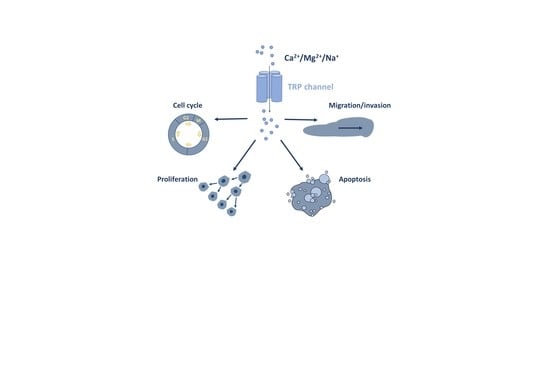
TRP Channels in Digestive Tract Cancers
Abstract
:1. Introduction
2. Oral Cancers
3. Esophgeal Cancer
4. Liver Cancer
5. Pancreatic Cancer
6. Gastric Cancer
7. Colorectal Cancer
8. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| ABCB1 | ATP-Binding Cassette Subfamily B Member 1 |
| Ca2+ | Calcium Ions |
| CAC | Colitis-Associated Cancer |
| CRC | Colorectal Cancer |
| Cryo-EM | Cryogenic Electron Microscopy |
| EAC | Esophageal Adenocarcinoma |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Endothelial–Mesenchymal Transition |
| ER | Endoplasmic Reticulum |
| ESCC | Esophageal Squamous Cell Carcinoma |
| HCC | Hepatocellular Carcinoma |
| IHC | Immunohistochemistry |
| La3+ | Lanthanum Ions |
| LCSLCs | Liver Cancer Stem-Like Cells |
| Mg2+ | Magnesium Ion |
| Na+ | Sodium Ions |
| NET | Neuroendocrine Tumor |
| ROS | Reactive Oxygen Species |
| SCC | Squamus Cell Carcinoma |
| TNM | Tumor Node Metastasis |
| TRP | Transient Receptor Potential |
| TRPA | Transient Receptor Potential Ankyrin |
| TRPC | Transient Receptor Potential Canonical |
| TRPM | Transient Receptor Potential Melastatin |
| TRPML | Transient Receptor Potential Mucolipin |
| TRPP | Transient Receptor Potential Polycystic |
| TRPV | Transient Receptor Potential Vanilloid |
| WB | Western-Blot |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; NouredDine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Griffin-Sobel, J.P. Gastrointestinal Cancers: Screening and Early Detection. Semin. Oncol. Nurs. 2017, 33, 165–171. [Google Scholar] [CrossRef]
- Jitender, S.; Sarika, G.; Varada, H.R.; Omprakash, Y.; Mohsin, K. Screening for oral cancer. J. Exp. Ther. Oncol. 2016, 11, 303–307. [Google Scholar]
- Olsen, J.R.; Apisarnthanarax, S.; Murphy, J.D.; Tait, D.; Huguet, F.; Hallemeier, C.L.; Jabbour, S.K. Gastrointestinal Cancers: Fine-Tuning the Management of Rectal, Esophageal, and Pancreas Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Kandimalla, R.; Huang, H.; Zhu, L.; Li, Y.; Gao, F.; Goel, A.; Wang, X. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin. Cancer Biol. 2019, 55, 37–52. [Google Scholar] [CrossRef]
- Ilson, D.H. Advances in the treatment of gastric cancer. Curr. Opin. Gastroenterol. 2018, 34, 465–468. [Google Scholar] [CrossRef]
- Aziz, M.A.; Yousef, Z.; Saleh, A.M.; Mohammad, S.; Al Knawy, B. Towards personalized medicine of colorectal cancer. Crit. Rev. Oncol. Hematol. 2017, 118, 70–78. [Google Scholar] [CrossRef]
- Jácome, A.A.; Coutinho, A.K.; Lima, E.M.; Andrade, A.C.; Dos Santos, J.S. Personalized medicine in gastric cancer: Where are we and where are we going? World J. Gastroenterol. 2016, 22, 1160–1171. [Google Scholar] [CrossRef]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in colorectal cancer: A challenge for personalized medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galun, D.; Srdic-Rajic, T.; Bogdanovic, A.; Loncar, Z.; Zuvela, M. Targeted therapy and personalized medicine in hepatocellular carcinoma: Drug resistance, mechanisms, and treatment strategies. J. Hepatocell. Carcinoma 2017, 4, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Yu, I.S.; Cheung, W.Y. Metastatic Colorectal Cancer in the Era of Personalized Medicine: A More Tailored Approach to Systemic Therapy. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9450754. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An Introduction to Trp Channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [Green Version]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Hung, C.Y.; Tan, C.H. TRP channels in nociception and pathological pain. In Advances in Pain Research: Mechanisms and Modulation of Chronic Pain. Adv. Exp. Med. Biol. 2018, 1099, 13–27. [Google Scholar] [PubMed]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, T.F.J.; Loch, S.; Lambert, S.; Straub, I.; Mannebach, S.; Mathar, I.; Düfer, M.; Lis, A.; Flockerzi, V.; Philipp, S.E.; et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nat. Cell Biol. 2008, 10, 1421–1430. [Google Scholar] [CrossRef]
- Roper, S.D. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 2014, 223, 827–871. [Google Scholar]
- Gao, Y.; Liao, P. TRPM4 channel and cancer. Cancer Lett. 2019, 454, 66–69. [Google Scholar] [CrossRef]
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional role of transient receptor potential channels in immune cells and epithelia. Front. Immunol. 2018, 9, 174. [Google Scholar] [CrossRef]
- Santoni, G.; Maggi, F.; Morelli, M.B.; Santoni, M.; Marinelli, O. Transient Receptor Potential Cation Channels in Cancer Therapy. Med. Sci. 2019, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.K.; Banham, A.H.; Yaacob, N.S.; Nur Husna, S.M. The oncogenic roles of TRPM ion channels in cancer. J. Cell. Physiol. 2019, 234, 14556–14573. [Google Scholar] [CrossRef]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 kinase activity is essential for T cell colonization and alloreactivity in the gut. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Nadolni, W.; Zierler, S. The Channel-Kinase TRPM7 as Novel Regulator of Immune System Homeostasis. Cells 2018, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, Z.; Lis, A.; Penner, R.; Fleig, A. TRPM7 is regulated by halides through its kinase domain. Cell. Mol. Life Sci. 2013, 70, 2757–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM family channels in cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterea, A.M.; Egom, E.E.; El Hiani, Y. TRP channels in gastric cancer: New hopes and clinical perspectives. Cell Calcium 2019, 82, 102053. [Google Scholar] [CrossRef]
- Fels, B.; Bulk, E.; Pethő, Z.; Schwab, A. The role of TRP channels in the metastatic cascade. Pharmaceuticals 2018, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Yang, D.; Myeong, J.; Ha, K.; Kim, S.H.; Park, E.J.; Kim, I.G.; Cho, N.H.; Lee, K.P.; Jeon, J.H.; et al. Regulation of calcium influx and signaling pathway in cancer cells via TRPV6-Numb1 interaction. Cell Calcium 2013, 53, 102–111. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Ouadid-Ahidouch, H.; Skryma, R.; Shuba, Y. Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lehen’kyi, V.; Flourakis, M.; Skryma, R.; Prevarskaya, N. TRPV6 channel controls prostate cancer cell proliferation via Ca 2+/NFAT-dependent pathways. Oncogene 2007, 26, 7380–7385. [Google Scholar] [CrossRef] [Green Version]
- Thebault, S.; Flourakis, M.; Vanoverberghe, K.; Vandermoere, F.; Roudbaraki, M.; Lehen’kyi, V.; Slomianny, C.; Beck, B.; Mariot, P.; Bonnal, J.L.; et al. Differential role of transient receptor potential channels in Ca 2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006, 66, 2038–2047. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yu, Y.; Yang, J. Structural Biology of TRP Channels. In Transient Receptor Potential Channels. Advances in Experimental Medicine and Biology; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 704, ISBN 978-94-007-0265-3. [Google Scholar]
- Huang, Y.; Fliegert, R.; Guse, A.H.; Lü, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85, 102111. [Google Scholar] [CrossRef]
- Saotome, K.; Singh, A.K.; Yelshanskaya, M.V.; Sobolevsky, A.I. Crystal structure of the epithelial calcium channel TRPV6. Nature 2016, 534, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; She, J.; Zeng, W.; Chen, Q.; Bai, X.-C.; Jiang, Y. Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 2017, 552, 205–209. [Google Scholar] [CrossRef]
- Autzen, H.E.; Myasnikov, A.G.; Campbell, M.G.; Asarnow, D.; Julius, D.; Cheng, Y. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 2018, 359, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinayagam, D.; Mager, T.; Apelbaum, A.; Bothe, A.; Merino, F.; Hofnagel, O.; Gatsogiannis, C.; Raunser, S. Electron cryo-microscopy structure of the canonical TRPC4 ion channel. Elife 2018, 7, e36615. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Choi, W.; Sun, W.; Du, J.; Lü, W.; Lu, W. Structure of the human lipid-gated cation channel TRPC3. Elife 2018, 7, e36852. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Z.; Li, J.; Santa-Cruz, A.; Sanchez-Martinez, S.; Zhang, J.; Clapham, D.E. Structure of full-length human TRPM4. Proc. Natl. Acad. Sci. USA 2018, 115, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.Y. Structure of the cold- And menthol-sensing ion channel TRPM8. Science 2018, 359, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.E.T.; Lodowski, D.T.; Huynh, K.W.; Yazici, A.; Del Rosario, J.; Kapoor, A.; Basak, S.; Samanta, A.; Han, X.; Chakrapani, S.; et al. Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM. Nat. Struct. Mol. Biol. 2018, 25, 53–60. [Google Scholar] [CrossRef]
- Duan, J.; Li, Z.; Li, J.; Hulse, R.E.; Santa-Cruz, A.; Valinsky, W.C.; Abiria, S.A.; Krapivinsky, G.; Zhang, J.; Clapham, D.E. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc. Natl. Acad. Sci. USA 2018, 115, E8201–E8210. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Paknejad, N.; Maksaev, G.; Sala-Rabanal, M.; Nichols, C.G.; Hite, R.K.; Yuan, P. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat. Struct. Mol. Biol. 2018, 25, 252–260. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Chen, G.L.; Ge, Y.; Liu, J.; Xie, K.; Peng, X.; Zhou, W.; Zhong, J.; Zhang, Y.; et al. Cryo-EM structure of TRPC5 at 2.8-Å resolution reveals unique and conserved structural elements essential for channel function. Sci. Adv. 2019, 5, eaaw7935. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Li, J.; Zeng, B.; Chen, G.L.; Peng, X.; Zhang, Y.; Wang, J.; Clapham, D.E.; Li, Z.; Zhang, J. Structure of the mouse TRPC4 ion channel. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; McGoldrick, L.L.; Sobolevsky, A.I. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 2018, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surh, Y.J.; Lee, E.; Lee, J.M. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 402, 259–267. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, C.; Tao, Y.; Wang, S.; Wei, Z.; Cao, Y.; Wu, H.; Fan, F.; Lin, C.; Shan, Y.; et al. Chemopreventive efficacy of menthol on carcinogen-induced cutaneous carcinoma through inhibition of inflammation and oxidative stress in mice. Food Chem. Toxicol. 2015, 82, 12–18. [Google Scholar] [CrossRef]
- Dwivedi, C.; Muller, L.A.; Goetz-Parten, D.E.; Kasperson, K.; Mistry, V.V. Chemopreventive effects of dietary mustard oil on colon tumor development. Cancer Lett. 2003, 196, 29–34. [Google Scholar] [CrossRef]
- Marincsák, R.; Tóth, B.; Czifra, G.; Márton, I.; Rédl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral Dis. 2009, 15, 328–335. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ohkubo, T.; Ikebe, T.; Yamazaki, J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int. J. Oncol. 2012, 40, 1431–1440. [Google Scholar]
- Sakakibara, A.; Sakakibara, S.; Kusumoto, J.; Takeda, D.; Hasegawa, T.; Akashi, M.; Minamikawa, T.; Hashikawa, K.; Terashi, H.; Komori, T. Upregulated expression of transient receptor potential cation channel subfamily v receptors in mucosae of patients with oral squamous cell carcinoma and patients with a history of alcohol consumption or smoking. PLoS ONE 2017, 12, e0169723. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Xu, W.L.; Xu, Z.Q.; Qi, C.; Li, Y.; Cheng, J.; Liu, L.K.; Wu, Y.N.; Gao, J.; Ye, J.H. The overexpressed functional transient receptor potential channel TRPM2 in oral squamous cell carcinoma. Sci. Rep. 2016, 6, 38471. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, C.B.; Kirma, N.B.; De La Chapa, J.J.; Chen, R.; Henry, M.A.; Luo, S.; Hargreaves, K.M. Vanilloids induce oral cancer apoptosis independent of TRPV1. Oral Oncol. 2014, 50, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Chapa, J.J.; Singha, P.K.; Lee, D.R.; Gonzales, C.B. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J. Oral Pathol. Med. 2018, 47, 674–682. [Google Scholar] [CrossRef]
- Ruparel, S.; Bendele, M.; Wallace, A.; Green, D. Released lipids regulate transient receptor potential channel (TRP)-dependent oral cancer pain. Mol. Pain 2015, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Fliegert, R.; Bauche, A.; Wolf Pérez, A.M.; Watt, J.M.; Rozewitz, M.D.; Winzer, R.; Janus, M.; Gu, F.; Rosche, A.; Harneit, A.; et al. 2′-Deoxyadenosine 5′-diphosphoribose is an endogenous TRPM2 superagonist. Nat. Chem. Biol. 2017, 13, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Kolisek, M.; Beck, A.; Fleig, A.; Penner, R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell 2005, 18, 61–69. [Google Scholar] [CrossRef]
- Zaidi, N.; Kelly, R.J. The management of localized esophageal squamous cell carcinoma: Western approach. Chin. Clin. Oncol. 2017, 6, 46. [Google Scholar] [CrossRef]
- Kim, J.J.; Bowlby, R.; Mungall, A.J.; Robertson, A.G.; Odze, R.D.; Cherniack, A.D.; Shih, J.; Pedamallu, C.S.; Cibulskis, C.; Dunford, A.; et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–174. [Google Scholar]
- Wang, K.; Johnson, A.; Ali, S.M.; Klempner, S.J.; Bekaii-Saab, T.; Vacirca, J.L.; Khaira, D.; Yelensky, R.; Chmielecki, J.; Elvin, J.A.; et al. Comprehensive Genomic Profiling of Advanced Esophageal Squamous Cell Carcinomas and Esophageal Adenocarcinomas Reveals Similarities and Differences. Oncologist 2015, 20, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Ding, X.; He, Z.H.; Zhou, K.C.; Wang, Q.; Wang, Y.Z. Critical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancer. Gut 2009, 58, 1443–1450. [Google Scholar] [CrossRef]
- Lan, X.; Zhao, J.; Song, C.; Yuan, Q.; Liu, X. TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, S.; Shiozaki, A.; Ichikawa, D.; Hikami, S.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; Kishimoto, M.; et al. Transient Receptor Potential Melastatin 7 as an Independent Prognostic Factor in Human Esophageal Squamous Cell Carcinoma. Anticancer Res. 2017, 37, 1161–1167. [Google Scholar]
- Kudou, M.; Shiozaki, A.; Yamazato, Y.; Katsurahara, K.; Kosuga, T.; Shoda, K.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. The expression and role of TRPV2 in esophageal squamous cell carcinoma. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Wang, F.; Yang, Y.; Ma, W.; Lin, Z.; Cheng, N.; Long, Y.; Deng, S.; Li, Z. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio 2019, 9, 206–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Boustany, C.; Bidaux, G.; Enfissi, A.; Delcourt, P.; Prevarskaya, N.; Capiod, T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 2008, 47, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Liu, G.G.; Xu, X.; Xie, C.; Sun, F.; Yang, Y.; Zhang, T.; Hua, S.; Fan, W.; Li, Q.; et al. High expression of vanilloid receptor-1 is associated with better prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2008, 186, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.G.; Xie, C.; Sun, F.; Xu, X.; Yang, Y.; Zhang, T.; Deng, Y.; Wang, D.; Huang, Z.; Yang, L.; et al. Clinical significance of transient receptor potential vanilloid 2 expression in human hepatocellular carcinoma. Cancer Genet. Cytogenet. 2010, 197, 54–59. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, G.G.; Xie, C.; Qian, K.; Lei, X.; Liu, Q.; Liu, G.G.; Cao, Z.; Fu, J.; Du, H.; et al. Pharmacological inhibition of TRPV4 channel suppresses malignant biological behavior of hepatocellular carcinoma via modulation of ERK signaling pathway. Biomed. Pharmacother. 2018, 101, 910–919. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Xie, R.; Liu, J.; Nie, X.; An, J.; Wen, G.; Liu, X.; Jin, H.; Tuo, B. The ncx1/trpc6 complex mediates tgfb-driven migration and invasion of human hepatocellular carcinoma cells. Cancer Res. 2018, 78, 2564–2576. [Google Scholar] [CrossRef] [Green Version]
- Waning, J.; Vriens, J.; Owsianik, G.; Stüwe, L.; Mally, S.; Fabian, A.; Frippiat, C.; Nilius, B.; Schwab, A. A novel function of capsaicin-sensitive TRPV1 channels: Involvement in cell migration. Cell Calcium 2007, 42, 17–25. [Google Scholar] [CrossRef]
- Vriens, J.; Janssens, A.; Prenen, J.; Nilius, B.; Wondergem, R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 2004, 36, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.P.; Chen, J.C.; Wu, C.C.; Chen, C.T.; Tang, N.Y.; Ho, Y.T.; Lo, C.; Lin, J.P.J.G.; Chung, J.G.; Lin, J.P.J.G. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009, 29, 165–174. [Google Scholar] [PubMed]
- Chen, W.T.; Lin, G.B.; Lin, S.H.; Lu, C.H.; Hsieh, C.H.; Ma, B.L.; Chao, C.Y. Static magnetic field enhances the anticancer efficacy of capsaicin on HepG2 cells via capsaicin receptor TRPV1. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, C.; Yin, S.; Liu, J.; Gao, C.; Lin, Z.; Huang, R.; Huang, J.; Li, Z. Novel role of TRPV2 in promoting the cytotoxicity of H2O2-mediated oxidative stress in Human hepatoma cells. Free Radic. Biol. Med. 2015, 89, 1003–1013. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, X.; Fang, Y.; Liu, G.; Xie, C.; Qian, K.; Lei, X.; Cao, Z.; Du, H.; Cheng, X.; et al. Transient receptor potential vanilloid-type 2 targeting on stemness in liver cancer. Biomed. Pharmacother. 2018, 105, 697–706. [Google Scholar] [CrossRef]
- Selli, C.; Erac, Y.; Kosova, B.; Erdal, E.S.; Tosun, M. Silencing of TRPC1 regulates store-operated calcium entry and proliferation in Huh7 hepatocellular carcinoma cells. Biomed. Pharmacother. 2015, 71, 194–200. [Google Scholar] [CrossRef]
- Wen, L.; Liang, C.; Chen, E.; Chen, W.; Liang, F.; Zhi, X.; Wei, T.; Xue, F.; Li, G.; Yang, Q.; et al. Regulation of Multi-drug Resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yu, Y.; Sun, S.; Wang, Z.; Liu, P.; Liu, S.; Jiang, J. Bradykinin promotes migration and invasion of hepatocellular carcinoma cells through TRPM7 and MMP-2. Exp. Cell Res. 2016, 349, 68–76. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA. Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Futur. Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Wang, Y.; Chen, Q.; Liu, Z.; Xiao, S.; Wang, B.; Shi, B. TRPM2 promotes the proliferation and invasion of pancreatic ductal adenocarcinoma. Mol. Med. Rep. 2018, 17, 7537–7544. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Strowski, M.Z.; Kaiser, S.; Plath, T.; Giesecke, Y.; Neumann, M.; Hosokawa, H.; Kobayashi, S.; Langrehr, J.; Neuhaus, P.; et al. Transient receptor potential channel TRPM8 agonists stimulate calcium influx and neurotensin secretion in neuroendocrine tumor cells. Neuroendocrinology 2007, 85, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Li, Q.; Kazi, A.; Yang, Z.; Berg, A.; Yee, R. Aberrantly Over-Expressed TRPM8 Channels in Pancreatic Adenocarcinoma: Correlation with Tumor Size/Stage and Requirement for Cancer Cells Invasion. Cells 2014, 3, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S.; Zhou, W.; Lee, M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett. 2010, 297, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Hu, G.H.; Gong, Y.J.; Yu, Q.L.; He, B.; Li, W.H.; He, Z.G.Z.C.; Hao, W.J.; He, Z.G.Z.C.; Liu, Y.P. Silencing of TRPM8 inhibits aggressive tumor phenotypes and enhances gemcitabine sensitivity in pancreatic cancer. Pancreatology 2018, 18, 935–944. [Google Scholar] [CrossRef]
- Du, J.D.; Zheng, X.; Chen, Y.L.; Huang, Z.Q.; Cai, S.W.; Jiao, H.B.; Zhu, Z.M.; Hu, B. Elevated transient receptor potential melastatin 8 (Trpm8) expression is correlated with poor prognosis in pancreatic cancer. Med. Sci. Monit. 2018, 24, 3720–3725. [Google Scholar] [CrossRef]
- Cucu, D.; Chiritoiu, G.; Petrescu, S.; Babes, A.; Stanica, L.; Duda, D.G.; Horii, A.; Dima, S.O.; Popescu, I. Characterization of functional transient receptor potential melastatin 8 channels in human pancreatic ductal adenocarcinoma cells. Pancreas 2014, 43, 795–800. [Google Scholar] [CrossRef]
- Ulăreanu, R.; Chiriţoiu, G.; Cojocaru, F.; Deftu, A.; Ristoiu, V.; Stănică, L.; Mihăilescu, D.F.; Cucu, D. N-glycosylation of the transient receptor potential melastatin 8 channel is altered in pancreatic cancer cells. Tumor Biol. 2017, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yee, N.S.; Zhou, W.; Liang, I.C. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. DMM Dis. Model. Mech. 2011, 4, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Yee, N.S.; Zhou, W.; Lee, M.; Yee, R.K. Targeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma. Cancer Lett. 2012, 318, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 2012, 131, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S.; Kazi, A.A.; Li, Q.; Yang, Z.; Berg, A.; Yee, R.K. Aberrant over-expression of TRPM7 ion channels in pancreatic cancer: Required for cancer cell invasion and implicated in tumor growth and metastasis. Biol. Open 2015, 4, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybarczyk, P.; Vanlaeys, A.; Brassart, B.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Ouadid-Ahidouch, H.; Gautier, M. The Transient Receptor Potential Melastatin 7 Channel Regulates Pancreatic Cancer Cell Invasion through the Hsp90α/uPA/MMP-2 pathway. Neoplasia (United States) 2017, 19, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Skrzypski, M.; Sassek, M.; Pietrzak, P.; Pucci, C.; Wiedenmann, B.; Strowski, M.Z. Thermo-sensitive transient receptor potential vanilloid channel-1 regulates intracellular calcium and triggers chromogranin A secretion in pancreatic neuroendocrine BON-1 tumor cells. Cell. Signal. 2012, 24, 233–246. [Google Scholar] [CrossRef]
- Skrzypski, M.; Sassek, M.; Abdelmessih, S.; Mergler, S.; Grötzinger, C.; Metzke, D.; Wojciechowicz, T.; Nowak, K.W.; Strowski, M.Z. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell. Signal. 2014, 26, 41–48. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Qiu, L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signalling in pancreatic cancer cells. Cell Biochem. Funct. 2020, 1–8. [Google Scholar] [CrossRef]
- Skrzypski, M.; Kołodziejski, P.A.; Mergler, S.; Khajavi, N.; Nowak, K.W.; Strowski, M.Z. TRPV6 modulates proliferation of human pancreatic neuroendocrine BON-1 tumour cells. Biosci. Rep. 2016, 36, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Dong, M.; Zhou, J.; Sheng, W.; Li, X.; Gao, W. Expression and prognostic significance of TRPV6 in the development and progression of pancreatic cancer. Oncol. Rep. 2018, 39, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Cai, R.; Ding, X.; Zhou, K.; Shi, Y.; Ge, R.; Ren, G.; Jin, Y.; Wang, Y. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int. J. Cancer 2009, 125, 2281–2287. [Google Scholar] [CrossRef]
- Ge, P.; Wei, L.; Zhang, M.; Hu, B.; Wang, K.; Li, Y.Y.; Liu, S.; Wang, J.; Li, Y.Y. TRPC1/3/6 inhibition attenuates the TGF-β1-induced epithelial–mesenchymal transition in gastric cancer via the Ras/Raf1/ERK signaling pathway. Cell Biol. Int. 2018, 42, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, H.; Qu, C.; Xu, F.; Zhu, Y.; Lv, G.; Lu, Y.; Zhou, Q.; Zhou, H.; Zeng, X.; et al. Pyrazolo[1,5-a]pyrimidine TRPC6 antagonists for the treatment of gastric cancer. Cancer Lett. 2018, 432, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells-Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Almasi, S.; Kennedy, B.E.; El-Aghil, M.; Sterea, A.M.; Gujar, S.; Partida-Sánchez, S.; El Hiani, Y. TRPM2 channel–mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J. Biol. Chem. 2018, 293, 3637–3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Park, E.J.; Lee, J.H.; Jeon, J.H.; Kim, S.J.; So, I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008, 99, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal Cancer Statistics, 2014. CA. Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef]
- Sozucan, Y.; Kalender, M.E.; Sari, I.; Suner, A.; Oztuzcu, S.; Arman, K.; Yumrutas, O.; Bozgeyik, I.; Cengiz, B.; Igci, Y.Z.; et al. TRP genes family expression in colorectal cancer. Exp. Oncol. 2015, 37, 208–212. [Google Scholar] [CrossRef]
- Ibrahim, S.; Dakik, H.; Vandier, C.; Chautard, R.; Paintaud, G.; Mazurier, F.; Lecomte, T.; Guéguinou, M.; Raoul, W. Expression profiling of calcium channels and calcium-activated potassium channels in colorectal cancer. Cancers (Basel) 2019, 11, 561. [Google Scholar] [CrossRef] [Green Version]
- Hou, N.; He, X.; Yang, Y.; Fu, J.; Zhang, W.; Guo, Z.; Hu, Y.; Liang, L.; Xie, W.; Xiong, H.; et al. TRPV1 Induced apoptosis of colorectal cancer cells by activating calcineurin-NFAT2-p53 signaling pathway. Biomed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Kappel, S.; Stokłosa, P.; Hauert, B.; Ross-Kaschitza, D.; Borgström, A.; Baur, R.; Galván, J.A.; Zlobec, I.; Peinelt, C. TRPM4 is highly expressed in human colorectal tumor buds and contributes to proliferation, cell cycle, and invasion of colorectal cancer cells. Mol. Oncol. 2019, 13, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhao, R.; Bai, B.; Wu, Y.; Xu, Y.; Lu, S.; Fang, Y.; Wang, Z.; Maswikiti, E.P.; Zhou, X.; et al. Identification of key tumorigenesis-related genes and their microRNAs in colon cancer. Oncol. Rep. 2018, 40, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Sobradillo, D.; Hernández-Morales, M.; Ubierna, D.; Moyer, M.P.; Núñez, L.; Villalobos, C. A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+remodeling and cancer hallmarks in colorectal carcinoma cells. J. Biol. Chem. 2014, 289, 28765–28782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guéguinou, M.; Harnois, T.; Crottes, D.; Uguen, A.; Deliot, N.; Gambade, A.; Chantôme, A.; Haelters, J.P.; Jaffrès, P.A.; Jourdan, M.L.; et al. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: A novel opportunity to modulate anti- EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget 2016, 7, 36168–36184. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chen, Z.; Zhu, Y.; Pan, Q.; Liu, Y.; Qi, X.; Jin, L.; Jin, J.; Ma, X.; Hua, D. Inhibition of transient receptor potential channel 5 reverses 5-fluorouracil resistance in human colorectal cancer cells. J. Biol. Chem. 2015, 290, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.F.; Anand, P.; Ghosh, S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef]
- Devesa, I.; Planells-Cases, R.; Fernández-Ballester, G.; González-Ros, J.M.; Ferrer-Montiel, A.; Fernández-Carvajal, A. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J. Inflamm. Res. 2011, 4, 67–81. [Google Scholar]
- Lee, J.; Yamamoto, T.; Kuramoto, H.; Kadowaki, M. TRPV1 expressing extrinsic primary sensory neurons play a protective role in mouse oxazolone-induced colitis. Auton. Neurosci. Basic Clin. 2012, 166, 72–76. [Google Scholar] [CrossRef]
- Massa, F.; Sibaev, A.; Marsicano, G.; Blaudzun, H.; Storr, M.; Lutz, B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J. Mol. Med. 2006, 84, 142–146. [Google Scholar] [CrossRef]
- Martelli, L.; Ragazzi, E.; Di Mario, F.; Martelli, M.; Castagliuolo, I.; Dal Maschio, M.; Palù, G.; Maschietto, M.; Scorzeto, M.; Vassanelli, S.; et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol. Motil. 2007, 19, 668–674. [Google Scholar] [CrossRef]
- Vinuesa, A.G.; Sancho, R.; García-Limones, C.; Behrens, A.; Ten Dijke, P.; Calzado, M.A.; Muñoz, E. Vanilloid receptor-1 regulates neurogenic inflammation in colon and protects mice from colon cancer. Cancer Res. 2012, 72, 1705–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, P.R.; Takahashi, N.; Harris, A.R.; Lee, J.J.; Bertin, S.; Jeffries, J.; Jung, M.; Duong, J.; Triano, A.I.; Lee, J.J.; et al. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J. Clin. Invest. 2014, 124, 3793–3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, J.; Jin, L.; Lei, K.; Liu, H.; Yang, Y. Fibulin-5 contributes to colorectal cancer cell apoptosis via the ROS/MAPK and Akt signal pathways by downregulating transient receptor potential cation channel subfamily V member 1. J. Cell. Biochem. 2019, 120, 17838–17846. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, W.H.; Park, J.Y.; Kang, J.H.; Kim, M.O.; Kawada, T.; Yoo, H.; Han, I.S.; Yu, R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor γ in HT-29 human colon cancer cells. J. Med. Food 2004, 7, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Launay, P.; Cheng, H.; Srivatsan, S.; Penner, R.; Fleig, A.; Kinet, J.P. TRPM4 regulates calcium oscillations after T cell activation. Science 2004, 306, 1374–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Launay, P.; Fleig, A.; Perraud, A.L.; Scharenberg, A.M.; Penner, R.; Kinet, J.P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Fleig, A.; Penner, R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol. Sci. 2004, 25, 633–639. [Google Scholar] [CrossRef]
- Holzmann, C.; Kappel, S.; Kilch, T.; Jochum, M.M.; Urban, S.K.; Jung, V.; Stöckle, M.; Rother, K.; Peinelt, C. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget 2015, 6, 41783–41793. [Google Scholar] [CrossRef]
- Pérez-Riesgo, E.; Gutiérrez, L.G.; Ubierna, D.; Acedo, A.; Moyer, M.P.; Núñez, L.; Villalobos, C. Transcriptomic analysis of calcium remodeling in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 922. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Nejsum, L.N.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TPRM6, a new member of the TPRM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camprubí-Robles, M.; Planells-Cases, R.; Ferrer-Montiel, A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009, 23, 3722–3733. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Pang, Z.; Liu, Q.F. Magnesium intake and risk of colorectal cancer: A meta-analysis of prospective studies. Eur. J. Clin. Nutr. 2012, 66, 1182–1186. [Google Scholar] [CrossRef]
- Nasulewicz, A.; Wietrzyk, J.; Wolf, F.I.; Dzimira, S.; Madej, J.; Maier, J.A.M.; Rayssiguier, Y.; Mazur, A.; Opolski, A. Magnesium deficiency inhibits primary tumor growth but favors metastasis in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2004, 1739, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Wolf, F.I.; Trapani, V.; Cittadini, A. Magnesium and the control of cell proliferation: Looking for a needle in a haystack. Magnes. Res. 2008, 21, 83–91. [Google Scholar]
- Trapani, V.; Arduini, D.; Cittadini, A.; Wolf, F.I. From magnesium to magnesium transporters in cancer: TRPM7, a novel signature in tumour development. Magnes. Res. 2014, 26, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Wolf, F.I.; Maier, J.A.M.; Nasulewicz, A.; Feillet-Coudray, C.; Simonacci, M.; Mazur, A.; Cittadini, A. Magnesium and neoplasia: From carcinogenesis to tumor growth and progression or treatment. Arch. Biochem. Biophys. 2007, 458, 24–32. [Google Scholar] [CrossRef]
- Su, F.; Wang, B.-F.; Zhang, T.; Hou, X.-M.; Feng, M.-H. TRPM7 deficiency suppresses cell proliferation, migration, and invasion in human colorectal cancer via regulation of epithelial-mesenchymal transition. Cancer Biomark. 2019, 26, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shrubsole, M.J.; Ness, R.M.; Schlundt, D.; Cai, Q.; Smalley, W.E.; Li, M.; Shyr, Y.; Zheng, W.; John, R.; et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 2007, 86, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermosura, M.C.; Nayakanti, H.; Dorovkov, M.V.; Calderon, F.R.; Ryazanov, A.G.; Haymer, D.S.; Garruto, R.M. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 11510–11515. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, S.; Cazzaniga, A.; Trapani, V.; Cappadone, C.; Farruggia, G.; Merolle, L.; Wolf, F.I.; Iotti, S.; Maier, J.A.M. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reimers, M.S.; Zeestraten, E.C.M.; Kuppen, P.J.K.; Liefers, G.J.; van de Velde, C.J.H. Biomarkers in precision therapy in colorectal cancer. Gastroenterol. Rep. 2013, 1, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.C.; Winter, D.C.; Heeney, A.; Gibbons, D.; Lugli, A.; Puppa, G.; Sheahan, K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 2016, 115, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlobec, I.; Lugli, A. Tumour budding in colorectal cancer: Molecular rationale for clinical translation. Nat. Rev. Cancer 2018, 18, 203–204. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. MAbs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Haustrate, A.; Hantute-Ghesquier, A.; Prevarskaya, N.; Lehen’kyi, V. Monoclonal antibodies targeting ion channels and their therapeutic potential. Front. Pharmacol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Edwards, W.; Fung-Leung, W.P.; Huang, C.; Chi, E.; Wu, N.; Liu, Y.; Maher, M.P.; Bonesteel, R.; Connor, J.; Fellows, R.; et al. Targeting the ion channel Kv1.3 with scorpion venom peptides engineered for potency, selectivity, and half-life. J. Biol. Chem. 2014, 289, 22704–22714. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Ataga, K.I.; Stocker, J. Senicapoc (ICA-17043): A potential therapy for the prevention and treatment of hemolysis-associated complications in sickle cell anemia. Expert Opin. Investig. Drugs 2009, 18, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Arulkumaran, N.; Unwin, R.J.; Tam, F.W.K. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin. Investig. Drugs 2011, 20, 897–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Hirte, H.; Welch, S.; Ilenchuk, T.T.; Lutes, T.; Rice, C.; Fields, N.; Nemet, A.; Dugourd, D.; Piha-Paul, S.; et al. First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Invest. New Drugs 2017, 35, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Type of Cancer | Channel | mRNA/Protein (+ Assessment Method) | Sample Size | Aim/Outcome + Reference |
|---|---|---|---|---|
| Oral | TRPV1–4 | mRNA (qPCR) | 37 oral SCC + tissues samples/compared to normal adjacent tissue | TRPV1–4 mRNA and protein expression upregulated in oral SCC tissue samples in comparison to normal tissue [61] |
| protein (IHC) | ||||
| TRPV1 | Protein (IHC + WB) | 18 tongue SCC + 8 leukoplakia + 7 normal tongue tissues samples | TRPV1 protein and mRNA expression upregulated in tongue SCC tissue samples in comparison to normal tissue [59] 1 | |
| mRNA (qPCR) | ||||
| Protein (IHC) | 3 oral SCC + 3 normal oral mucosa tissue samples | TRPV1 protein is expressed in oral SCC [63] | ||
| TRPM2 | Protein (IHC) | 9 normal tongue + 12 papilloma tongue + 23 tongue carcinoma tissue samples | TRPM2 protein is overexpressed in tongue carcinoma in comparison to normal and papilloma samples [62] |
| Type of Cancer | Channel | mRNA/Protein (+ Assessment Method) | Sample Size | Aim/Outcome + Reference |
|---|---|---|---|---|
| Esophageal | TRPC6 | mRNA (in situ hybridization) | 55 paraffin-embedded ESSC + 21 fresh ESCC tissue samples/compared to normal adjacent tissue | TRPC6 mRNA and protein are overexpressed compared to normal adjacent tissue [71] |
| Protein (IHC) | ||||
| TRPM8 | mRNA (qPCR) | 10 ESCC tissue samples/compared to normal adjacent tissue | TRPM8 mRNA and protein are overexpressed compared to normal adjacent tissue [72] | |
| Protein (WB) | ||||
| TRPM7 | Protein (IHC) | 52 ESCC tissue samples/compared to non-cancerous esophageal epithelia | TRPM7 protein is overexpressed compared to non-cancerous esophageal epithelia (no TRPM7 expression detected) [73] 1 | |
| TRPV2 | Protein (IHC) | 62 ESCC tissue samples | Analysis of TRPV2 expression (low/high); worse overall survival and 5 year survival of patients with high TRPV2 protein expression [74] |
| Type of Cancer | Channel | mRNA/Protein (+ Assessment Method) | Sample Size | Aim/Outcome + Reference |
|---|---|---|---|---|
| Liver | TRPV1 | mRNA (RT-PCR) | 6 pairs of HCC tissue samples/compared to normal adjacent tissue | 6 non-tumor tissues showed TRPV1 mRNA overexpression; HCC tissue samples showed downregulation in 4/6 tested [77] |
| mRNA (in situ hybridization) | 15 HCC samples/compared to normal adjacent tissue | TRPV1 expressed in some HCC and normal tissue samples; data non-conclusive [77] | ||
| Protein (IHC) | 62 HCC tissue samples + 62 non-tumor control tissues | High TRPV1 expression was observed in 30/62 HCC samples; high TRPV1 expression was associated with longer disease-free survival [77] | ||
| TRPV2 | Protein (IHC) | 55 HCC cancer tissue samples | Upregulation of TRPV2 on mRNA and protein levels inversely correlated with histopathologic differentiation [78] | |
| mRNA (RT-PCR) | 13 paired HCC tumor mRNA extracts | |||
| TRPV4 | Protein (IHC) | 45 HCC tissue samples/compared to normal adjacent tissue | TRPV4 protein and mRNA levels higher in HCC tissues than in normal tissues; positive correlation between TRPV4 expression; the histological grade and number of tumors [79] | |
| mRNA (qPCR) | ||||
| TRPC6 | Protein (IHC) | 150 HCC tissue samples/compared to normal tissues | TRPC protein upregulated in HCC tissues in comparison to normal tissues [80] |
| Type of Cancer | Channel | mRNA/Protein (+ Assessment Method) | Sample Size | Aim/Outcome + Reference |
|---|---|---|---|---|
| Pancreatic | TRPM2 | mRNA (analysis of previously published cancer genome studies) | 91 pancreatic cancer patients | High TRPM2 expression correlated with lower overall survival [92] |
| TRPM8 | Protein (IHC) | 280 pancreatic adenocarcinoma tissue microarrays | Moderate or high level of TRPM8 protein expression in 92% of pancreatic adenocarcinoma; the expression levels of TRPM8 positively correlate with the size of the primary tumor and tumor stages [94] | |
| Protein (IHC) | 5 pancreatic adenocarcinoma tissue samples/compared to normal adjacent tissue | TRPM8 protein expression upregulated compared to normal tissue [95] | ||
| Protein (IHC) mRNA (qPCR) | 44 pancreatic adenocarcinoma tissue samples/compared to normal adjacent tissue | TRPM8 protein and mRNA upregulated compared to normal tissue [96] | ||
| mRNA (qPCR) | 110 pancreatic adenocarcinoma tissue samples/compared to normal adjacent tissue | TRPM8 mRNA upregulated compared to normal tissue; high TRPM8 protein expression was found to be associated with lower overall survival and poor disease free survival values for pancreatic cancer patients [97] | ||
| TRPM7 | Protein (IHC) | 5 pancreatic adenocarcinoma tissue samples/compared to normal pancreatic tissue samples | TRPM7 protein upregulated compared to normal tissue [100] | |
| Protein (IHC) | 282 pancreatic adenocarcinoma tissue microarrays/compared to normal pancreatic tissue microarrays | TRPM7 protein upregulated compared to normal tissue; TRPM7 expression correlates with the tumor stage [103] | ||
| Protein (IHC) mRNA (RT-PCR) | 8 tumor pancreatic ductal adenocarcinoma/compared to 6 normal pancreatic tissues | TRPM7 protein and mRNA upregulated compared to normal pancreatic tissue [102] | ||
| TRPV6 | Protein (IHC) | 76 tumor pancreatic tissue samples compared to adjacent normal pancreatic tissues | TRPV6 protein upregulated compared to normal pancreatic tissue [109] |
| Type of Cancer | Channel | mRNA/Protein (+ Assessment method) | Sample Size | Aim/Outcome + Reference |
|---|---|---|---|---|
| Gastric | TRPC6 | Protein (IHC) | 25 primary gastric cancer samples/compared to 4 gastritis samples | TRPC6 mRNA and protein expression upregulated compared to gastritis samples [111] |
| mRNA (in situ hybridization) | 10 primary gastric cancer samples | |||
| TRPM2 | mRNA (analysis of online gastric cancer databases) | 896 gastric cancer patients; analysis of low TRPM2 vs high TRPM2 expression | High TRPM2 mRNA expression high expression negatively associated with the overall survival of patients [115] |
| Type of Cancer | Channel | mRNA/Protein (+ Assessment Method) | Sample Size | Aim/Outcome + Reference |
|---|---|---|---|---|
| Colorectal (CRC) | TRPC1 | mRNA (analysis of CRC datasets, available from public databases) | 656 CRC samples including 47 normal samples | High TRPC1 expression correlated with poor prognosis for the patients [120] |
| 585 CRC samples including 19 normal samples | ||||
| TRPV1 | Protein (IHC) | 10 CRC tissue samples, 10 CRC-adjacent tissue samples, and 6 normal subjects | TRPV1 protein expression decreased in CRC tissues compared to normal tissues [121] | |
| TRPM4 | Protein (IHC) | CRC tumor tissue microarrays from 379 patients | High TRPM4 protein expression was associated with unfavorable tumor features characteristic for epithelial-mesenchymal transition and infiltrative growth patterns [122] | |
| TRPM6 | mRNA (analysis of CRC datasets, available from public databases) | 656 CRC samples including 47 normal samples | TRPM6 mRNA expression decreased compared to normal tissue [120] | |
| 585 CRC samples including 19 normal samples | ||||
| mRNA (analysis of CRC dataset, available from public databases) | 585 CRC samples including 19 normal samples | TRPM6 mRNA expression decreased compared to normal tissue; high TRPM6 mRNA expression positively correlated with overall survival [123]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stokłosa, P.; Borgström, A.; Kappel, S.; Peinelt, C. TRP Channels in Digestive Tract Cancers. Int. J. Mol. Sci. 2020, 21, 1877. https://doi.org/10.3390/ijms21051877
Stokłosa P, Borgström A, Kappel S, Peinelt C. TRP Channels in Digestive Tract Cancers. International Journal of Molecular Sciences. 2020; 21(5):1877. https://doi.org/10.3390/ijms21051877
Chicago/Turabian StyleStokłosa, Paulina, Anna Borgström, Sven Kappel, and Christine Peinelt. 2020. "TRP Channels in Digestive Tract Cancers" International Journal of Molecular Sciences 21, no. 5: 1877. https://doi.org/10.3390/ijms21051877
APA StyleStokłosa, P., Borgström, A., Kappel, S., & Peinelt, C. (2020). TRP Channels in Digestive Tract Cancers. International Journal of Molecular Sciences, 21(5), 1877. https://doi.org/10.3390/ijms21051877