Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds
Abstract
1. Introduction
2. Results
2.1. Germination and Subsequent Seedling Growth Characteristics under Aging and Melatonin Priming in Oat Seeds
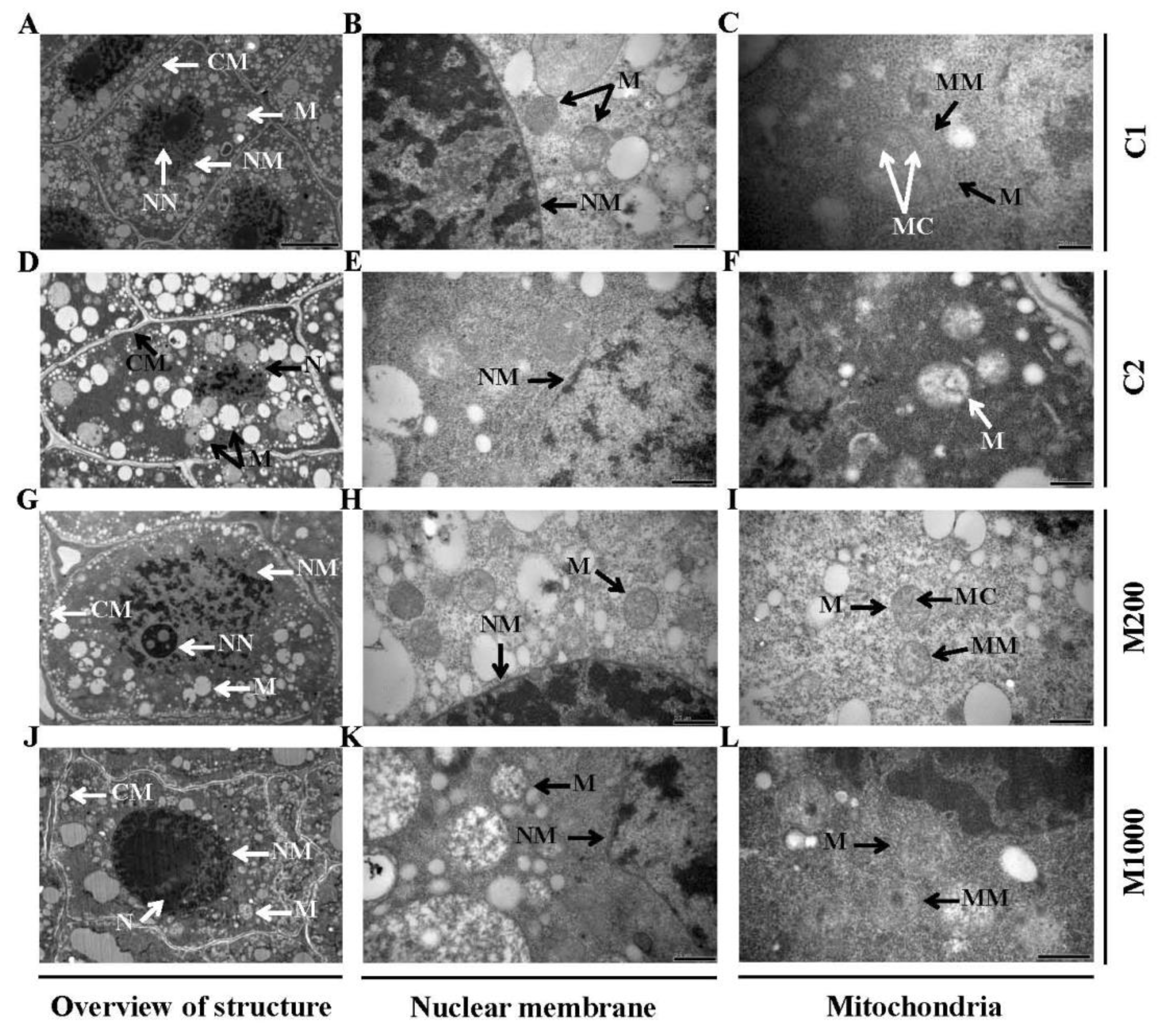
2.2. Ultrastructural Alterations in Embryos of Oat Seeds under Aging and Melatonin Priming
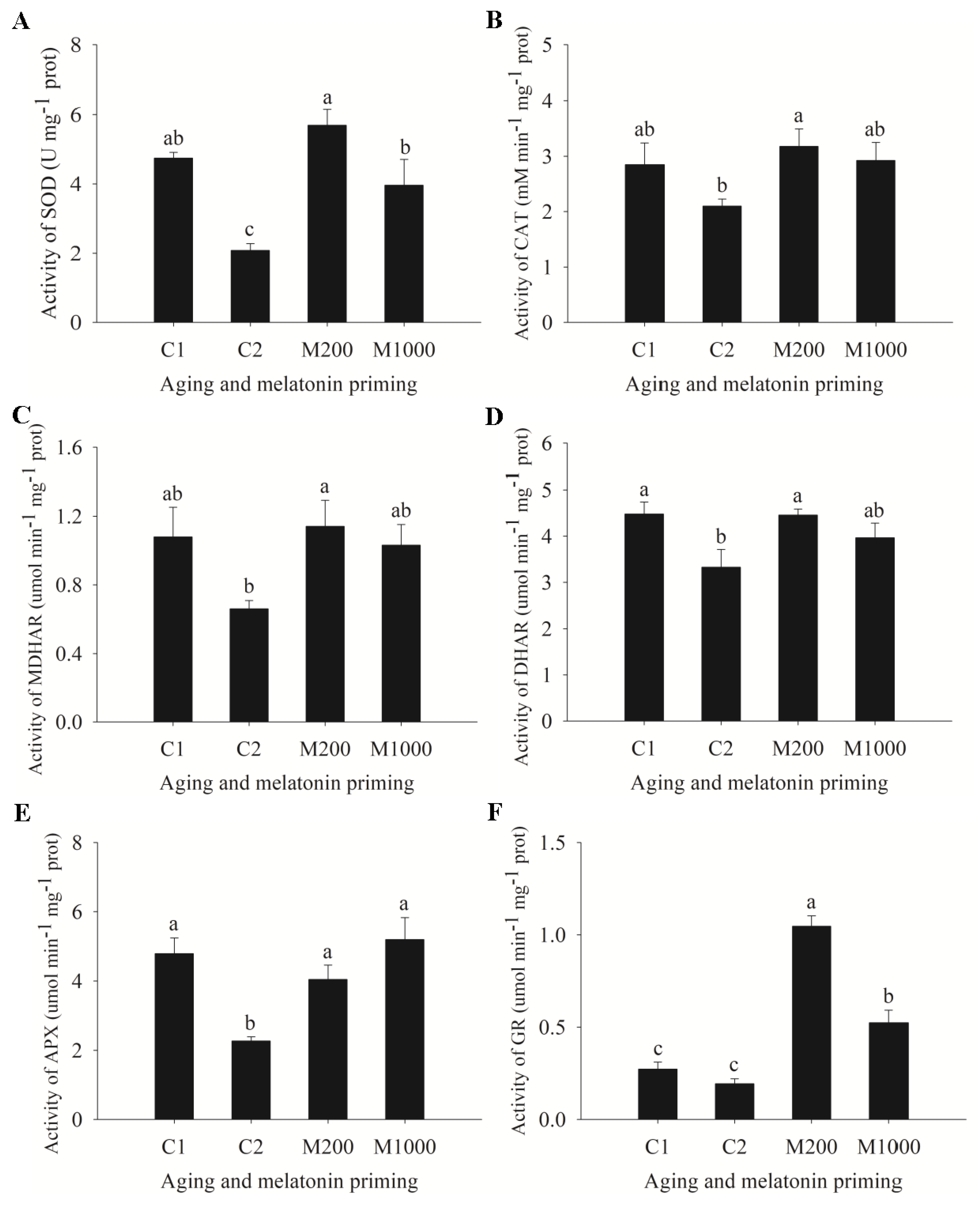
2.3. ROS Generation, Lipid Peroxidation, and Antioxidases’ Changes in Embryos of Oat Seeds under Aging and Melatonin Priming
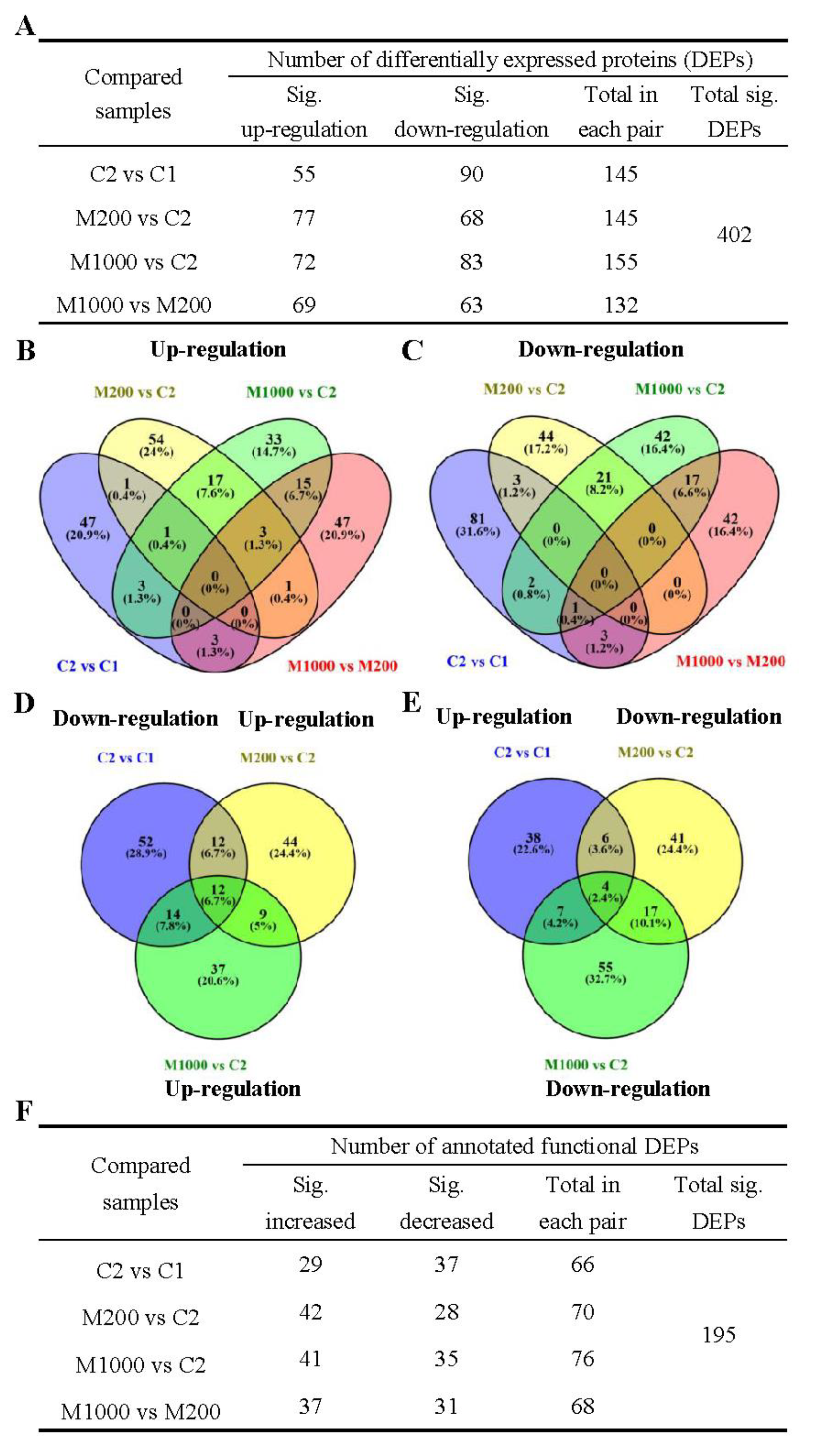
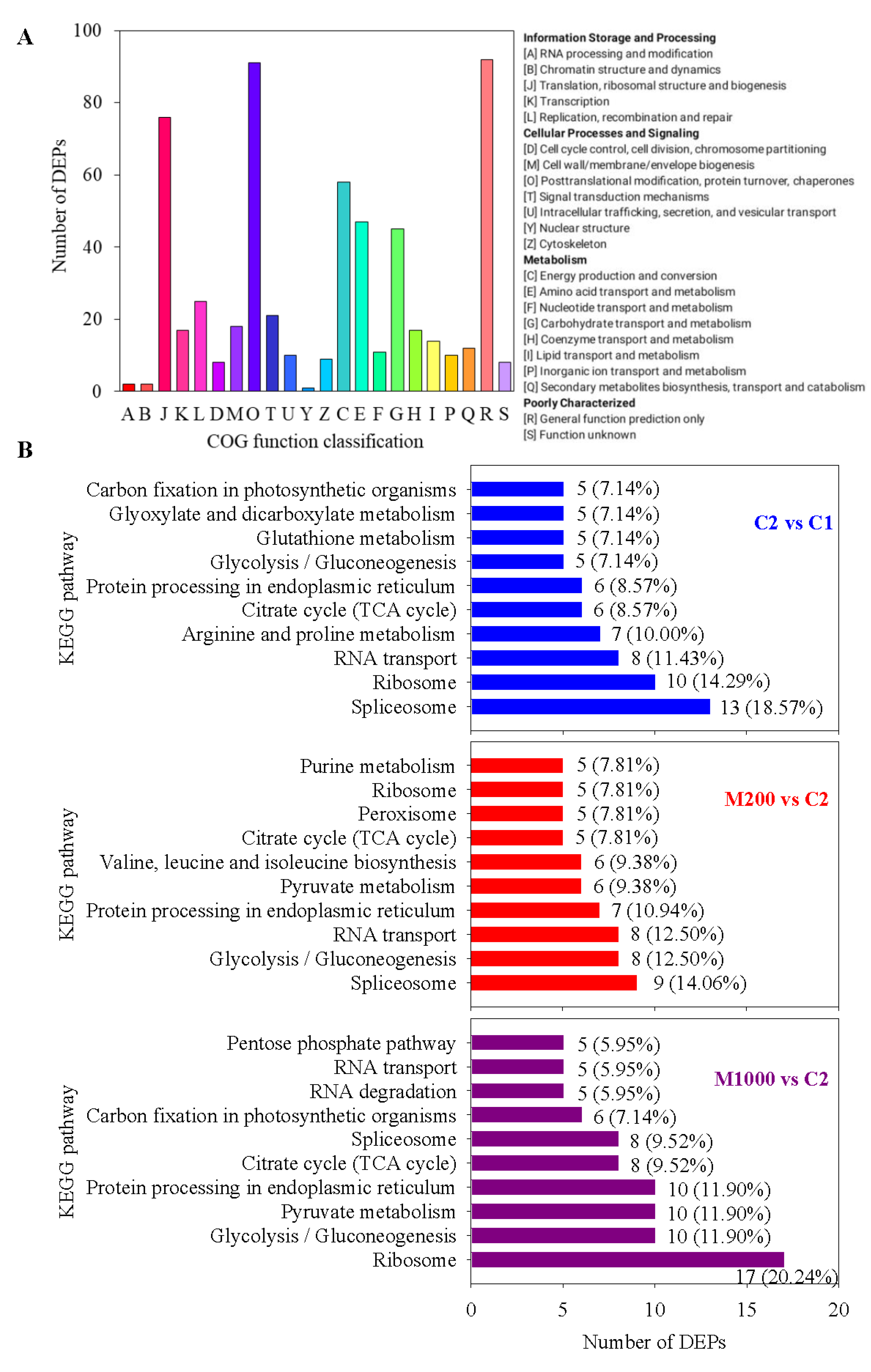
2.4. Proteomics Overview of Embryos in Oat Seeds
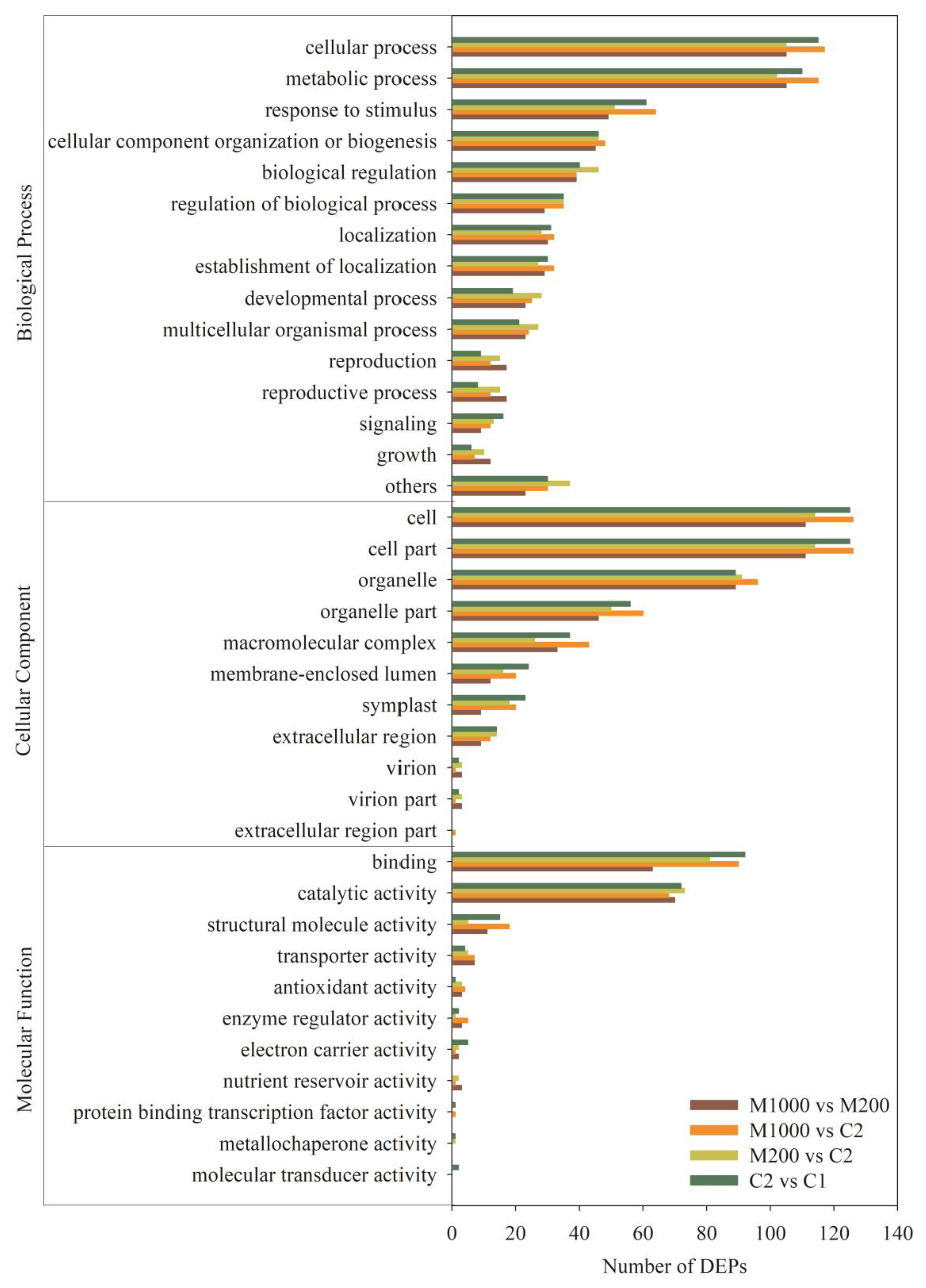
2.5. Functional Annotation Analysis of DEPs in Embryos
2.6. Validation of Transcriptional Expression Analysis of Selected DEPs by qRT-PCR
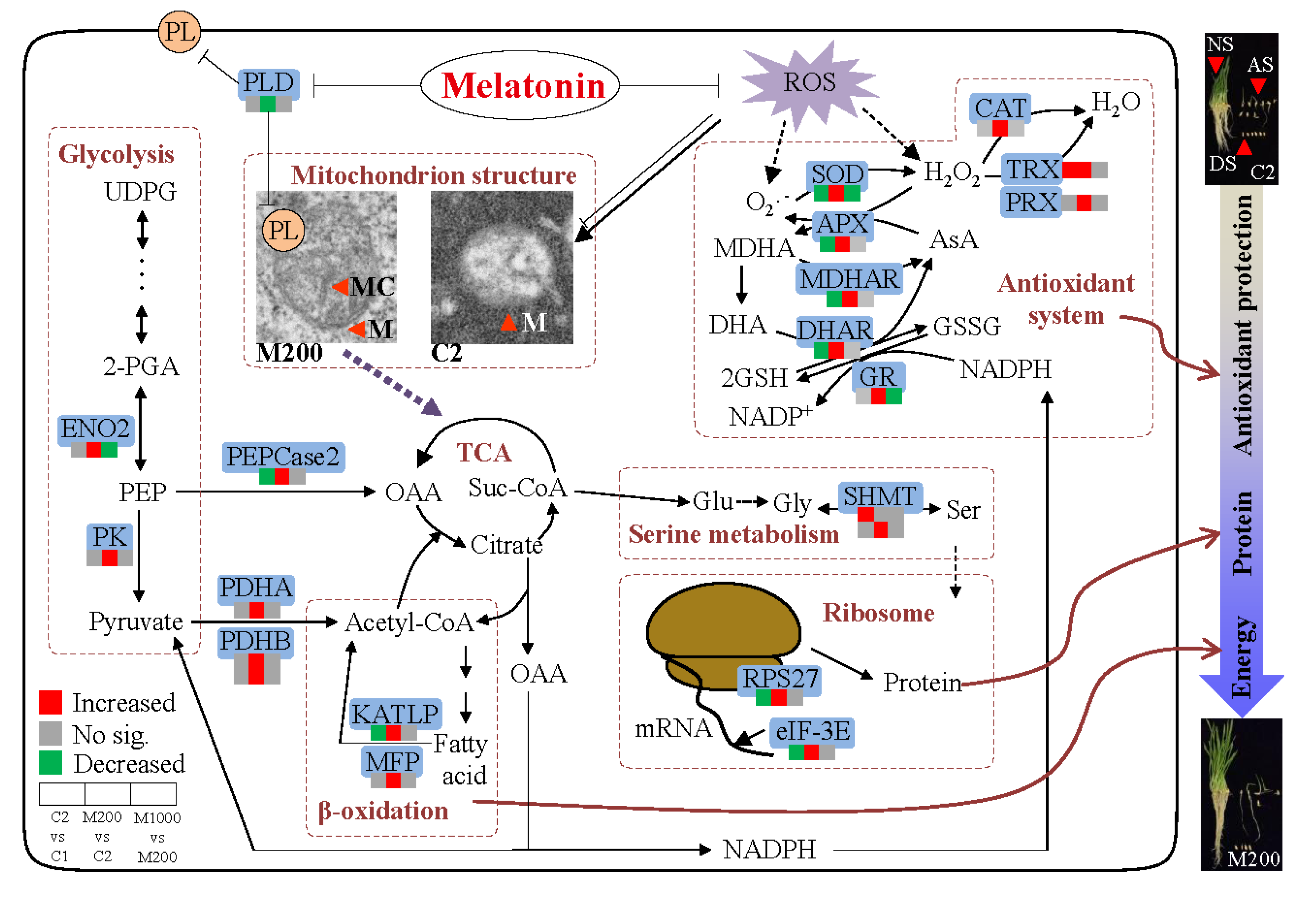
3. Discussion
4. Materials and Methods
4.1. Seed Material
4.2. Experimental Treatments
4.3. Germination Test and Seedling Growth Assay
4.4. Ultrastructure by Transmission Electron Microscopy (TEM)
4.5. Determination of Physiological Parameters
4.6. Embryonic Protein Extraction and Quantification
4.7. Protein Reduction, Digestion, and iTRAQ Labeling
4.8. NanoLC-MS/MS Analysis
4.9. Database Searching, Protein Identification, Annotation, and Functional Analysis
4.10. RNA Extraction and qRT-PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APX | Ascorbate peroxidase |
| AsA-GSH | Ascorbate-glutathione |
| CAT | Catalase |
| DHAR | Dehydroascorbate reductase |
| GI | Germination index |
| GP | Germination percentage |
| GR | Glutathione reductase |
| H2O2 | Hydrogen peroxide |
| iTRAQ | Isobaric tags for relative and absolute quantification |
| KATLP | 3-Ketoacyl-CoA thiolase-like protein |
| LPO | Lipid peroxidation |
| MDA | Malondialdehyde |
| MDHAR | Monodehydroascorbate reductase |
| O2−· | Superoxide anion |
| OH· | Hydroxyl radical |
| ROS | Reactive oxygen species |
| SL | Shoot length |
| SOD | Superoxide dismutase |
| SVI | Seedling vigor index |
| SW | Shoot weight |
| TEM | Transmission electron microscopy |
References
- Wang, W.Q.; Liu, S.J.; Song, S.Q.; Møller, I.M. Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol. Bioch. 2015, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M. Review on physiological aspects of seed deterioration. Intl. J. Agri. Crop Sci. 2013, 6, 627–631. [Google Scholar]
- Wojtyla, L.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, S.; Wang, J.; Hu, Y. Quantitative proteomic analysis of wheat seeds during artificial ageing and priming using the isobaric tandem mass tag labeling. PLoS ONE 2016, 11, e0162851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Xu, H.H.; Liu, S.J.; Li, N.; Wang, W.Q.; Møller, I.M.; Song, S.Q. Proteomic analysis reveals different involvement of embryo and endosperm proteins during aging of Yliangyou 2 hybrid rice seeds. Front. Plant Sci. 2016, 7, 1394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-García, F.; González-Benito, M.E.; Gómez-Campo, C. High viability recorded in ultra-dry seeds of 37 species of Brassicaceae after almost 40 years of storage. Seed Sci. Technol. 2007, 35, 143–153. [Google Scholar] [CrossRef]
- Zanotti, R.F.; Motta, L.B.; Bragatto, J.; Labate, C.A.; Salomão, A.N.; Vendrame, W.A.; Cuzzuol, G.R.F. Germination, carbohydrate composition and vigor of cryopreserved Caesalpinia echinata seeds. Braz. Arch. Biol. Technol. 2012, 55, 661–669. [Google Scholar] [CrossRef]
- Deepa, G.T.; Chetti, M.B.; Khetagoudar, M.C.; Adavirao, G.M. Influence of vacuum packaging on seed quality and mineral contents in chilli (Capsicum annuum L.). J. Food Sci. Tech. 2013, 50, 153–158. [Google Scholar] [CrossRef]
- Siadat, S.A.; Moosavi, A.; Zadeh, M.S. Effects of seed priming on antioxidant activity and germination characteristics of maize seeds under different ageing treatment. Res. J. Seed Sci. 2012, 5, 51–62. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Guan, Y.; Cao, D.; Li, J.; Nawaz, A.; Hu, Q.; Hu, W.; Ning, M.; Hu, J. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 5, 14278. [Google Scholar]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Pouramir-Dashtmian, F.; Khajeh-Hosseini, M.; Esfahani, M. Improving chilling tolerance of rice seedling by seed priming with salicylic acid. Arch. Agron. Soil Sci. 2014, 60, 1291–1302. [Google Scholar] [CrossRef]
- Ahmadiyan, K.; Mir-Mahmoodi, T.; Yazdanseta, S. Effect of seed priming on morpho-physiological traits of wheat in drought stress conditions. Int. J. Biosci. 2015, 6, 90–97. [Google Scholar]
- Krainart, C.; Siri, B.; Vichitphan, K. Effects of accelerated aging and subsequent priming on seed quality and biochemical change of hybrid cucumber (Cucumis sativa Linn.) seeds. Int. J. Agric. Technol. 2015, 11, 165–179. [Google Scholar]
- Nguyen, T.P.; Cueff, G.; Hegedus, D.D.; Rajjou, L.; Bentsink, L. A role for seed storage proteins in Arabidopsis seed longevity. J. Exp. Bot. 2015, 66, 6399–6413. [Google Scholar] [CrossRef]
- Byeon, Y.; Park, S.; Kim, Y.S.; Park, D.H.; Lee, S.; Back, K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J. Pineal Res. 2012, 53, 107–111. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in plants-diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotox. Environ. Safe. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Cheng, Y.; Lu, G.; Fu, G.; Ma, H.; Liu, Q.; Zhang, X.; Zou, X.; Li, C. Exogenous melatonin alleviates damage from drought stress in Brassica napus L. (rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 43. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Nojadeh, M.S.; Ebrahimzadeh, A. Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol. Tec. 2019, 148, 184–191. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef]
- Su, X.; Xin, L.; Li, Z.; Zheng, H.; Mao, J.; Yang, Q. Physiology and transcriptome analyses reveal a protective effect of the radical scavenger melatonin in aging maize seeds. Free Radical Res. 2018, 52, 1094–1109. [Google Scholar] [CrossRef]
- Xu, D.; Ren, G.Y.; Liu, L.L.; Zhu, W.X.; Liu, Y.H. The influences of drying process on crude protein content of naked oat cut herbage (Avena nuda L.). Dry Technol. 2014, 32, 321–332. [Google Scholar]
- Klose, C.; Arendt, E.K. Proteins in oats; their synthesis and changes during germination: A review. Crit. Rev. Food Sci. 2012, 52, 629–639. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Giménez, M.J.; Real, A.; Dolores García-Molina, M.; Sousa, C.; Barro, F. Characterization of celiac disease related oat proteins: Bases for the development of high quality oat varieties suitable for celiac patients. Sci. Rep. 2017, 7, 42588. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Xin, X.; Fu, S.; An, M.; Wu, S.; Chen, X.; Zhang, J.; He, J.; Whelan, J.; Lu, X. Proteomic and carbonylation profile analysis at the critical node of seed ageing in Oryza sativa. Sci. Rep. 2017, 7, 40611. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhu, Y.; Cheng, H.; Yan, H.; Zhao, L.; Tang, J.; Ma, X.; Mao, P. Nitric oxide regulates seedling growth and mitochondrial responses in aged oat seeds. Int. J. Mol. Sci. 2018, 19, 1052. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; David, A.; Yadav, S.; Baluška, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol Plantarum. 2014, 152, 714–728. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.; Li, D.B.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Cui, G.; Sun, F.; Gao, X.; Xie, K.; Zhang, C.; Liu, S.; Xi, Y. Proteomic analysis of melatonin-mediated osmotic tolerance by improving energy metabolism and autophagy in wheat (Triticum aestivum L.). Planta. 2018, 248, 69–87. [Google Scholar] [CrossRef]
- Szafrańska, K.; Glińska, S.; Janas, K.M. Ameliorative effect of melatonin on meristematic cells of chilled and re-warmed Vigna radiata roots. Biol. Plantarum. 2013, 57, 91–96. [Google Scholar] [CrossRef]
- Yang, M.; Geng, M.; Shen, P.; Chen, X.; Li, Y.; Wen, X. Effffect of post-silking drought stress on the expression profifiles of genes involved incarbon and nitrogen metabolism during leaf senescence in maize (Zea mays L.). Plant Physiol. Bioch. 2019, 135, 304–309. [Google Scholar] [CrossRef]
- Xu, M.; He, D.; Teng, H.; Chen, L.; Song, H.; Huang, Q. Physiological and proteomic analyses of coix seed aging during storage. Food Chem. 2018, 260, 82–89. [Google Scholar] [CrossRef]
- Jeoung, N.H.; Harris, C.R.; Harris, R.A. Regulation of pyruvate metabolism in metabolic-related diseases. Rev. Endocr. Metab. Dis. 2014, 15, 99–110. [Google Scholar] [CrossRef]
- Dong, K.; Zhen, S.; Cheng, Z.; Cao, H.; Ge, P.; Yan, Y. Proteomic analysis reveals key proteins and phosphoproteins upon seed germination of wheat (Triticum aestivum L.). Front. Plant Sci. 2015, 6, 1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, H.; Song, L.; Shu, Y.; Gu, W. Comparative proteomics analysis reveals the mechanism of pre-harvest seed deterioration of soybean under high temperature and humidity stress. J. Proteom. 2012, 75, 2109–2127. [Google Scholar] [CrossRef] [PubMed]
- Mira, S.; González-Benito, M.E.; Hill, L.M.; Walters, C. Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. J. Exp. Bot. 2010, 61, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Permana, H.; Kumada, R.; Shinozaki, Y.; Tanabata, T.; Yamada, T.; Hirasawa, T.; Kanekatsu, M. Proteomic analysis of embryonic proteins synthesized from long-lived mRNAs during germination of rice seeds. Plant Cell Physiol. 2012, 53, 687–698. [Google Scholar] [CrossRef]
- Izard, T.; Aevarsson, A.; Allen, M.D.; Westphal, A.H.; Perham, R.N.; de Kok, A.; Hol, W.G.J. Principles of quasi-equivalence and Euclidean geometry govern the assembly of cubic and dodecahedral cores of pyruvate dehydrogenase complexes. Proc. Natl. Acad. Sci. USA 1999, 96, 1240–1245. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Sun, Q.Q.; Cao, Y.Y.; Li, X.; Zhao, B.; Wu, P.; Guo, Y. Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci. Rep. 2017, 7, 503. [Google Scholar] [CrossRef]
- Yang, Y.; Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotech. 2018, 49, 191–198. [Google Scholar] [CrossRef]
- Pinfield-Wells, H.; Rylott, E.L.; Gilday, A.D.; Graham, S.; Job, K.; Larson, T.R.; Graham, I.A. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 2005, 43, 861–872. [Google Scholar] [CrossRef]
- Thompson, J.E.; Froese, C.D.; Madey, E.; Smith, M.D.; Hong, Y. Lipid metabolism during plant senescence. Prog. Lipid Res. 1998, 37, 119–141. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Kong, L.; Xia, F.; Yan, H.; Zhu, Y.; Mao, P. Proteomic and physiological analysis of the response of oat (Avena sativa) seeds to heat stress under different moisture conditions. Front. Plant Sci. 2016, 7, 896. [Google Scholar] [CrossRef]
- Choudhary, N.L.; Sairam, R.K.; Tyagi, A. Expression of Δ1-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.). Indian J. Biochem. Bio. 2005, 42, 366–370. [Google Scholar]
- Lam, H.M.; Coschigano, K.; Schultz, C.; Melo-Oliveira, R.; Tjaden, G.; Oliveira, I.; Ngai, N.; Hsieh, M.H.; Coruzzi, G. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell. 1995, 7, 887–898. [Google Scholar] [PubMed]
- Zhuang, Y.; Ren, G.; He, C.; Li, X.; Meng, Q.; Zhu, C.; Wang, R.; Zhang, J. Cloning and characterization of a maize cDNA encoding glutamate decarboxylase. Plant Mol. Biol. Rep. 2010, 28, 620–626. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. The metabolism and function of γ-aminobutyric acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef]
- Engel, N.; Ewald, R.; Gupta, K.J.; Zrenner, R.; Hagemann, M.; Bauwe, H. The presequence of Arabidopsis serine hydroxymethyltransferase SHM2 selectively prevents import into mesophyll mitochondria. Plant Physiol. 2011, 157, 1711–1720. [Google Scholar] [CrossRef][Green Version]
- Fulneček, J.; Matyášek, R.; Votruba, I.; Holý, A.; Křížová, K.; Kovařík, A. Inhibition of SAH-hydrolase activity during seed germination leads to deregulation of flowering genes and altered flower morphology in tobacco. Mol. Genet. Genom. 2011, 285, 225–236. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar]
- Catusse, J.; Meinhard, J.; Job, C.; Strub, J.M.; Fischer, U.; Pestsova, E.; Westhoff, P.; Dorsselaer, A.V.; Job, D. Proteomics reveals potential biomarkers of seed vigor in sugarbeet. Proteomics 2011, 11, 1569–1580. [Google Scholar] [CrossRef]
- Light, S.H.; Anderson, W.F. The diversity of allosteric controls at the gateway to aromatic amino acid biosynthesis. Protein Sci. 2013, 22, 395–404. [Google Scholar] [CrossRef]
- Carroll, A.J. The Arabidopsis cytosolic ribosomal proteome: From form to function. Front. Plant Sci. 2013, 4, 32. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Bussiere, C.; Pallesen, J.; West, M.; Johnson, A.W.; Frank, J. Characterization of the nuclear export adaptor protein Nmd3 in association with the 60S ribosomal subunit. J. Cell Biol. 2010, 189, 1079. [Google Scholar] [CrossRef] [PubMed]
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Soós, V.; Sebestyén, E.; Juhász, A.; Light, M.E.; Kohout, L.; Szalai, G.; Tandori, J.; Staden, J.V.; Balázs, E. Transcriptome analysis of germinating maize kernels exposed to smoke-water and the active compound KAR1. BMC Plant Biol. 2010, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Sathish, S.; Ahamed, R.; Natesan, S.; Arulkumar, N.; Park, H.S.; Kalaiselvi, S.; Umarani, R.; Raveendran, M.; Bhaskaran, M.; Kim, G.S. Proteomic analysis of ageing in black gram (Vigna mungo L.) seeds and its relation to seed viability. Plant Omics. 2015, 8, 201–211. [Google Scholar]
- Tsunezuka, H.; Fujiwara, M.; Kawasaki, T.; Shimamoto, K. Proteome analysis of programmed cell death and defense signaling using the rice lesion mimic mutant cdr2. Mol. Plant Microbe Interact. 2005, 18, 52–59. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Xiong, L.M.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef]
- Dos Santos, C.V.; Rey, P. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci. 2006, 11, 329–334. [Google Scholar] [CrossRef]
- Rouhier, N.; Gelhaye, E.; Sautiere, P.E.; Brun, A.; Laurent, P.; Tagu, D.; Gerard, J.; de Faÿ, E.; Meyer, Y.; Jacquot, J.P. Isolation and characterization of a new peroxiredoxin from poplar sieve tubes that uses either glutaredoxin or thioredoxin as a proton donor. Plant Physiol. 2001, 127, 1299–1309. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56. [Google Scholar] [CrossRef] [PubMed]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2015. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigour determination in soybean seed multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Yan, H.F.; Mao, C.L.; Zhu, Y.Q.; Cheng, H.; Mao, P.S. Exogenous glutathione pre-treatment improves germination and resistance of Elymus sibiricus seeds subjected to different ageing conditions. Seed Sci. Technol. 2017, 45, 607–621. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Changes in malondialdehyde content and superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plantarum. 1996, 97, 104–111. [Google Scholar] [CrossRef]
- Schickler, H.; Caspi, H. Response of antioxidative enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum. Physiol. Plantarum. 1999, 105, 39–44. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Arrigoni, O.; Dipierro, S.; Borraccino, G. Ascorbate free radical reductase: A key enzyme of the ascorbic acid system. FEBS Lett. 1981, 125, 242–244. [Google Scholar] [CrossRef]
- Dalton, D.A.; Baird, L.M.; Langeberg, L.; Taugher, C.Y.; Anyan, W.R.; Vance, C.P.; Sarath, G. Subcellular localization of oxygen defense enzymes in soybean (Glycine rnax [L.] Merr.) root nodules. Plant Physiol. 1993, 102, 481–489. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Madamanchi, N.R.; Alscher, R.G. Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol. 1991, 97, 88–93. [Google Scholar] [CrossRef]


| Compared Samples | Accession | Description | FC of DEPs | FC in qRT-PCR |
|---|---|---|---|---|
| C2 vs. C1 | W5DQ12 | Proteasome subunit beta type | 0.093 | 0.497 ± 0.082 |
| B6TJX6 | PTI1-like tyrosine-protein kinase 3 | 0.057 | 6.306 ± 0.812 | |
| A0A0D3GV84 | Lon protease homolog, mitochondrial | 0.065 | 2.413 ± 0.472 | |
| A0A1Y1HNC9 | Eukaryotic translation initiation factor eIF-4A | 15.348 | 2.312 ± 0.268 | |
| M200 vs. C2 | A9UIF0 | Phospholipase D | 0.089 | 1.197 ± 0.040 |
| A0A1D5RZK8 | Nascent polypeptide-associated complex subunit beta | 7.442 | 1.749 ± 0.143 | |
| Q10P35 | Enolase 2, putative, expressed | 7.780 | 2.061 ± 0.063 | |
| M1Q6S1 | Starch synthase, chloroplastic/amyloplastic | 4.453 | 0.279 ± 0.070 | |
| W5AA91 | Importin subunit alpha | 5.507 | 15.181 ± 1.293 | |
| M1000 vs. C2 | A0A072VAH0 | Peroxidase | 25.234 | 0.591 ± 0.093 |
| A0A1D5RZK8 | Nascent polypeptide-associated complex subunit beta | 6.870 | 1.808 ± 0.206 | |
| A0A1D5VEP2 | Obg-like ATPase 1 | 0.106 | 2.596 ± 0.309 | |
| F2DCZ4 | Aconitate hydratase | 0.240 | 1.590 ± 0.076 | |
| A0A1D5RU17 | Guanosine nucleotide diphosphate dissociation inhibitor | 0.061 | 61.430 ± 12.310 | |
| M1000 vs. M200 | A0A072VAH0 | Peroxidase | 15.909 | 0.873 ± 0.113 |
| A9UIF0 | Phospholipase D | 5.824 | 0.668 ± 0.054 | |
| Q10P35 | Enolase 2, putative, expressed | 0.293 | 0.888 ± 0.049 | |
| M1Q6S1 | Starch synthase, chloroplastic/amyloplastic | 0.125 | 2.267 ± 0.585 | |
| A0A1D5VEP2 | Obg-like ATPase 1 | 0.100 | 0.686 ± 0.059 | |
| F2DCZ4 | Aconitate hydratase | 0.049 | 1.068 ± 0.011 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Jia, S.; Mao, P. Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds. Int. J. Mol. Sci. 2020, 21, 1898. https://doi.org/10.3390/ijms21051898
Yan H, Jia S, Mao P. Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds. International Journal of Molecular Sciences. 2020; 21(5):1898. https://doi.org/10.3390/ijms21051898
Chicago/Turabian StyleYan, Huifang, Shangang Jia, and Peisheng Mao. 2020. "Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds" International Journal of Molecular Sciences 21, no. 5: 1898. https://doi.org/10.3390/ijms21051898
APA StyleYan, H., Jia, S., & Mao, P. (2020). Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds. International Journal of Molecular Sciences, 21(5), 1898. https://doi.org/10.3390/ijms21051898






