell Sci. 1996, 109 Pt 6, 1427–1436. [Google Scholar]
- Tang, C.W.; Maya-Mendoza, A.; Martin, C.; Zheng, K.; Chen, S.; Feret, D.; Wilson, S.A.; Jackson, D.A. The integrity of a lamin-B1-Dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J. Cell Sci. 2008, 121 Pt 7, 1014–1024. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Isla, T.; Hollister, R.; West, H.; Mui, S.; Growdon, J.H.; Peterson, R.C.; Parisi, J.E.; Hyman, B.T. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 1997, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.K.; Zhu, X.; Rottkamp, C.A.; Monteiro, M.; Takeda, A.; Smith, M.A. Cyclin’ toward dementia: Cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J. Neurosci. Res. 2000, 61, 128–133. [Google Scholar] [CrossRef]
- Nagy, Z.; Esiri, M.M.; Cato, A.M.; Smith, A.D. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. 1997, 94, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mufson, E.J.; Herrup, K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J. Neurosci. 2003, 23, 2557–2563. [Google Scholar] [CrossRef] [Green Version]
- Currais, A.; Hortobágyi, T.; Soriano, S. The neuronal cell cycle as a mechanism of pathogenesis in Alzheimer’s disease. Aging (Albany NY) 2009, 1, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Siedlak, S.L.; Wang, Y.; Perry, G.; Castellani, R.J.; Cohen, M.L.; Smith, M.A. Neuronal binucleation in Alzheimer disease hippocampus. Neuropathol. Appl. Neurobiol. 2008, 34, 457–465. [Google Scholar] [CrossRef]
- Arendt, T.; Brückner, M.K.; Mosch, B.; Losche, A. Selective cell death of hyperploid neurons in Alzheimer’s disease. Am. J. Pathol. 2010, 177, 15–20. [Google Scholar] [CrossRef]
- Moir, R.D.; Montag-Lowy, M.; Goldman, R.D. Dynamic properties of nuclear lamins: Lamin B is associated with sites of DNA replication. J. Cell Biol. 1994, 125, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Kill, I.R.; Hutchison, C.J. S-Phase phosphorylation of lamin B2. FEBS Lett. 1995, 377, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Spann, T.P.; Moir, R.D.; Goldman, A.E.; Stick, R.; Goldman, R.D. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol. 1997, 136, 1201–1212. [Google Scholar] [CrossRef]
- Anachkova, B.; Djeliova, V.; Russev, G. Nuclear matrix support of DNA replication. J. Cell Biochem. 2005, 96, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Esiri, M.M.; Smith, A.D. Expression of cell division markers in the hippocampus in Alzheimer’s disease and other neurodegenerative conditions. Acta Neuropathol. 1997, 93, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Donahue, G.; Otte, G.L.; Capell, B.C.; Nelson, D.M.; Cao, K.; Aggarwala, V.; Cruickshanks, H.; Rai, T.S.; McBryan, T.; et al. Lamin B1 depletion in senescent cells triggers large-Scale changes in gene expression and the chromatin landscape. Genes Dev. 2013, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrio-Alonso, E.; Hernández-Vivanco, A.; Walton, C.C.; Perea, G.; Frade, J.M. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci. Rep. 2018, 8, 14316. [Google Scholar] [CrossRef]
- Cheung, I.; Shulha, H.P.; Jiang, Y.; Matevossian, A.; Wang, J.; Weng, Z.; Akbarian, S. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 8824–8829. [Google Scholar] [CrossRef] [Green Version]
- Håkelien, A.M.; Delbarre, E.; Gaustad, K.G.; Buendia, B.; Collas, P. Expression of the myodystrophic R453W mutation of lamin A in C2C12 myoblasts causes promoter-specific and global epigenetic defects. Exp. Cell Res. 2008, 314, 1869–1880. [Google Scholar] [CrossRef]
- Columbaro, M.; Capanni, C.; Mattioli, E.; Novelli, G.; Parnaik, V.K.; Squarzoni, S.; Maraldi, N.M.; Lattanzai, G. Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment. Cell Mol. Life Sci. 2005, 62, 2669–2678. [Google Scholar] [CrossRef] [Green Version]
- Sabatelli, P.; Lattanzi, G.; Ognibene, A.; Columbaro, M.; Capanni, C.; Merlini, L.; Maraldi, N.M.; Squarzoni, S. Nuclear alterations in autosomal-dominant Emery-Dreifuss muscular dystrophy. Muscle Nerve 2001, 24, 826–829. [Google Scholar] [CrossRef]
- Capanni, C.; Cenni, V.; Mattioli, E.; Sanatelli, P.; Ognibene, A.; Columbaro, M.; Parnaik, V.K.; Wehnert, M.; Maraldi, N.M.; Squarzoni, S.; et al. Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy fibroblasts: Altered intermolecular interaction with emerin and implications for gene transcription. Exp. Cell Res. 2003, 291, 122–134. [Google Scholar] [CrossRef]
- Filesi, I.; Gullotta, F.; Lattanzi, G.; D’Apice, M.R.; Capanni, C.; Nardone, A.M.; Columbaro, M.; Scarano, G.; Mattioli, E.; Sabatelli, P.; et al. Alterations of nuclear envelope and chromatin organization in mandibuloacral dysplasia, a rare form of laminopathy. Physiol. Genom. 2005, 23, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, N.; Denegri, M.; Chiodi, I.; Corioni, M.; Valgardsdottir, R.; Cobianchi, F.; Riva, S.; Biamonti, G. Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock. Mol. Biol. Cell 2004, 15, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourmouli, N.; Jeppesen, P.; Mahadevhaiah, S.; Burgoyne, P.; Wu, R.; Gilbert, D.M.; Bongiorni, S.; Prantera, G.; Fanti, L.; Pimpinelli, S.; et al. Heterochromatin and tri-methylated lysine 20 of histone H4 in animals. J. Cell Sci. 2004, 117 Pt 12, 2491–2501. [Google Scholar] [CrossRef] [Green Version]
- Martens, J.H.; O’Sullivan, R.J.; Braunschweig, U.; Opravil, S.; Radolf, M.; Steinlein, P.; Jenuwein, T. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 2005, 24, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winick-Ng, W.; Rylett, R.J. Into the Fourth Dimension: Dysregulation of Genome Architecture in Aging and Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Amari, M.; Fukuda, T.; Suzuki, K.; Takatama, M. Comparison of AT8 immunoreactivity in the locus ceruleus and hippocampus of 154 brains from routine autopsies. Neuropathology 2017, 37, 306–310. [Google Scholar] [CrossRef]
- Regalado-Reyes, M.; Furcila, D.; Hernández, F.; Ávila, J.; DeFelipe, J.; León-Espinosa, G. Phospho-Tau Changes in the Human CA1 During Alzheimer’s Disease Progression. J. Alzheimers Dis. 2019, 69, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Jeganathan, S.; Hascher, A.; Chinnathambi, S.; Biernat, J.; Mandelkow, E.M.; Maldelkow, E. Proline-Directed pseudo-phosphorylation at AT8 and PHF1 epitopes induces a compaction of the paperclip folding of Tau and generates a pathological (MC-1) conformation. J. Biol. Chem. 2008, 283, 32066–32076. [Google Scholar] [CrossRef] [Green Version]
- Qu, M.H.; Li, H.; Tian, R.; Nie, C.L.; Liu, Y.; Han, B.S.; He, R.Q. Neuronal tau induces DNA conformational changes observed by atomic force microscopy. Neuroreport 2004, 15, 2723–2727. [Google Scholar]
- Nagy, Z.; Esiri, M.M.; Smith, A.D. The cell division cycle and the pathophysiology of Alzheimer’s disease. Neuroscience 1998, 87, 731–739. [Google Scholar]
- Li, W.; Wang, X.S.; Qu, M.H.; Liu, Y.; He, R.Q. Human protein tau represses DNA replication in vitro. Biochim. Biophys. Acta. 2005, 1726, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Federico, C.; Gil, L.; Bruno, F.; D’Amico, A.G.; D’Agata, V.; Saccone, S. Phosphorylated nucleolar Tau protein is related to the neuronal in vitro differentiation. Gene 2018, 664, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Pietrzak, M.; Rempala, G.; Nelson, P.T.; Hetman, M. Neurodegeneration-associated instability of ribosomal DNA. Biochim. Biophys. Acta 2014, 1842, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verde, P.; Casalino, L.; Talotta, F.; Yaniv, M.; Weitzman, J.B. Deciphering AP-1 function in tumorigenesis: Fra-Ternizing on target promoters. Cell Cycle 2007, 6, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Zatsepina, O.V.; Todorov, I.T.; Philipova, R.N.; Krachmarov, C.P.; Trendelenburg, M.F.; Jordan, E.G. Cell cycle-Dependent translocations of a major nucleolar phosphoprotein, B23, and some characteristics of its variants. Eur. J. Cell Biol. 1997, 73, 58–70. [Google Scholar]
- Matthews, D.A. Adenovirus protein V induces redistribution of nucleolin and B23 from nucleolus to cytoplasm. J. Virol. 2001, 75, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.; Zhang, H.; Li, S.; Jiang, W.; Liu, D. Three major nucleolar proteins migrate from nucleolus to nucleoplasm and cytoplasm in root tip cells of Vicia faba L. exposed to aluminum. Environ. Sci. Pollut. Res. Int. 2014, 21, 10736–10743. [Google Scholar] [CrossRef]
- Ranade, D.; Pradhan, R.; Jayakrishnan, M.; Hegde, S.; Sengupta, K. Lamin A/C and Emerin depletion impacts chromatin organization and dynamics in the interphase nucleus. BMC Mol. Cell Biol. 2019, 20, 11. [Google Scholar] [CrossRef]
- Kumeta, M.; Yoshimura, S.H.; Hejna, J.; Takeyasu, K. Nucleocytoplasmic shuttling of cytoskeletal proteins: Molecular mechanism and biological significance. Int. J. Cell Biol. 2012. [Google Scholar] [CrossRef] [Green Version]
- West, M.J.; Coleman, P.D.; Flood, D.G.; Troncoso, J.C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 1994, 344, 769–772. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Burgess, R.C.; Misteli, T.; Oberdoerffer, P. DNA damage, chromatin, and transcription: The trinity of aging. Curr. Opin. Cell Biol. 2012, 24, 724–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s Disease and Cancer: When Two Monsters Cannot Be Together. Front. Neurosci. 2019, 13, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Antibody/Supplier/Catalog Number/Manufacture | Species | Dilution |
|---|---|---|
| AT100 (antiphospho Tau S212-T214)/abcam/Thermo Fisher/MN1060/Waltham, MA, USA | mouse monoclonal | 1:100 |
| AT8 (antiphospho Tau S205-T205)/Thermo Fisher/MN1020/Waltham, MA, USA | mouse monoclonal | 1:20 |
| AH36 (antiphospho Tau S205-T205) Stress Marq/SMC-601/Victoria, B.C., Canada | rabbit monoclonal | 1:100 |
| Anti-Lamin A/abcam/ab26300/Cambridge, UK | rabbit polyclonal | 1:500 |
| Anti-Lamin B1/abcam/ab133741/Cambridge, UK | rabbit monoclonal | 1:200 |
| Anti-Lamin B2/abcam/ab151735/Cambridge, UK | rabbit monoclonal | 1:200 |
| Anti-Lamin C/abcam/ab125679/Cambridge, UK | rabbit polyclonal | 1:20 |
| B23 (anti-nucleophosmin)/abcam/ab10530/Cambridge, UK | mouse monoclonal | 1:50 |
| Anti- H4K20me3/abcam/ab9053/Cambridge, UK | rabbit monoclonal | 1:100 |
| Alexa –Fluor 488 secondary antibody/Life Technologies/CA, USA | goat anti-rabbit | 1:300 |
| Alexa –Fluor 633 secondary antibody/Life Technologies/Carlsbad, CA, USA | goat anti-mouse | 1:200 |
| Sytox Orange nucleic acid stain/Molecular Probes/Eugene, OR, USA | 1:1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, L.; Niño, S.A.; Chi-Ahumada, E.; Rodríguez-Leyva, I.; Guerrero, C.; Rebolledo, A.B.; Arias, J.A.; Jiménez-Capdeville, M.E. Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2020, 21, 1841. https://doi.org/10.3390/ijms21051841
Gil L, Niño SA, Chi-Ahumada E, Rodríguez-Leyva I, Guerrero C, Rebolledo AB, Arias JA, Jiménez-Capdeville ME. Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression. International Journal of Molecular Sciences. 2020; 21(5):1841. https://doi.org/10.3390/ijms21051841
Chicago/Turabian StyleGil, Laura, Sandra A. Niño, Erika Chi-Ahumada, Ildelfonso Rodríguez-Leyva, Carmen Guerrero, Ana Belén Rebolledo, José A. Arias, and María E. Jiménez-Capdeville. 2020. "Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression" International Journal of Molecular Sciences 21, no. 5: 1841. https://doi.org/10.3390/ijms21051841
APA StyleGil, L., Niño, S. A., Chi-Ahumada, E., Rodríguez-Leyva, I., Guerrero, C., Rebolledo, A. B., Arias, J. A., & Jiménez-Capdeville, M. E. (2020). Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression. International Journal of Molecular Sciences, 21(5), 1841. https://doi.org/10.3390/ijms21051841







