Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. STZ Induced Severe Diabetes in Rats
2.2. Pregestational Diabetes Resulted in Fetal Growth Restriction and Decreased Placental Efficiency in Rats
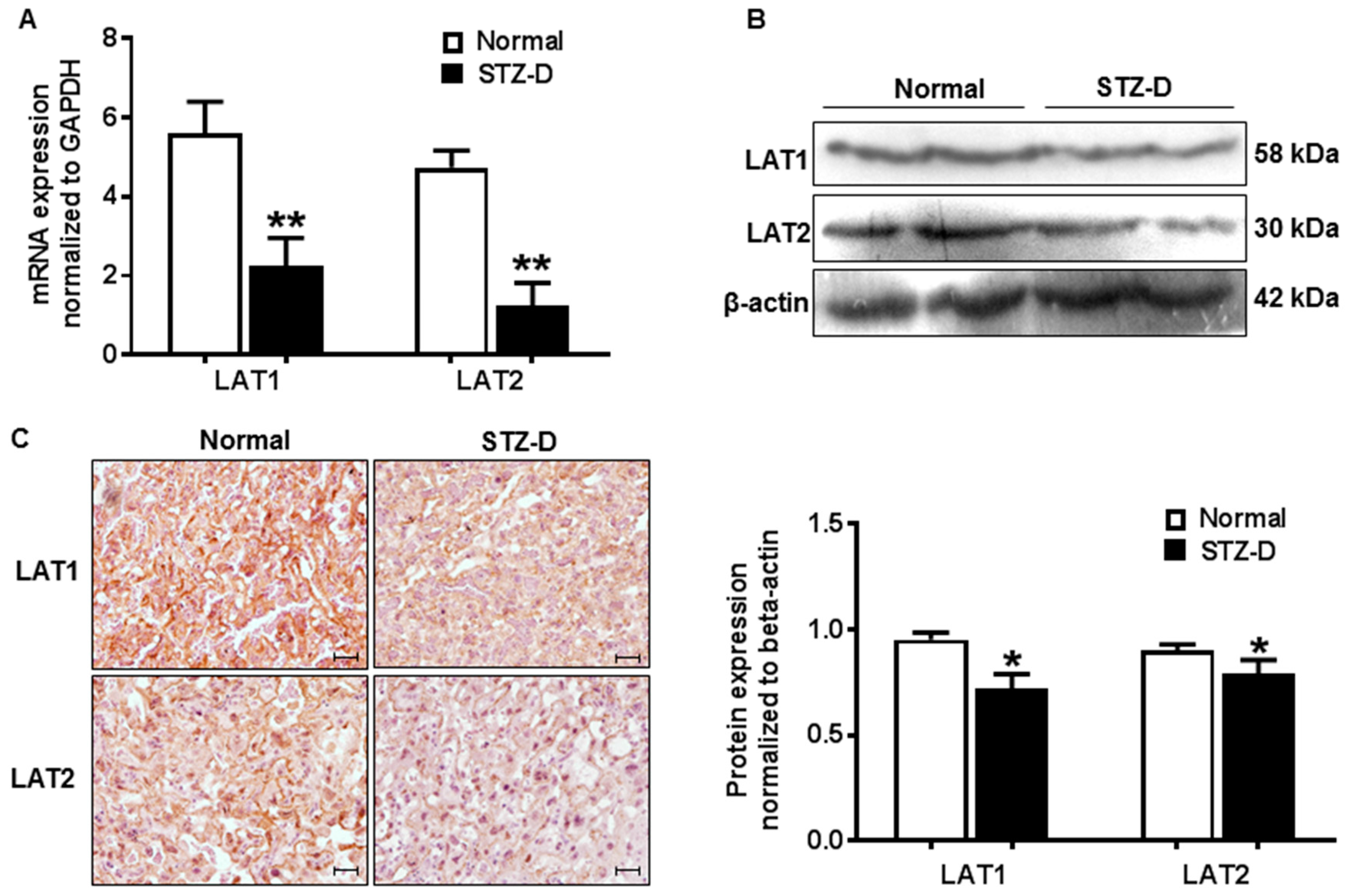
2.3. Pregestational Diabetes Resulted in Decreased Placental Amino Acid Transporter Expression in Rats
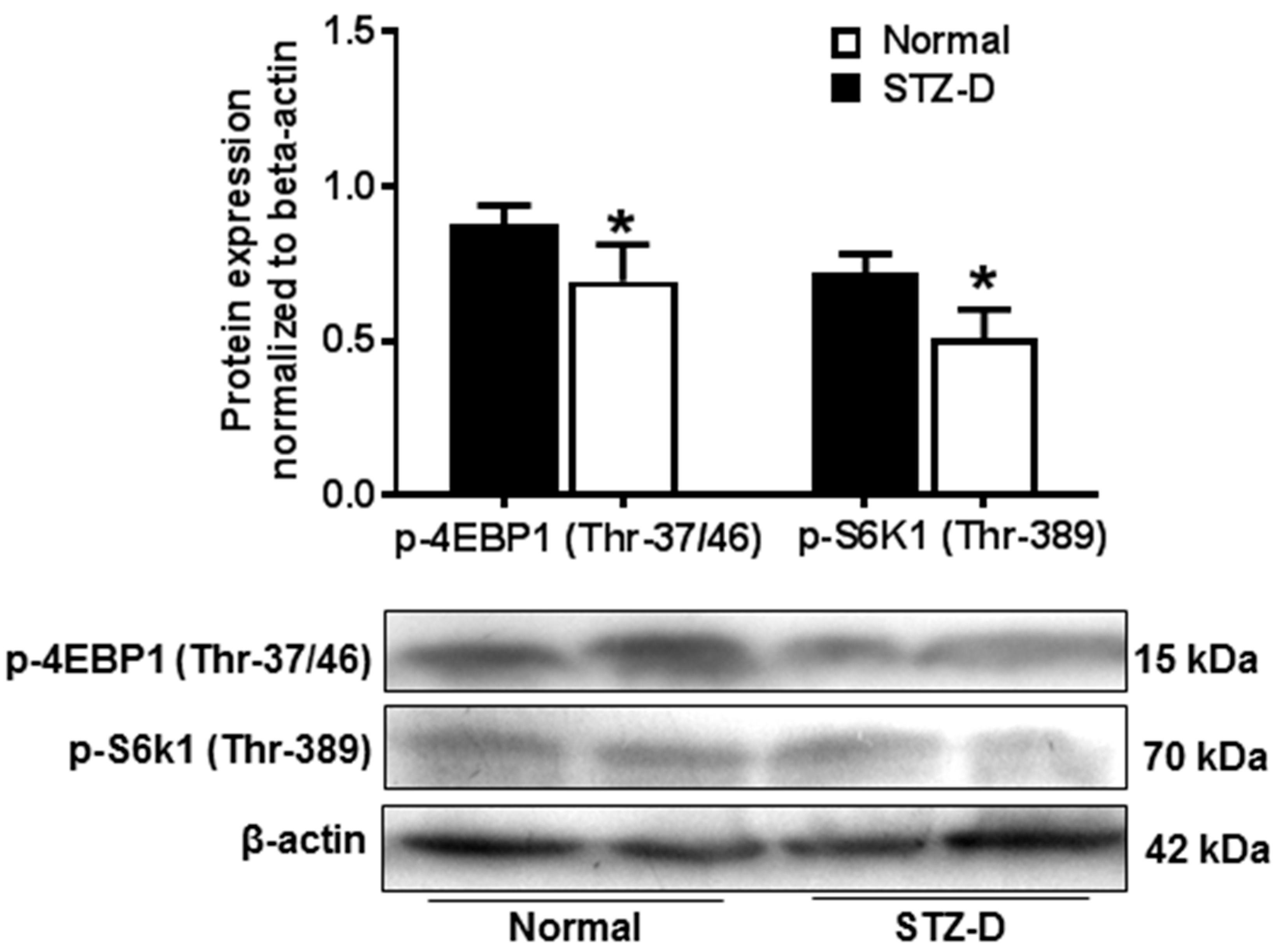
2.4. Pregestational Diabetes Reduced Placental mTORC1 Activity in Rats
2.5. Hyperglycemia in Vitro Down-Regulated Amino Acid Transporter Expression and mTORC1 Activity in JEG-3 Trophoblast Cells
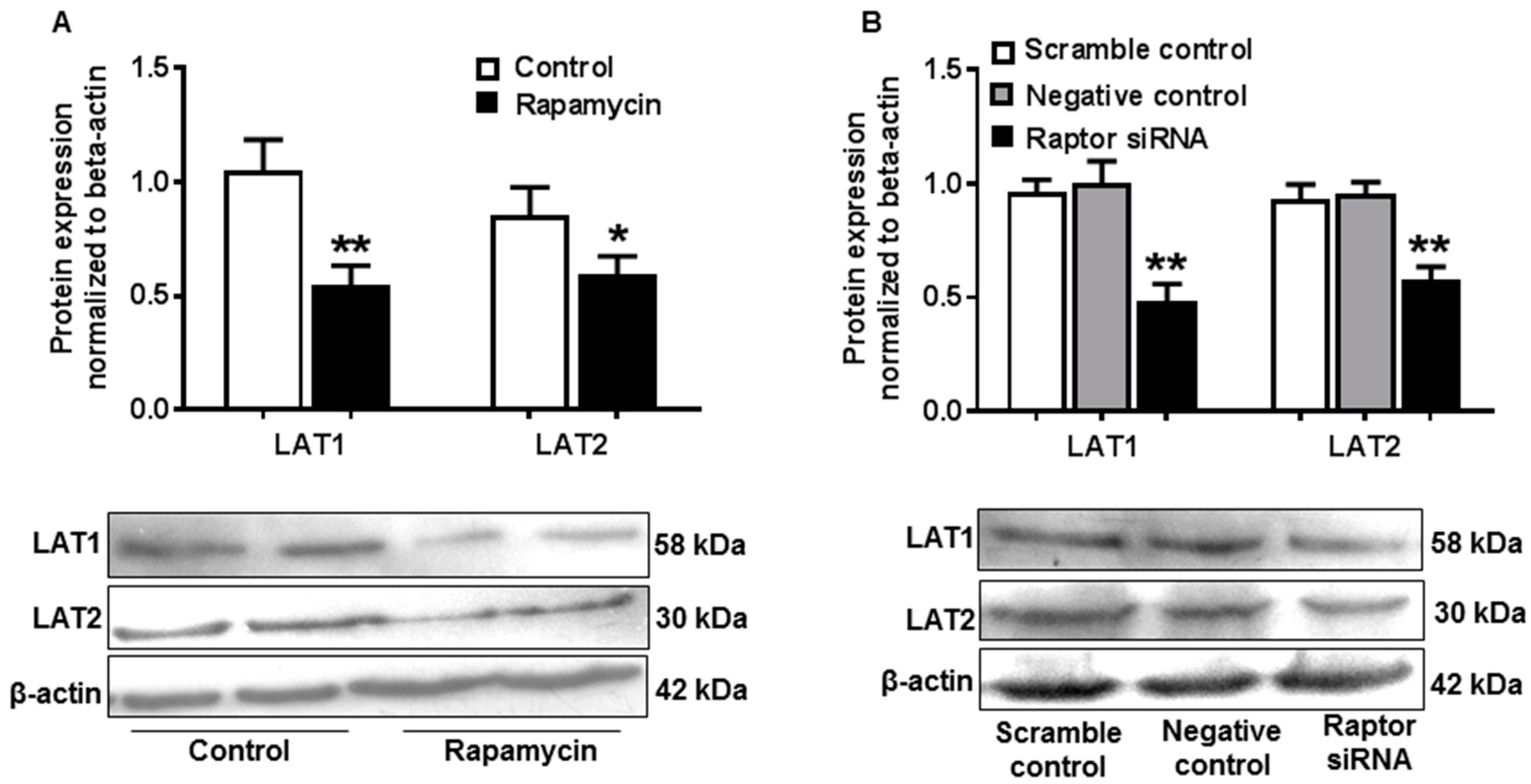
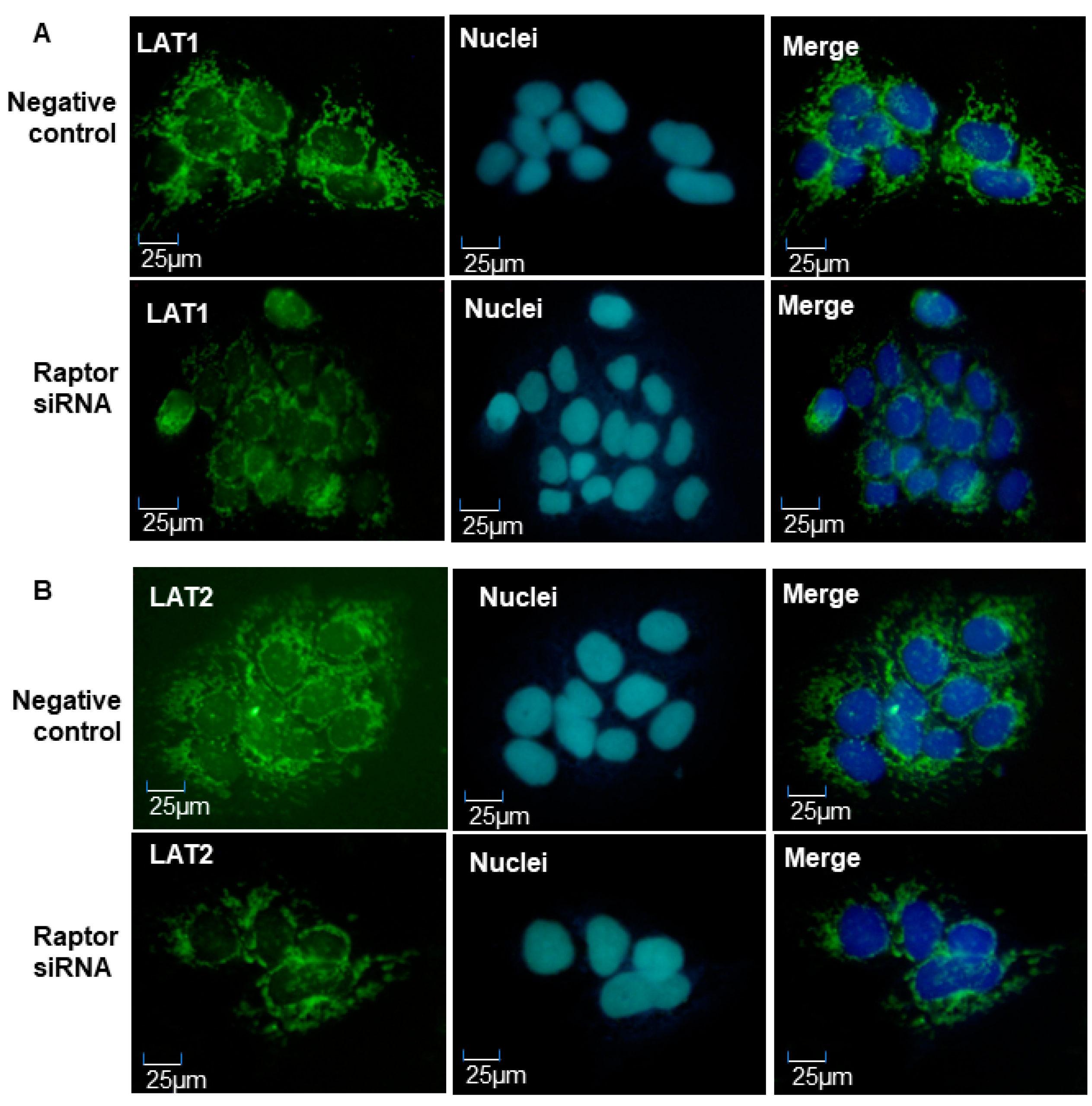
2.6. Inhibition of mTORC1 Activity Resulted in Decreased Amino Acid Transporter Expression in JEG-3 Trophoblast Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Collection of Blood and Placenta Samples of Rat
4.3. Human Trophoblast Cell Culture
4.4. mTORC1 Inhibition in Placental Trophoblast
4.5. RNA Isolation and Quantitative Real-Time PCR
4.6. Western Blot Analysis
4.7. Immunohistochemistry
4.8. Immunofluorescence
4.9. Data Presentation and Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shand, A.W.; Bell, J.C.; McElduff, A.; Morris, J.; Roberts, C.L. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998–2002. Diabet. Med. 2008, 25, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Glazier, J.D.; Cetin, I.; Perugino, G.; Ronzoni, S.; Grey, A.M.; Mahendran, D.; Marconi, A.M.; Pardi, G.; Sibley, C.P. Association between the activity of the system a amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 1997, 42, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Ylven, K.; Wennergren, M.; Powell, T.L. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 2002, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, C.; Wang, J.; Zhang, R.; Qian, X.; Zhu, H. Regulation of Human Trophoblast GLUT3 Glucose Transporter by Mammalian Target of Rapamycin Signaling. Int. J. Mol. Sci. 2015, 16, 13815–13828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 2013, 591, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jansson, N.; Palmberg, I.; Saljo, K.; Powell, T.L.; Jansson, T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007, 582, 449–459. [Google Scholar] [CrossRef]
- Arroyo, J.A.; Brown, L.D.; Galan, H.L. Placental mammalian target of rapamycin and related signaling pathways in an ovine model of intrauterine growth restriction. Am. J. Obstet. Gynecol. 2009, 201, 616.e1-7. [Google Scholar] [CrossRef] [Green Version]
- Jansson, N.; Rosario, F.J.; Gaccioli, F.; Lager, S.; Jones, H.N.; Roos, S.; Jansson, T.; Powell, T.L. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 2013, 98, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Taricco, E.; Radaelli, T.; de Santis, M.S.N.; Cetin, I. Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta 2003, 24, 343–347. [Google Scholar] [CrossRef]
- Vambergue, A.; Fajardy, I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J. Diabetes 2011, 2, 196–203. [Google Scholar] [CrossRef]
- Weiss, U.; Cervar, M.; Puerstner, P.; Schmut, O.; Haas, J.; Mauschitz, R.; Arikan, G.; Desoye, G. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia 2001, 44, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, N.; Pettersson, J.; Haafiz, A.; Ericsson, A.; Palmberg, I.; Tranberg, M.; Ganapathy, V.; Powell, T.L.; Jansson, T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006, 576, 935–946. [Google Scholar]
- Kusinski, L.C.; Stanley, J.L.; Dilworth, M.R.; Hirt, C.J.; Andersson, I.J.; Renshall, L.J.; Baker, B.C.; Baker, P.N.; Sibley, C.P.; Wareing, M.; et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R86–R93. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.M.; Vaughan, O.R.; Sekita, Y.; Finn, S.L.; Burton, G.J.; Constancia, M.; Fowden, A.L. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J. Physiol. 2010, 588, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Horgan, G.W.; Bhattacharya, S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta 2012, 33, 611–618. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ogawa, M.; Nakai, A.; Hayashi, M.; Satoh, S.; Matsubara, S. Fetal/Placental weight ratio in term Japanese pregnancy: Its difference among gender, parity, and infant growth. Int. J. Med. Sci. 2015, 12, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Rosario, F.J.; Jansson, N.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 2011, 152, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, A.G.; D’Souza, S.W.; Glazier, J.D.; Mahendran, D.; Maresh, M.J.; Sibley, C.P. Altered activity of the system a amino acid transporter in microvillous membrane vesicles from placentas of macrosomic babies born to diabetic women. J. Clin. Investig. 1994, 94, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Ekstrand, Y.; Bjorn, C.; Wennergren, M.; Powell, T.L. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 2002, 51, 2214–2219. [Google Scholar] [CrossRef] [Green Version]
- Ericsson, A.; Saljo, K.; Sjostrand, E.; Jansson, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J. Physiol. 2007, 581, 1323–1332. [Google Scholar] [CrossRef]
- Mejia, C.; Lewis, J.; Jordan, C.; Mejia, J.; Ogden, C.; Monson, T.; Winden, D.; Watson, M.; Reynolds, P.R.; Arroyo, J.A. Decreased activation of placental mTOR family members is associated with the induction of intrauterine growth restriction by secondhand smoke in the mouse. Cell Tissue Res. 2017, 367, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lager, S.; Samulesson, A.M.; Taylor, P.D.; Poston, L.; Powell, T.L.; Jansson, T. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Physiol. Rep. 2014, 2, e00242. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, Y.; Lewis, D.F.; Cooper, D.B.; McCathran, C.E.; Wang, Y. Downregulation of vitamin D receptor and miR-126-3p expression contributes to increased endothelial inflammatory response in preeclampsia. Am. J. Reprod. Immunol. 2019, 82, e13172. [Google Scholar] [CrossRef] [PubMed]
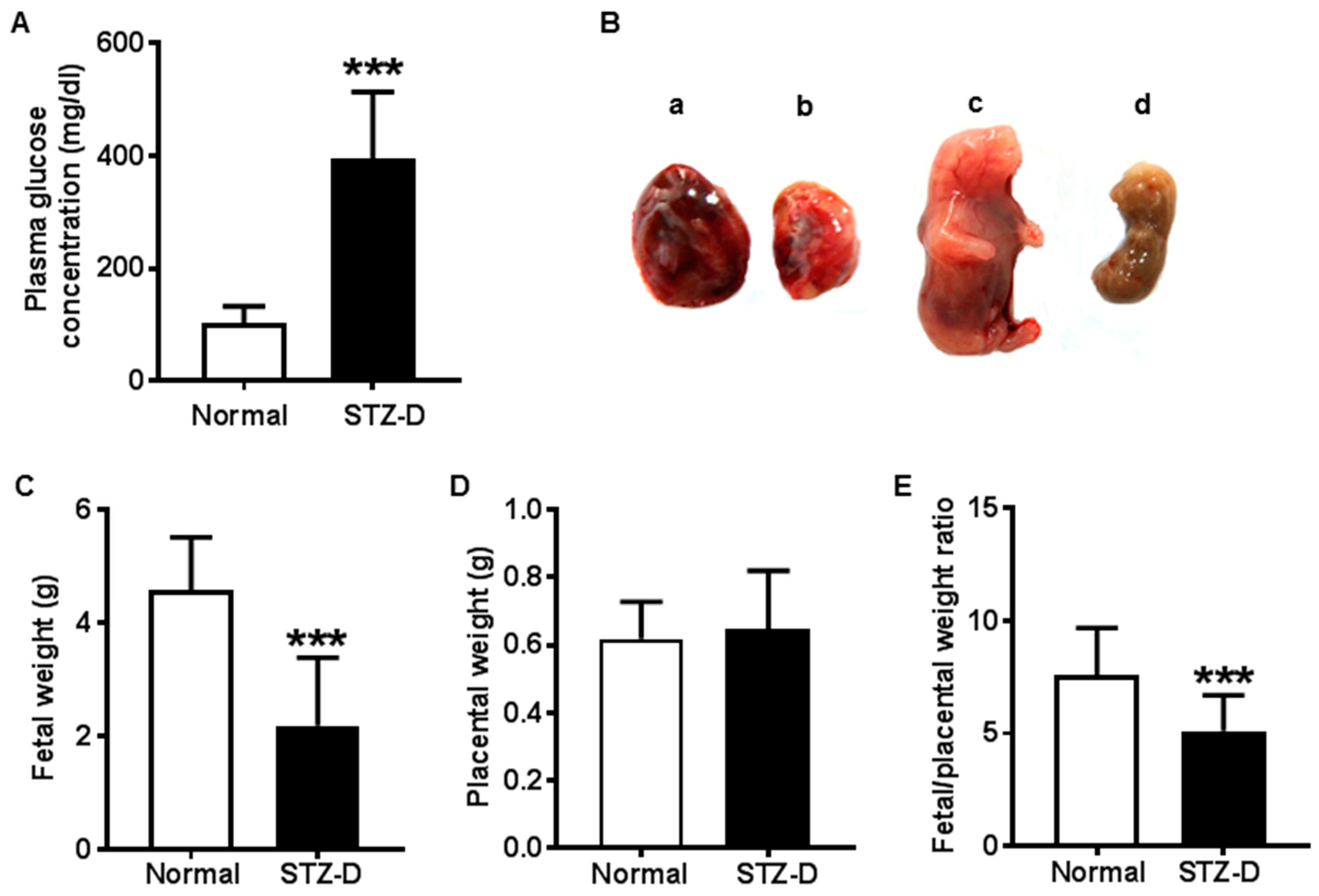

| Group | Normal | STZ-D | n | p-Value |
|---|---|---|---|---|
| Maternal glucose concentration (mg/dL) | 103 ± 29.78 | 395 ± 118.3 | 13 | <0.001 |
| Fetal weight (g) | 4.57 ± 0.95 | 2.19 ± 1.21 | 30 | <0.001 |
| Placental weight (g) | 0.62 ± 0.11 | 0.65 ± 0.17 | 30 | 0.42 |
| Fetal/placental weight ratio | 7.60 ± 2.01 | 5.11 ± 1.59 | 30 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, J.; Cao, Y.; Jia, X.; Huang, Y.; Cai, M.; Lu, C.; Zhu, H. Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 1849. https://doi.org/10.3390/ijms21051849
Xu J, Wang J, Cao Y, Jia X, Huang Y, Cai M, Lu C, Zhu H. Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats. International Journal of Molecular Sciences. 2020; 21(5):1849. https://doi.org/10.3390/ijms21051849
Chicago/Turabian StyleXu, Jie, Jiao Wang, Yang Cao, Xiaotong Jia, Yujia Huang, Minghui Cai, Chunmei Lu, and Hui Zhu. 2020. "Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats" International Journal of Molecular Sciences 21, no. 5: 1849. https://doi.org/10.3390/ijms21051849
APA StyleXu, J., Wang, J., Cao, Y., Jia, X., Huang, Y., Cai, M., Lu, C., & Zhu, H. (2020). Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats. International Journal of Molecular Sciences, 21(5), 1849. https://doi.org/10.3390/ijms21051849




